2019 Volume 88 Issue 2 Pages 276-283
2019 Volume 88 Issue 2 Pages 276-283
The postharvest physiology of cut astilbe inflorescences (Astilbe × arendsii), which consist of many small florets with a short vase life, was studied in response to treatments to extend their vase life. Exogenous ethylene treatment at 0.3 μL·L−1 for 7 h did not affect the senescence of inflorescences or leaves of five cultivars and 0.2 mM silver thiosulfate for 2 h did not improve the quality of inflorescences of three cultivars, which indicated that ethylene is not a critical factor for senescence in astilbe florets. Continuous treatment with 2% sucrose or 2% trehalose solutions prolonged the cut inflorescence vase life of one or two of five astilbe cultivars, respectively. Pulse treatment with 2% trehalose in combination with 6% sucrose increased total soluble sugar contents from 11.4 to 57.6 mg·g−1 FW and raised the respiration rate of inflorescences from 15.3 to 28.4 μmol CO2·h−1·g−1 FW at 2 days after harvest (DAH) in the cultivar ‘Gloria Purpurea’. However, the effects of pulse treatment diminished at 4 DAH and extended the vase life from 4.0 days to a maximum of 5.6 days, which suggested that pulse treatments were inadequate to maintain sugar contents and respiration activity. Continuous treatment with 6% sucrose extended the vase life from 4.3 to 10.0 days and raised the chroma (C*) value of florets from 28.7 to 54.9 at 8 DAH. Continuous treatment with 2% trehalase + 4% sucrose markedly prolonged the vase life to 11.5 days and increased the C* value to 53.9 at 8 DAH. Under stereomicroscopic observation, continuous treatment with 2% trehalase + 4% sucrose maintained more vivid pink color of petals, styles, filaments and receptacles than those in control florets at 9 DAH. Combined treatment with 2% trehalose and 30 μM validamycin A, a potent inhibitor of trehalose metabolizing activity, induced severe wilting of florets and necrotic spots on leaves. Exogenous trehalose may be hydrolyzed by trehalose metabolizing activity in cut astilbe inflorescences. The results suggest that continuous treatment with trehalose and sucrose solutions is effective to maintain development and delay senescence of florets to extend the vase life of cut astilbe inflorescences.
Astilbe (Astilbe × arendsii) is a perennial plant that blooms in early summer and is cultivated as an ornamental garden plant. It is native to eastern Asia and commercial cultivars have been bred in Europe. The vase life of cut inflorescences is relatively short partly due to vascular occlusion, which occurs as a physiological response to the stress of cutting or by microbial colonization in the vascular system (Kalkman, 1986; Loubaud and van Doorn, 2004; van Doorn et al., 1993).
To rehydrate cut astilbe after 24 h of dry storage, surfactants are effective in improving water uptake and flower quality, but they do not significantly improve the vase life if the cut inflorescences are kept in water (Kalkman, 1986; van Doorn et al., 1993). Loubaud and van Doorn (2004) reported that inhibitors of peroxidase and catechol oxidase improve the vase life of astilbe after dry storage for 24 h. Antioxidants such as sodium benzoate and n-propyl gallate prolong the vase life of cut carnation flowers (Baker et al., 1977). In preliminary experiments, however, antioxidants such as sodium benzoate and n-propyl gallate did not prolong the vase life of the astilbe ‘Amethyst’ (data not shown), which suggests that antioxidants are effective only for dry storage or transportation.
Although the ethylene sensitivity of Astilbe spp. is reported to be ‘very little’ (Minnesota commercial flower growers, 1994), it has not been rigorously evaluated owing to the incidence of vascular blockage (Woltering and van Doorn, 1988).
