2019 Volume 88 Issue 2 Pages 293-298
2019 Volume 88 Issue 2 Pages 293-298
Pyridinedicarboxylic acid (PDCA) analogs extend the vase life of spray-type (SP) carnation flowers by accelerating flower opening and retarding senescence. Among the PDCA analogs, 2,3-PDCA and 2,4-PDCA are equally and highly effective. In the present study, we characterized the promotion of flower opening by PDCAs and their analogs, pyridinecarboxylic acids. The present results showed that 3-pyridinecarboxylic acid (3-PCA), and its derivative, 3-PCA amide, are most active in accelerating flower opening of ‘Light Pink Barbara’ carnation cut flowers. 3-PCA treatment promoted flower opening in cut flowers of many SP carnation cultivars, that had been stored dry at 2–4°C for 3 weeks and resulted in extension of their display time.
Vlad et al. (2010) reported that 2,4-pyridinedicarboxylic acid (2,4-PDCA) inhibited ethylene production in detached flowers of ‘White Sim’ carnations, a standard type of carnation flower with only one flower per stalk (stem). Senescence was delayed, resulting in extension of the vase life of the flowers. This action of 2,4-PDCA was later confirmed in spray-type (SP) carnation flowers, such as ‘Light Pink Barbara (LPB)’ and ‘Mule’, which have a main stalk with several offshoots, each having one or two flowers at their tips (Satoh et al., 2014). Sugiyama and Satoh (2015) revealed that PDCA analogs, including 2,3-, 2,4-, 2,5-, 2,6-, 3,4- and 3,5-PDCA, accelerated flower opening in addition to prolonging vase life in ‘LPB’ carnation flowers. To the best of our knowledge, this is the first report of chemicals with dual effects on cut carnation flowers. PDCA analogs were effective in a wide range of SP carnation cultivars other than ‘LPB’ and ‘Mule’, including ‘Barbara’, ‘Beam Cherry’, ‘Candle’, ‘Collin’, ‘Rascal Green’, and ‘Scarlet Ostara’ (Satoh and Nomura, 2016). Among the PDCA analogs, 2,3-PDCA and 2,4-PDCA were the most effective in extending the vase life and accelerating flower bud opening in ‘LPB’ carnation flowers. These results suggested that these PDCAs may be useful as flower care agents in the near future.
Extension by 2,4-PDCA of the vase life of cut carnation flowers is mainly due to reduced ethylene production (Satoh et al., 2014; Vlad et al., 2010). Regarding the action mechanism of 2,4-PDCA for reducing ethylene production in senescing carnation flowers, Vlad et al. (2010) hypothesized that it inhibits 1-aminocyclopropane-1-carboxylate (ACC) oxidase by competing with ascorbate, a co-substrate of the enzyme action. Actually, the inhibition of in vitro activity of ACC oxidase by 2,4-PDCA was shown using an enzyme prepared from tomato pericarp tissues (Fragkostefanakis et al., 2013) and a recombinant enzyme produced in Escherichia coli cells from the carnation ACC oxidase gene, DcACO1 cDNA (Satoh et al., 2014). These observations supported the hypothesis that 2,4-PDCA inhibits ACC oxidase action in carnation flowers and reduces ethylene production. However, it is uncertain whether this hypothesis is applicable to PDCA analogs other than 2,4-PDCA. It is therefore necessary to test whether or not each chemical actually inhibits the action of ACC oxidase.
Satoh et al. (2014) hypothesized the association of gibberellin (GA) with the promoting action of 2,4-PDCA on flower bud opening in cut carnation flowers. This hypothesis arose from the notion that 2,4-PDCA is a structural analog of 2-oxoglutarate, which is a co-substrate for enzymes acting in GA biosynthesis and inactivation, such as GA 3β-dioxygenase (GA 3β-hydroxylase), GA-44 dioxygenase, and GA 2β-dioxygenase (GA 2β-hydroxylase) (Hedden and Kamiya, 1997; Lange et al., 1994a, b; Smith and MacMillan, 1984, 1986). Recently, however, Morita et al. (2017) reported that GA is not involved in the enhancing effect of 2,4-PDCA on flower opening of ‘LPB’ carnations. Thus, the action mechanism of PDCAs for accelerating flower opening in carnation remains unclear.
