2019 Volume 88 Issue 2 Pages 202-213
2019 Volume 88 Issue 2 Pages 202-213
Phytohormones play major roles in the berry maturation process. Gibberellic acid (GA) and cytokinin (CK) are phytohormones used in seedless table grape production. Several studies have been conducted on the effects of GA and CK application on berry development. However, the detailed mechanisms underlying their physiological effects on berry maturation after the veraison stage have not been clarified. Skin browning during maturation is a major commercial problem in yellow-green skinned grape cultivars including ‘Shine Muscat’, and expanding our knowledge of these mechanisms is a necessary step towards addressing this problem. In this study, we investigated the effects of GA and CK treatments from the veraison stage to the subsequent developmental stages of this grape berry. Both treatments resulted in enlarged berries and the suppression of increases in sugar content. Chlorophyll in the berry skin was less decomposed after GA/CK treatment, and the occurrence of skin browning in the maturation stage was reduced, as expression of the VvPP2Cs gene decreased. GA/CK treatment at the veraison stage (45–50 DAFB) reduced the expression levels of phytohormone-related genes, particularly those of VvGID1 and VvCHKs, which are involved in GA and CK signaling, respectively. These similar changes in gene expression patterns suggest phytohormonal crosstalk and a common expressional regulatory mechanism. VvACO2 and VvYUC1 expressions were significantly increased in skin browning samples, regardless of treatment, indicating involvement of the ethylene and auxin biosynthesis pathways in skin browning. Therefore, GA/CK treatment at the veraison stage may broadly affect phytohormone biosynthesis and signaling pathways in subsequent developmental stages, although the effect size greatly differs depending on the experimental conditions, including year and plant.
The maturation of fruits such as grapes and other berries is accompanied by marked changes in morphology and physiology. Internal and external factors including phytohormones, light, temperature, and plant water status play major roles in the maturation process (Azuma, 2018; Gao-Takai et al., 2017; Sugiura et al., 2018). The effects of phytohormones are particularly important. Many studies have investigated the mechanisms driving the effects of phytohormones on berry development.
Abscisic acid (ABA) is a well-studied phytohormone in grapes. Its content in colored grapes rapidly increases around the veraison stage (Coombe and Hale, 1973), and the accumulation of sugars and anthocyanins is stimulated by its external application (Koyama et al., 2010; Mori et al., 2005). Ethylene is another phytohormone related to fruit maturation in a broad range of plant species. Grape berries are classified as non-climacteric fruits, in which ethylene is not responsible for triggering ripening; however, low levels of ethylene are produced during the maturation process (Chervin et al., 2004; Coombe and Hale, 1973; El-Kereamy et al., 2003; Mehta and Chundawat, 1979). Furthermore, ABA and ethylene function interactively at the onset of grape berry ripening (Sun et al., 2010).
Gibberellic acid (GA) and cytokinin (CK) are phytohormones used in seedless table grape production, and several studies have investigated their effects on berry development (Asano et al., 2001; Coombe, 1960; Hirano et al., 1996; Maoz et al., 2014; Mochida et al., 2013; Ohara et al., 2008; Okamoto et al., 2003; Peppi and Fidelibus, 2008; Ravest et al., 2017; Wang et al., 1993). Optimum methods for GA and CK application have been developed for different seedless grape cultivars (Asano et al., 2001; Fujishima et al., 2012; Fukunaga and Kurooka, 1988; Ishikawa and Baba, 2004; Ishikawa et al., 2003; Lu et al., 1997; Mochida et al., 2013; Thanarut et al., 2010; Zabadal and Bukovac, 2006). There are many reports on the effects of GA and CK on berry enlargement throughout the developmental stage (Acheampong et al., 2015; Hirano et al., 1996; Peppi and Fidelibus, 2008; Reynolds et al., 1992; Zabadal and Bukovac, 2006; Zoffoli et al., 2009). Levels of isopentenyladenine, a CK, rapidly increase with berry maturation and physiological roles have been suggested for this compound in the later developmental stage (Böttcher et al., 2015). One study conclusively demonstrated that GA and CK inhibit grape berry maturation (Fortes et al., 2015).
‘Shine Muscat’ is a yellow-green skinned table grape cultivar grown in Japan (Yamada et al., 2008). Berry skin browning, a physiological disorder, usually develops at the maturation stage about 70–80 days after full bloom (DAFB) (Mochida et al., 2013; Suehiro et al., 2014). Skin browning markedly decreases the market value of the product, and is therefore a problem in table grape production in Japan. Hence, there is an incentive to develop a procedure to reduce or prevent skin browning. Progress in this field demands a better understanding of the berry maturation process in yellow-green skinned grape cultivars.
GA and CK may inhibit the maturation of grape berries (Fortes et al., 2015). However, the details of their involvement in berry maturation after the veraison stage have not been explored. In addition, few studies have examined the anti-senescence effects of GA or CK treatment on grape berries, although Lichter (2016) reported that high concentrations of these compounds suppress rachis browning.