Sucrose is often used to extend the vase life of cut flowers (Halevy and Mayak, 1979; Ichimura and Hisamatsu, 1999; Nichols and Ho, 1975; Ranwala and Miller, 2009). Norikoshi et al. (2016) reported that in cut rose flowers sucrose treatment increases glucose and fructose concentrations in the vacuole, which may reduce the osmotic potential of the symplast and increase water uptake leading to cell expansion during flower opening. Sucrose treatment extends the vase life, and gene expression, of sucrose transporters and invertase is involved in maintaining quality in the petals of cut peony (Xue et al., 2018). Given that the astilbe inflorescence comprises numerous small florets and is often harvested at the stage when 50% of the florets are open, the supply of soluble sugars, such as sucrose and glucose, may contribute to opening of florets in the distal portion of the inflorescence after harvest.
Trehalose is an atypical disaccharide composed of α-d-glucopyranosyl-α-d-glucopyranoside (Sussich et al., 1998). Trehalose is found in bacteria, yeast, fungi, insects, and invertebrate animals but is only rarely present in higher plants (Müller et al., 1995b). However, trehalose prolonged the vase life of cut flowers of several species, including the gladiolus (Otsubo and Iwaya-Inoue, 2000; Yamada et al., 2003; Yamane et al., 2005) and tulip (Iwaya-Inoue and Takata, 2001). Trehalose maintains turgor in petal tissues of cut tulip flowers (Iwaya-Inoue and Takata, 2001). In addition, trehalose has been reported to be a retardant of programmed cell death in gladiolus (Yamada et al., 2003). Astilbe has a short vase life and water deficit in inflorescences is thought to be a cause of the short vase life in astilbe as describe above (Kalkman, 1986; Loubaud and van Doorn, 2004; van Doorn et al., 1993). Therefore, we expected trehalose to maintain the water content in petals and prolong vase life.
Trehalase is synthesized in higher plants and hydrolyzes a trehalose molecule into two glucose molecules (Müller et al., 1995a, b; Paul et al., 2008). In cut tulip flowers, trehalose treatment increased fructose and sucrose contents (Ranwala and Miller, 2009). However, whether cut flowers in general are capable of metabolizing trehalose is not well understood.
In the present study, we examined the effects of ethylene and sugar treatments on physiological processes in cut astilbe inflorescences with the aim of improving their short vase life. Especially, we tried to combine treatments of trehalose and sucrose to improve water balance and supply of carbohydrate resources to open buds and maintain respiration substrates. Validamycin A, a potent inhibitor of trehalase metabolizing activity, was used to elucidate the function of trehalose metabolizing in astilbe tissues. The role of trehalose and sucrose in cut astilbe inflorescences is discussed.
Astilbe plants (Astilbe × arendsii) ‘Gloria Purpurea’, ‘Mainz’, ‘Diamond’, ‘Amethyst’, and ‘Erica’ were grown in an experimental field of Utsunomiya University (36°32'55" N, 139°54'42" E). Cut inflorescences were harvested when 50% of their florets were open with or without leaves. Cut flowers were stored and evaluated at 20–21°C and 60% relative humidity under cool white fluorescent light of 15 μmol·m−2·s−1 photosynthetic photon flux density in the following experiments.
Experiment 1: Effect of exogenous ethylene and silver thiosulfate on vase lifeCut inflorescences with leaves of ‘Mainz’, ‘Diamond’, ‘Amethyst’, ‘Gloria Purpurea’, and ‘Erica’ were placed in distilled water to evaluate the ethylene sensitivity of florets and leaves. Five or six cut inflorescences were treated with 0.3 μL·L−1 exogenous ethylene in a desiccator for 7 h at 20°C. Control flowers were not treated with ethylene.
Five cut inflorescences that lacked leaves from ‘Amethyst’, ‘Gloria Purpurea’, and ‘Erica’ were treated with or without 0.2 mM silver thiosulfate for 2 h at 21°C, then placed in distilled water and incubated at 21°C.
Vase life was evaluated daily until 50% of the florets on the inflorescence were wilted or faded.
Experiment 2: Effects of sucrose and trehalase on vase lifeCut inflorescences without leaves from ‘Mainz’, ‘Diamond’, ‘Amethyst’, ‘Gloria Purpurea’, and ‘Erica’ were kept in distilled water, 2% sucrose solution, or 2% trehalose solution. Three to six cut inflorescences were used for each treatment. Vase life was evaluated daily until 50% of the florets on the inflorescence were wilted or faded.