In the course of our previous study to test whether 2,3-PDCA and 2,4-PDCA have GA-like activity, we used a bioassay system, in which exogenously applied GA promotes hypocotyl elongation of lettuce seedlings (Satoh and Nomura, 2017). Unexpectedly, we found that 2,3-PDCA promoted root elongation, whereas 2,4-PDCA inhibited it in lettuce, carrot and rice seedlings. Further analysis using rice seedlings revealed that, among PDCAs and pyridinecarboxylic acids (PCAs), 3-PCA was the most effective at promoting root elongation, suggesting that a carboxyl group substituted on position 3 of the pyridine ring is necessary to exert its activity on root elongation.
In the present study we investigated the effects of 3-PCA and its amide, in comparison with those of PDCA analogs including 2,3- and 2,4-PDCA, on flower bud opening in cut flowers of ‘LPB’ carnations. Moreover, we investigated whether or not 3-PCA treatment promotes flower opening in some cultivars after long-term chilling, which reduces their flower opening ability.
The effects of pyridinedicarboxylic acid (PDCA) and pyridinecarboxylic acid (PCA) analogs on acceleration of flower opening and extension of vase life were examined using Dianthus caryophyllus L. ‘Light Pink Barbara (LPB)’, a spray-type (SP) carnation flower. Flowers at the usual commercial stage of flowering were harvested with 65-cm-long stalks, when the first flower out of six to eight flower buds on a stalk was partially open, at the nursery of a commercial grower in Miyagi Prefecture, Japan. The carnation flowers were harvested in the afternoon, immediately placed in plastic containers with their cut stalk ends in tap water, and dispatched the next morning to the Faculty of Agriculture of Ryukoku University, Otsu, Shiga Prefecture, with no water supplied during transportation. The flowers were not treated with any flower preservatives, such as sodium silverthiosulfate anionic complex (STS), after harvest. Upon arrival the next day, the flowers were placed in plastic buckets with their cut stalk ends in tap water under continuous light from white fluorescent lamps (14 μmol·m−2·s−1 PPFD) at 23°C and 40–60% relative humidity, for several hours before the start of experiments. Each experiment was repeated three times on April and May in 2017, and May in 2018. A typical result from the three experiments is shown in the text as Figure 2, and results of two other experiments can be found in Supplementary data.
In the experiments to examine the effects of 3-PCA on flower opening after long-term chilling (as described below), carnation flowers of different cultivars were used, i.e. ‘Aragon’, ‘Kim’, ‘Eufolia’, ‘Ornella’, ‘Pigeon’, ‘Scarlette Plus’, ‘Skyline’, and ‘Sirasi’. Flowers of these cultivars were harvested in the nursery of Sagaro Flowers, Bogota, Colombia at the usual commercial stages and treated with STS by conventional commercial procedures. One sample of the flowers was stored at 2°C for 3 weeks under dry conditions (without supplying water), and they were transported by air to Japan. The flowers reached our laboratory at Ryukoku University seven days after they were sent. Another sample of the flowers was transported to Japan immediately after harvest and STS treatment. After arrival at our laboratory, the flowers were stored at 4°C for 3 weeks under dry conditions. The storage under these conditions was called ‘long-term chilling’. The experiments were carried out in March–May, 2018.