To date, the effects of GA/CK treatment have mainly been examined for application in the full bloom stage. In this study, we investigated the effects of GA and CK application at the veraison stage to determine their effects on skin browning and berry maturation in ‘Shine Muscat’ table grapes. Physiological changes were detected by analyzing the expression of phytohormone-related genes in the berry skin.
In 2012, we selected two 7-year-old ‘Shine Muscat’ (Vitis labruscana Bailey × V. vinifera L.) plants for our experiments (vines Nos. 24 and 25). These vines had been grown under forcing culture at the Shimane Agricultural Technology Center. Forcing cultivation advances the harvest time by about 1 month compared to the rain protected (normal) cultivation system. Flower clusters were treated with 200 ppm streptomycin (Meiji Seika Pharma, Tokyo, Japan), which was sprayed onto the plants 10 days before full bloom. The flower clusters were then immersed in 25 ppm gibberellic acid (GA3; Kyowa Hakko Bio Co., Ltd., Tokyo, Japan) solution containing 5 ppm 1-(2-chloro-4-pyridyl)-3-phenylurea (CPPU, Fulmet; Kyowa Hakko Bio Co., Ltd.) at full bloom to promote seedless berry production. The developing grape berry clusters were treated again by immersion in 25 ppm GA3 solution 10–15 DAFB. The final number of berries was adjusted to 40–45 per cluster. The yield of grapes per plant was on average 2,293 and 2,298 kg/10a in vines 24 and 25, respectively. We subjected grapes at the veraison stage (45 DAFB) to GA treatment by hand spraying the berry clusters with 25 ppm GA3 (Kyowa Hakko Bio Co., Ltd.). Spraying was continued until the berry clusters were fully wetted. In the CK treatment, we sprayed 10 ppm Fulmet (Kyowa Hakko Bio Co., Ltd.), using three clusters per plant (i.e., six clusters in total) for each treatment. After the treatments, we periodically observed the appearance of whole berry clusters and harvested two berries from each cluster (i.e., six berries per plant) at 75, 90, and 120 DAFB. Berry weights and degree of browning were measured on each harvest date.
In 2013, we selected two 8-year-old plants as experimental material (vines Nos. 14 and 15). These vines had been grown under semi-forced and rain protected culture at the Shimane Agricultural Technology Center. The streptomycin, GA3, and Fulmet treatments for seedless berry production were identical to those used in 2012. The final number of berries was adjusted to 40–45 per cluster. The yield of grapes per plant was on average 1,870 and 2,274 kg/10a in vines 14 and 15, respectively. GA/CK treatments at the veraison stage (45 DAFB) were performed using GA3 and Fulmet at final equivalent concentrations of 50 ppm and 20 ppm, respectively. Nine berry clusters per plant were subjected to each treatment. After the treatments, the appearance of whole berry clusters was observed periodically, and three berries were randomly collected from the upper, middle, and lower portions of each berry cluster: i.e., nine berries in total at 70, 90, and 110 DAFB (for vine 15)/120 DAFB (for vine 14) for measurement and analyses. The berry skins were collected and immediately frozen in liquid nitrogen, and then stored at −80°C until later use.
In 2014, we used three 9-year-old plants (vines Nos. 14, 15, and 16) grown under rain protected culture at the Shimane Agricultural Technology Center. The procedures for streptomycin, GA3, and Fulmet treatments for seedless berry production were identical to those used in previous years. The final number of berries was adjusted to 40 per cluster. The yield of grapes per plant was on average 1,420, 1,640, and 1,400 kg/10a for vines 14, 15, and 16, respectively. At the veraison stage (50 DAFB), GA/CK treatments were applied as in previous years using the general reagents of GA3 (Tokyo Chemical Industry Co., Ltd. [TCI], Tokyo, Japan) and 1-(2-Chloro-4-pyridyl)-3-phenylurea (CPPU; TCI) at concentrations of 50 ppm and 20 ppm, respectively. The GA spraying solution was prepared by dissolving GA3 in a small amount of ethanol, followed by dilution in an aqueous solution (0.1% v/v, final concentration) of Tween 20 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and adjusting the pH to 7.0 using HCl. The CK spraying solution was prepared in the same manner as the GA solution. The control spray contained 0.1% (v/v) Tween 20. Ten clusters per plant were used for each treatment. Skin browning was first observed 90 DAFB in all plants. Four berries from each cluster were periodically harvested at 50 (pre-treatment) and 70 DAFB. After skin browning, 4–8 berries with or without brown skin were harvested separately at 90 and 110 DAFB (120 DAFB for vine 16) for measurement and analysis. The berry skins were collected, immediately frozen in liquid nitrogen, and then stored at −80°C until later use.