Experiment 3: Effects of pulse treatment with sugar on vase life, sugar contents, and respiration rateCut inflorescences without leaves from ‘Gloria Purpurea’ were pulse-treated for 24 h with the following solutions: distilled water (control), sucrose at either 2, 4, 6, 8, 10, or 12% (w/v), 2% trehalose alone, and 2% trehalose plus sucrose at all the concentrations mentioned above. A higher trehalose concentration was not used in this experiment, since pulse treatments with 4% trehalose plus 2, 4, or 6% sucrose did not improve the vase life in ‘Gloria purpurea’ in a preliminary experiment. Cut inflorescences (five replicates per treatment) were placed at 5°C in the dark for 24 h as a precooling stage. To prevent bacterial contamination, all treatments were treated with 1% (v/v) bactericide with inorganic ions (Misaki Farm BC; Otsuka Chemical, Tokyo, Japan). After the pulse treatments, vase solutions were discarded and the inflorescences were placed in distilled water at 20°C. Vase solutions were replaced and stems of inflorescences were recut at 5 and 10 days after harvest (DAH). Chroma (C*) of inflorescences was measured using a colorimeter (NR-3000; Nippon Denshoku Industries, Tokyo, Japan).
Soluble sugar contentContents of soluble sugars were measured in florets of cut inflorescences pulse-treated with 2% trehalose, 6% sucrose, and a combination of 2% trehalose and 6% sucrose, and in control florets at 0, 2, 4, and 6 DAH. Approximately 0.5 g florets were sampled from the middle portion of the inflorescences and dried with a freeze dryer. Four replicates of dried samples were weighed and 5% rhamnose was added as an internal standard. Soluble sugars in the samples were extracted with 80% ethanol at 80°C for 30 min. Samples were centrifuged at 11,000 × g for 10 min and the supernatant was recovered. Extraction was repeated three times. Ethanol and water were removed by a centrifugal evaporator (CVE-200D; EYELA, Tokyo, Japan). Resultant pellets were dissolved in 50 μL of pure water (RFD 250NB; ADVANTEC, Toyo Roshi Kaisha, Tokyo, Japan) and 50 μL of 75% acetonitrile, and filtered through a 20 μm Millipore filter. Samples were analyzed by high-performance liquid chromatography (SCL-6B; Shimadzu, Kyoto, Japan) using a refractive index detector (RI-8022; Tosoh, Tokyo, Japan). The separation column (TSK-gel Amide 80; Tosoh, Tokyo, Japan) was eluted with 75% acetonitrile at a flow rate of 1 mL·min−1, at an oven temperature of 80°C. Glucose, fructose, sucrose, and trehalose were identified and quantified by comparison with authentic standards.
Respiration rateRespiration rate of inflorescences, which were pulse-treated with 2% trehalose, 6% sucrose, or a combination of 2% trehalose and 6% sucrose, and control inflorescences were measured at 0, 2, 4, and 6 DAH. Four branches of each replicate inflorescence were cut and weighed, and then placed in 15 mL screw-capped centrifuge tubes containing 1 mL distilled water. The tubes were tightly capped and incubated in a water bath at 25°C. From the tubes, 1 mL gas samples were taken with a syringe through a rubber septum on the cap at 0 and 30 min after incubation. The CO2 content in the gas samples was determined using a gas chromatograph (GC2014; Shimadzu, Kyoto, Japan) equipped with a thermal conductivity detector and a PorapakTM Q column (1.0 m × 3.0 mm I.D., Shinwa Chemical Industries, Kyoto, Japan).