Profile of flower opening and senescence of carnation flowers treated with PDCA and PCA analogsThree samples (bunches) of five flower stalks (trimmed to 60-cm long), each having five flower buds (25 buds in total per sample), were put in 0.9 L glass jars with their stalk ends in 300 mL of test solution (one sample per glass jar). Flower samples were treated with PDCA or PCA analogs at 5 mM for 24 h, and kept in distilled water thereafter. PDCA analogs were 2,3-PDCA, 2,4-PDCA, and 3,4-PDCA, and PCA analogs used were 2-PCA, 3-PCA, 3-PCA amide, and 4-PCA. The pH of these test solutions was adjusted to 7 with 1 M NaOH. The flowers were kept under continuous light from white fluorescent lamps (14 μmol·m−2·s−1 PPFD) at 23°C and 40–60% relative humidity for 30 days (Fig. 2) or 20 days (Fig. 3). During the 30-day period, the distilled water was replaced once a week. Fully-open and non-senescent (not wilted or turgid) flowers (FONS flowers), at flower opening stages Os 6 to Ss 2 (Harada et al., 2010; Morita et al., 2011), were counted daily and the percentage of these flowers from the total number (25) of initial flower buds per sample was calculated. Data are presented as the percentages of fully-open and non-senescent (FONS) flowers during experiments. Flower samples having 20% or more FONS flowers were regarded having display value. The time to flower opening was determined as the number of days from the start of the experiment to the time when the percentage of FONS flowers reached 20% (Sugiyama and Satoh, 2015). The vase life of cut flowers is shown by the number of days during which the percentage of FONS flowers was 20% or more. Data are shown by the mean ± SE. All the chemicals used were purchased from Wako Pure Chemical Industries, Ltd., Osaka, Japan.
Previously, the vase life of SP carnation cut flowers was defined as the period during which 40% or more flowers were fully-open and non-senescent (FONS) (Satoh et al., 2005, 2014). In the present study, the maximum percentage of FONS flowers in the untreated control, which did not surpass 20% in two out of the three experiments, were much lower than those obtained previously with ‘LPB’ flowers. For example, 66–88% (Satoh et al., 2014) compared with 100% (Satoh et al., 2005). The percentage of FONS flowers was also low in ‘LPB’ and ‘Mule’ carnations (Satoh et al., 2014; Sugiyama and Satoh, 2015), probably due to the differences in the vigor to open among flower samples, which were cultivated and harvested in different years and seasons. Therefore, as described in the Materials and Methods section, flower samples with 20% or more FONS flowers were regarded having display value. To show percentages of flower opening, flower samples with 16% and 68% FONS flowers, respectively, are shown in Figure 1. Owing to the low percentage of FONS in the untreated control, the promotive effect of PCA and PDCA analogs on flower opening and senescence was more clearly demonstrated in the present study.

‘Light Pink Barbara’ flowers with 16% (A) and 68% (B) fully-open and non-senescent (FONS) flowers, respectively. Bunches of five flower stalks with 25 buds in total were left in water (A: control) and in 5 mM 3-PCA for one day then in water (B: 3-PCA treated). Photographs were taken six days after the start of the experiment. The control sample with a 16% value had only four FONS flowers, but the treated sample with a 68% value had 17 FONS flowers, out of 25 flower buds, respectively. The data were from the experiment shown in Supplementary Figure 2.
We first examined the effect of PCA analogs, 2-, 3-, 4-PCA and 3-PCA amide, in comparison with PDCAs, 2,3-, 2,4-, and 3,4-PDCA, on the time to flower opening and the vase life of ‘LPB’ carnation flowers. We repeated this experiment three times, and obtained similar results; a typical result is shown in Figure 1.
Figure 1 shows the changes in the percentage of FONS flowers of ‘LPB’ carnations that were treated for the first day with PCA and PDCA analogs at 5 mM, then left in water for another 29 days. The effects of PCA analogs and PDCA analogs are shown in Figure 2A and Figure 2B, respectively, and the latter included the effect of 3-PCA as a reference.
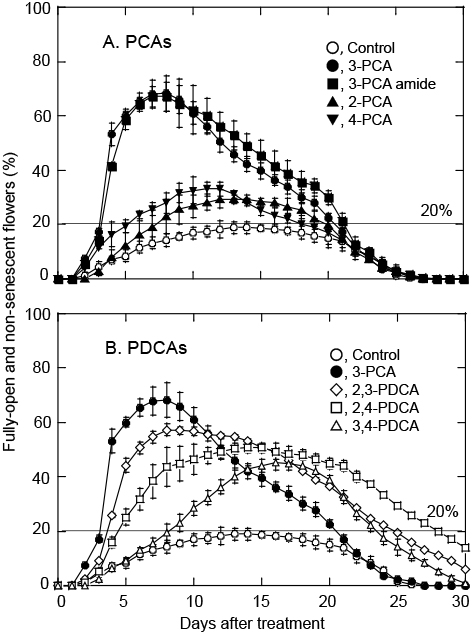
Changes in the percentage of fully-open and non-senescent cut ‘LPB’ flowers treated with or without PCA (A) and PDCA (B) analogs at 5 mM for one day then kept in water until day 30. Data show the mean ± SE of three replicates, each with 25 flower buds. Data for the control and 3-PCA treatment are shown in both figures for comparison.