Experiment 4: Effects of continuous treatment with sugar on vase lifeCut inflorescences with five replications were continuously treated with the following solutions: distilled water (control), sucrose at 2, 4, 6, 8, 10, or 12% (w/v), 2% trehalose alone, or 2% trehalose plus sucrose at all the concentrations mentioned above. All treatment solutions contained a bactericide (Legend MK; Chuokasei, Osaka, Japan) at 0.5 mL·L−1. The inflorescences were placed at 5°C in the dark for 24 h. Vase life of the inflorescences was evaluated at 20°C as previously described. All vase solutions were replaced at 5 and 10 DAH and stems of inflorescences were recut at those times.
Observation of florets under a stereomicroscopeFlorets on the cut inflorescences treated continuously with 2% trehalose, 4% sucrose, 2% trehalose in combination with 4% sucrose solution, or distilled water (control) were observed under a stereomicroscope (MZ16F; Leica Microsystems) at 0 and 9 DAH.
Experiment 5: Effects of trehalose on trehalase activity and sugar contentsCut inflorescences with five replications were continuously treated with the following solutions: distilled water (control), 30 μM validamycin A (a potent inhibitor of trehalose metabolizing activity), 0.5%, 2%, or 4% trehalose alone, or a combination of 2% trehalose and 30 μM validamycin A. All treatment solutions contained a bactericide (Legend MK; Chuokasei, Osaka, Japan) at 0.5 mL·L−1. The inflorescences were incubated at 20°C as described above.
The fresh weight of the cut inflorescences was measured daily and is presented as the difference from that at harvest, i.e., 0 DAH. Soluble sugar contents were determined at 3 DAH as described for Experiment 3.
Statistical analysisData were analyzed by one-way or two-way analysis of variance (ANOVA) and the Tukey–Kramer honest significant difference (HSD) test using the JMP Ver.6.0 software package (SAS Institute Inc., Cary, NC, USA).
The symptoms of flower senescence on cut astilbe inflorescences are wilting and fading of florets on the inflorescence. Exogenous ethylene treatment at 0.3 μL·L−1 for 7 h did not affect the senescence of inflorescences or leaves of the five cultivars (Table S1). Treatment with 0.2 mM silver thiosulfate for 2 h did not improve the quality of inflorescences of the three cultivars (Table S2). These results indicated that ethylene is not a critical factor for senescence in astilbe florets and are consistent with a previous report (Minnesota Commercial Flower Growers, 1994). Woltering and van Doorn (1988) could not evaluate the ethylene sensitivity of astilbe flowers owing to the incidence of vascular blockage. In the present study, ethylene sensitivity was evaluated without vascular blockage because cut inflorescences were recut under water immediately after harvest, and then transferred to the incubation room on the same campus without dry transportation. Woltering and van Doorn (1988) applied 3 μL·L−1 exogenous ethylene for 24 h to evaluate its sensitivity, but the ethylene was treated under mild conditions in this study. Therefore, a more severe ethylene treatment is needed to confirm the ethylene sensitivity of astilbe.
Effects of trehalose and sucrose on the quality, respiration rate, and soluble sugar contentsSignificant effects on vase life were observed among the sugar treatments and cultivars (Table 1). Among the five cultivars, 2% trehalose significantly extended the vase life of ‘Mainz’ and ‘Amethyst’ (Table 1). Treatment with 2% sucrose extended the vase life of ‘Amethyst’ but not the other cultivars (Table 1). In this experiment, 1% bactericide with inorganic ions was employed, but this product did not show any effect on cut astilbes compared to other bactericides. These results indicated that effects of the sugar treatments varied among the cultivars and that the concentrations tested may have been insufficient to extend the vase life of astilbe inflorescences.

Vase life of cut astilbe flowers continiuously treated with 2% sucrose and 2% trehalose.
Pulse treatments of sucrose and trehalose slightly improved the vase life of ‘Gloria Purpurea’ (Fig. 1A; Table S3). Pulse treatment with 2% trehalose + 6% sucrose increased total soluble sugar contents from 11.4 to 57.6 mg·g−1 FW (Fig. 2A) and raised the respiration rate of inflorescences from 15.3 to 28.4 μmol CO2·h−1·g−1 FW at 2 DAH (Fig. 2B). These results were indicative of high metabolic activity in opening florets from the middle to the distal portion of the inflorescences. The substantial increase in total soluble sugar content with sucrose and trehalose treatment was reported previously for gladiolus (Yamane et al., 2005) and tulip (Ranwala and Miller, 2009). However, the effects of pulse treatment diminished at 4 DAH (Fig. 2), which suggested that pulse treatments were inadequate to maintain the sugar contents and respiration activity.

Effects of treatments on cut inflorescences of the astilbe ‘Gloria Purpurea’. A: Inflorescences pulse-treated with 2% trehalose (Tre) + 4% sucrose (Suc) for 24 h and untreated inflorescences (Control). The photograph was taken at 6 days after harvest (DAH). B: Inflorescences treated continuously with 2% Tre + 4% Suc and untreated inflorescences (Control). The photograph was taken at 14 DAH. C: Florets on the cut inflorescences treated continuously with 2% Tre, 4% Suc, 2% Tre + 4% Suc solution, and untreated inflorescences (Control) at 0 and 9 DAH. D: Inflorescences treated continuously with 30 μM validamycin A, 2% Tre, 2% Tre + validamycin A, and untreated inflorescences (Control). The photograph was taken at 4 DAH.

Total soluble sugar contents in florets (A) and respiration rate of inflorescences (B) of cut inflorescences of the astilbe ‘Gloria Purpurea’ pulse-treated for 24 h with 2% trehalose (Tre), 6% sucrose (Suc), and 2% Tre + 6% Suc. Vertical bars indicate the SE (n = 4). Means with the same letter on the same day for a physiological parameter are not significantly different according to the Tukey–Kramer HSD test (P < 0.05).
Continuous treatment with 6% sucrose markedly extended the vase life from 4.3 to 10.0 d and raised the chroma (C*) value of florets from 28.7 to 54.9 at 8 DAH, preventing the fading of petals and receptacles in florets (Table 2). Continuous treatment with 2% trehalose in combination with 4% sucrose markedly prolonged the vase life to 11.5 d and increased the C* value to 53.9 at 8 DAH (Table 2; Fig. 1B). Under stereomicroscopic observation, continuous treatments of 2% trehalase + 4% sucrose maintained a more vivid pink color of petals, styles, filaments and receptacles than those in control florets at 9 DAH (Fig. 1C). These results suggested that anthocyanin contents in not only the petals, but also in styles, filaments and the receptacle, were maintained in response to treatment with high sugar concentrations. In this study, we did not examine any combinations of trehalose at more than 2% with sucrose in continuous treatment and this requires further investigation.

Vase life and C* of cut flowers in ‘Gloria Purpurea’ continiuously treated with sucrose (Suc) and trehalose (Tre).
Sucrose at higher than 10% or in combination with trehalose supplied as pulse and continuous treatments did not significantly prolong the vase life and in some cases shortened it (Table 2). Excessive concentrations of sugar in floral preservatives may be harmful, especially to petals, since less water was absorbed to petals presumably due to the low water potential of sugar solutions (Halevy and Mayak, 1979).
Effects of trehalose and validamycin A on quality of inflorescencesTreatment with 0.5, 2, or 4% trehalose maintained the fresh weight of cut inflorescences; the fresh weight was elevated with increases in trehalose concentration (Fig. S1). These results were consistent with the suggestion that trehalose may play a role in promoting water retention in cut flowers (Otsubo and Iwaya-Inoue, 2000).
Contents of sucrose and total soluble sugars, except trehalose, in the leaves of cut inflorescences were significantly raised by the 2% and 4% trealose treatments (Table 3). This finding is consistent with the increase in fructose and sucrose contents in cut tulip flowers treated with trehalose (Ranwala and Miller, 2009). Pulse treatment with 2% trehalose raised the respiration rate of cut astilbe inflorescences from 15.3 to 21.2 μmol CO2·h−1·g−1 FW at 2 DAH (Fig. 2), which suggested that trehalose treatments increased the sugar content and thus raised the respiration rate.