3-PCA and 3-PCA amide markedly promoted the opening of cut ‘LPB’ flowers and reduced the days to flower opening (Fig. 2A). 2-PCA and 4-PCA also accelerated flower opening, but their effects were weaker than those of 3-PCA and 3-PCA amide. Moreover, 2,3-PDCA, 2,4-PDCA, and 3,4-PDCA accelerated flower opening and the magnitude of their promotive effects decreased in this order, as reported previously (Sugiyama and Satoh, 2015).
Table 1 summarizes the days to flower opening and the vase life of the flowers as affected by the treatment with PCA and PDCA analogs, which were determined in the three experiments shown Figure 1 and Supplementary Figures 1 and 2. Flower opening was earliest in the flowers treated with 3-PCA and 3-PCA amide, 3.1 days, followed by 4-PCA (4.2 days), 2,3-PDCA (4.2 days), and 2-PCA (6.8 days) in this order. Among the PCA analogs, 3-PCA was more effective than 4-PCA and 2-PCA in promoting flower opening. These finding suggested that the carboxyl group at position 3 of the pyridine ring is needed for the promotion of flower opening. This agreed with a previous experiment in which 3-carboxylic acid was necessary to promote root elongation in rice seedlings (Satoh and Nomura, 2017). However, in the present study, both 3-PCA and 3-PCA amide were equally effective in promoting flower opening. This result was in contrast with the previous experiment, in which 3-PCA promoted, while 3-PCA amide inhibited, the root elongation of rice seedlings. Moreover, 3-PCA and 3,4-PDCA similarly promoted root elongation in rice seedlings (Satoh and Nomura, 2017). However, they had markedly different effects on the promotion of flower opening in ‘LPB’ carnations; 3-PCA was much more effective than 3,4-PDCA. The discrepancy in the promotion effect between 3-PCA and 3-PCA amide, or 3-PCA and 3,4-PDCA suggest differences in the mechanism of action of 3-PCA between the promotion of flower opening in carnations and that of root elongation in rice seedlings. The difference in the action mechanism between the two phenomena remains to be elucidated.
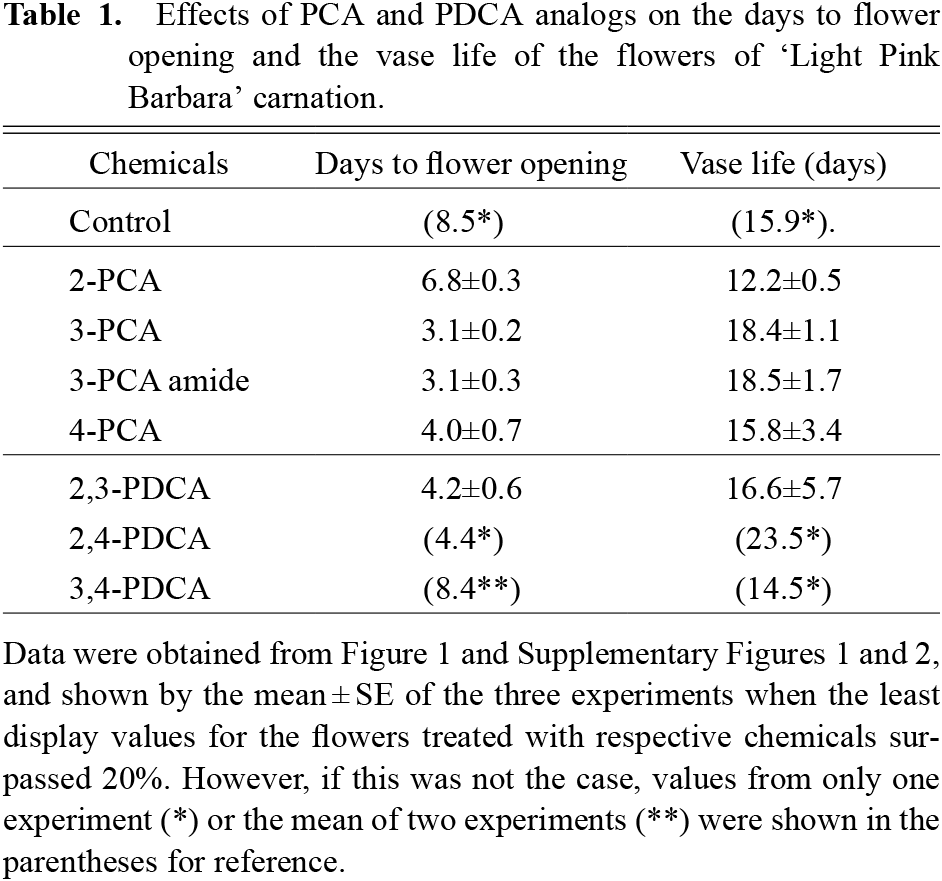
Effects of PCA and PDCA analogs on the days to flower opening and the vase life of the flowers of ‘Light Pink Barbara’ carnation.