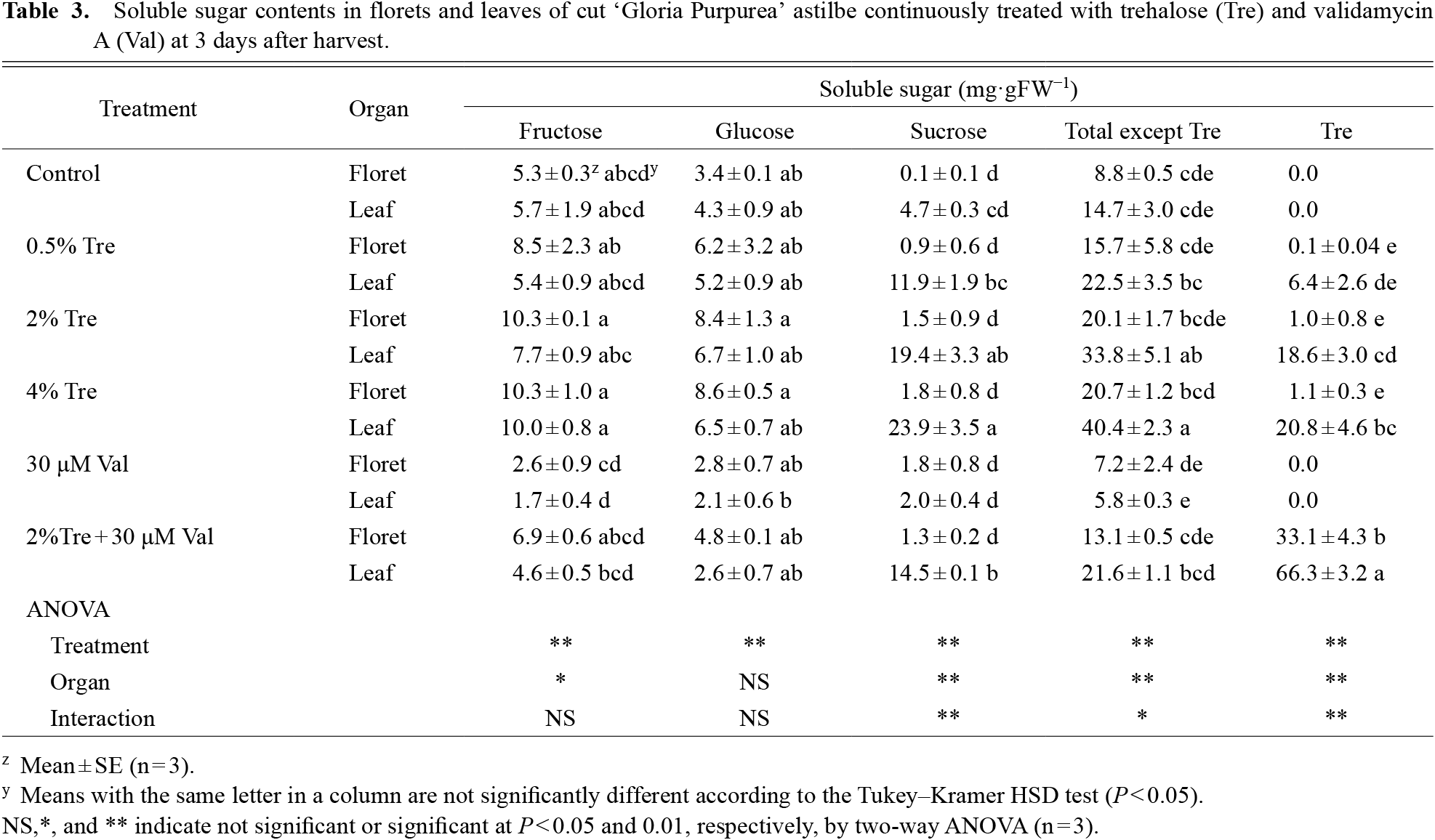
Soluble sugar contents in florets and leaves of cut ‘Gloria Purpurea’ astilbe continuously treated with trehalose (Tre) and validamycin A (Val) at 3 days after harvest.
Trehalose treatments significantly increased sucrose and trehalose contents in leaves, but to a much lesser degree in florets (Table 3). These results were consistent with a previous study on cut tulip flowers (Ranwala and Miller, 2009). Exogenous trehalose application caused substantial accumulation of trehalose in leaves (as high as 80 mg·g−1 FW) and to a much lesser extent in tepals (less than 10 mg·g−1 FW) (Ranwala and Miller, 2009). These results indicated that exogenous trehalose was metabolized by trehalase and converted to sucrose, and disaccharides accumulated especially in the leaves of cut astilbe inflorescences.
Combined treatment of 2% trehalose and 30 μM validamycin A significantly increased trehalose contents in the florets and leaves (Table 3), and induced severe wilting of the florets (Fig. 1D) and necrotic spots on the leaves (data not shown). Validamycin A inhibits trehalose metabolizing activity and excessive amounts of trehalose accumulated in floret and leaf tissues. López et al. (2009) reported that validamycin A improves the response to salt stress by inducing trehalose accumulation in root nodules of Medicago truncatula. However, excessive accumulation of trehalose, i.e., 66.3 mg·g−1 FW, induced by exogenous trehalose and validamycin A application showed that the treatment had adverse effects on florets and leaves of astilbe in the present study (Table 3). Trehalose has been found to be toxic to plants, and plant trehalase is functional in metabolizing trehalose from exogenous and endogenous sources (Müller et al., 1995a). Daily maximum water absorption of cut inflorescences of the astilbe ‘Gloria Purpurea’ treated with 2% trehalose and 2% sucrose exceeded 55% of the fresh weight at harvest of the cut inflorescence, even in inflorescences lacking leaves (data not shown). Accumulation of excess sucrose and trehalose increases the risk of wilting or necrosis, and their concentrations should be carefully adjusted depending on the treatment condition.
Roles of sucrose and trehaloseSucrose treatment improves water balance and extends the vase life of a variety of cut flowers (Halevy and Mayak, 1979). Developing flowers are active sink organs that require a continuous supply of carbohydrates as an energy source, as building blocks for cell wall synthesis, and to maintain osmotic potential (Halevy and Mayak, 1979; Ichimura and Hisamatsu, 1999; Nichols and Ho, 1975; Ranwala and Miller, 2009). In the present study, continuous treatment with sucrose tended to extend the vase life of astilbe inflorescences (Table 2), whereas pulse treatments were not useful to prolong the vase life (Table S3). Probably, cut astilbe inflorescences do not accumulate sufficient amounts of carbohydrates in the thin stems and small florets during sugar pulse treatments.
Trehalose was reported to be more effective in stabilizing both unilamellar vesicles and biological membranes than other sugars such as sucrose and glucose (Crowe et al., 1987). Trehalose also alleviates chilling stress in pepper membranes (Ding and Wang, 2018). In cut flowers, exogenous trehalose increases soluble sugar contents and prolongs the vase life of cut astilbe inflorescences (Table 3), gladiolus (Yamane et al., 2005), and tulips (Ranwala and Miller, 2009). Trehalose treatment also suppressed water loss (Fig. S1) and enhanced viability in petals, although slight wilting of the bracts of cut gladiolus has been observed (Otsubo and Iwaya-Inoue, 2000). Yamada et al. (2003) reported that trehalose has a suppressive effect on apoptotic cell death. In this context, trehalose may play several roles in retaining membrane integrity and turgor in petal cells and in delaying programmed cell death.
In conclusion, the application of trehalose and sucrose is effective to maintain development and delay senescence of florets, extending the vase life of cut astilbe inflorescences. Analysis of gene expression after treatments with trehalose and sucrose is needed to understand the roles of these sugars in the senescence of astilbe florets.
The authors thank Mr. Shin Takahashi and Miyoshi Group & Co., Ltd. for providing materials, and Dr. Masaru Matsuda of the Bioscience Education and Research Center, Utsunomiya, for supporting microscopic observations.