The vase lifves were 18.4 and 18.5 days in the flowers treated with 3-PCA and 3-PCA amide, respectively. In the flowers treated with 2-PCA and 4-PCA, they were 12.2 and 15.8 days, respectively. The flowers treated with 2,4-PDCA had a long vase life of 23.5 days, although the value was obtained from a single experiment. Previously, it was shown that 2,4-PDCA has dual actions in carnation flowers; one action is to promote flower opening and the other is to inhibit ethylene production in flowers by inhibiting the action of 1-aminocyclopropane-1-carboxylate oxidase (Satoh et al., 2014; Sugiyama and Satoh, 2015; Vlad et al., 2010). The longer vase life of the flowers treated with 2,4-PDCA may be caused by the pronounced inhibition of ethylene production as compared with the acceleration of flower opening. Recently, Sun et al. (2017) reported that 2-PCA blocks ethylene biosynthesis by inhibiting the ACC oxidase action, but 3-PCA does not. Therefore, 3-PCA, and probably 3-PCA amide, promoted only flower opening, but did not inhibit ethylene production, resulting in a shorter vase life than that of 2,4-PDCA-treated flowers. Overall, the present findings revealed that some PCA analogs, represented by 3-PCA, promoted flower opening in spray-type ‘LPB’ carnations, similar to the previously shown promotion of root elongation in rice seedlings (Satoh and Nomura, 2017).
Promotion by 3-PCA treatment of flower opening in various SP carnation cultivars that had been stored chilled for 3 weeksIn Japan, the demand for cut carnation flowers changes largely depending on religious, seasonal and special events such as New Year, Spring and Autumn Equinox Weeks, Bon Holiday Days, entrance and graduation ceremonies, bridal and funeral ceremonies, and so on. If we could store pre-harvested cut carnation flowers for a long period at low temperature to bring more balance to the supply of the flowers, it would great benefit the carnation flower industry in Japan.
However, we found in preliminary experiments that long-term chilling of SP carnation sometimes reduces the ability of the flowers to open fully after they are returned to room temperature. We assumed that application of 3-PCA could promote flower opening that was inhibited by long-term chilling. Therefore, we examined the effect of 3-PCA on the flowering of SP carnation flowers, which were stored at a chilling temperature for a long period. Actually, we used cut SP carnation flowers harvested in Colombia and stored at 2°C for three weeks before shipping or at 4°C for three weeks after arrival in Japan. The former storage was conducted at a nursery in Colombia, and the latter at the laboratory of Ryukoku University, Otsu, Shiga Prefecture.
In the first experiments, carnation flowers of various cultivars, ‘Aragon’, ‘Euforia’, ‘Kim’, ‘Ornella’, ‘Pigeon’, ‘Scarlette Plus’, ‘Sirasi’, and ‘Skyline’, were harvested at the commercial stage in a nursery in Colombia, and stored at 2°C for three weeks after harvest, then shipped by air to our laboratory at Ryukoku University. The transportation took one week. After arrival, the flowers were treated without (control) or with 3-PCA at 5 mM for one day, then supplied water for 19 days at 23–25°C. Figure 3A shows changes in the percentage of FONS flowers in carnation cultivars.
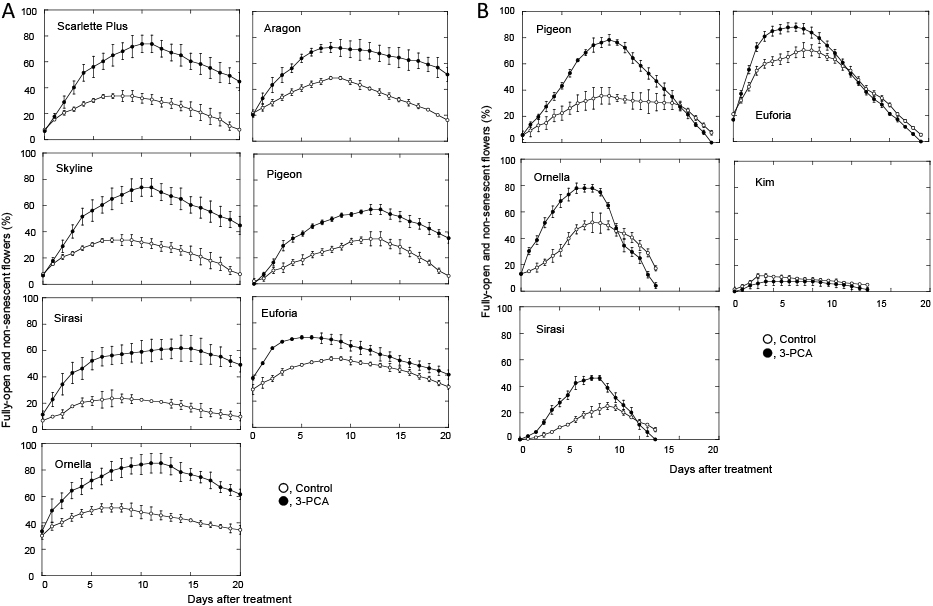
Changes in the percentage of fully-open and non-senescent flowers in different cultivars. A: Flowers of respective cultivars were harvested at a nursery in Colombia, and immediately stored at 2°C for three weeks, then flown to Japan, taking one week. After arrival, the flowers were treated as described in the legend for Figure 2, but observation of flowers was terminated on day 20. B: Flowers of the respective cultivars were harvested at a nursery in Colombia, and immediately flown to Japan, taking one week. After arrival, the flowers were stored at 4°C for three weeks, then observed for flower opening as described above.
The effect of 3-PCA to promote flower opening was strong with ‘Scarlette Plus’, medium with ‘Skyline’ and ‘Sirasi’, and weak with ‘Ornella’, ‘Pigeon’, ‘Euforia’, and ‘Aragon’. The magnitude of promotion was defined by the maximum difference in the percentage of FONS flowers between the control and 3-PCA-treated flowers, and the differences were determined just before the percentage in the control flowers reached the maximum; differences of > 40%, 40–31%, and 30–20% were regarded as strong, medium and weak effects, respectively.
On the other hand, in the flowers transported immediately after harvest and stored in Japan, 3-PCA promoted flower opening the most in ‘Pigeon’, followed by ‘Ornella’, slightly in ‘Sirasi’ and ‘Euforia’, and not at all in ‘Kim’. These results showed that 3-PCA promoted flower opening in most of the tested cultivars, although the magnitude of promotion varied with the carnation cultivar, as well as the method of chilling. We did not investigate the precise mechanisms underlying these differences.
In conclusion, the present findings suggested that 3-PCA treatment would be useful to promote flower opening after long-term chilling. This procedure for the treatment of cut carnation flowers will be tested more widely and precisely using carnation cultivars cultivated in Japan, and may be practically applicable in the near future.
We are grateful to Glores Co., Ltd., Ritto, Shiga Prefecture, Japan for the use of carnation flowers imported from Colombia.