Measurement of berry characteristicsBerries were weighed, after which the skin colors at their apices were determined using a color reader (CR-10; Konica Minolta Sensing Inc., Tokyo, Japan); this device provides Commission Internationale de l’Elcairage (CIE) L*, a*, and b* values. The total soluble solid content (TSSC; °Brix) of the juice extracted from the berries was measured using refractometry (N-1E; Atago Co., Ltd., Tokyo, Japan). The degree of skin browning in each whole berry cluster was determined based on criteria developed by Mochida et al. (2013). Individual berries were assigned to categories of browning as indicated in Suehiro et al. (2019).
Measurement of chlorophyll contents in berry skinChlorophyll content was measured using samples collected at 110–120 DAFB in 2013 and 2014. We extracted total chlorophyll following the methods of Downey et al. (2004) and Yoshida et al. (2008). Total chlorophyll was extracted from frozen berry skin (1.0 g each) using 80% acetone to which 0.2 g CaCO3 was added. The absorbance of the extract was recorded at 646 and 663 nm by spectrophotometry (UV-1800; Shimadzu, Kyoto, Japan). Chlorophyll A and B contents were calculated using the following formulas (Lichtenthaler and Wellburn, 1983):
Total RNA samples were prepared from berry skins using the hot borate extraction method described by Wan and Wilkins (1994). Each total RNA sample was quantified using a spectrophotometer and adjusted to 200 ng·L−1. cDNA samples were synthesized from 1.0 μg of total RNA after treatment with DNase I (TaKaRa Bio Inc., Shiga, Japan). Reverse transcription was performed in 20 μL reaction volumes using ReverTra Ace (Toyobo Co., Ltd., Osaka, Japan) with mixed oligo (dT) 20 and random primers (9 mer) following the manufacturer’s protocol. After the reactions were completed, 20 μL of water was added to each sample. Thus, a 1.0 μL cDNA sample was equivalent to 25 ng of total RNA. Extractions were performed on three samples from each berry cluster sampling point.
The expression levels of target genes were quantified by real-time quantitative reverse transcription PCR (qRT-PCR) using the TaKaRa Thermal Cycler Dice device (TP8000; TaKaRa Bio Inc.) as described in our previous study (Suehiro et al., 2014). Primer sets were prepared for the following phytohormone-related genes: the 9-cis-epoxycarotenoid dioxygenase (NCED)1 and NCED2 genes, which encode enzymes that are involved in ABA biosynthesis (Speirs et al., 2013); type 2C protein phosphatases (PP2Cs) genes, which are negative regulators of ABA signaling (Boneh et al., 2012; Gambetta et al., 2010); 1-aminocyclopropane-1-carboxylate oxidase (ACO) genes from the ethylene biosynthesis pathway (Muñoz-Robredo et al., 2013); VvEIN3, which encodes a transcription factor in the ethylene signaling pathway (Chervin and Deluc, 2010); GA 2-oxidase (GA2ox), GA3ox, and GA20ox from the GA biosynthesis pathway (Giacomelli et al., 2013); the gibberellic acid insensitive (GAI)1 gene, which encodes a transcription factor with a DELLA domain that is involved in GA signaling (Boss and Thomas, 2002); the gibberellic acid insensitive (GAI)1 gene, which encodes a transcription factor with a DELLA domain that is involved in GA signaling (Boss and Thomas, 2002); the gibberellin-insensitive dwarf 1 (GID1) gene, which encodes one of the GA receptors (Acheampong et al., 2015); the cytokinin histidine kinase receptors (CHKs) genes, which encode membrane-bound homodimeric proteins that are involved in cytokinin signaling (Böttcher et al., 2015); the VvYUC1 gene, which encodes a key enzyme that is involved in auxin biosynthesis (Böttcher et al., 2013); and the auxin response factor (ARF)1 gene, which encodes a transcription factor that is involved in auxin signaling (Wan et al., 2014). Primer sets for the VvPP2Cs, VvGA2ox, VvGA20ox, VvGA3ox, VvGAI1, VvGID1, and VvCHKs were newly designed by Suehiro et al. (2019) based on DNA sequence information provided by previous studies (Acheampong et al., 2015; Boneh et al., 2012; Boss and Thomas, 2002; Böttcher et al., 2015; Giacomelli et al., 2013). The primer sequences for each of the target genes are listed in Supplementary Table 1. The Elongation Factor 1 (EF1) (Hanana et al., 2007) and Ubiquitin (Ubq) (Czemmel et al., 2009) genes were used as internal control (reference) genes. The reactions were performed at least twice for each sample. Threshold cycle (Ct) values were measured using software provided by the manufacturer (version 5.11; TaKaRa Bio Inc.), and expression levels were calculated after normalization to the two internal control genes.
TSSC was lower after GA/CK treatment compared to the control (Fig. 1A; Table 1). The final berry size was significantly larger following GA/CK treatment than in controls at 110–120 DAFB (Fig. 1B; Table 1). Berry skin colors at each sampling point were displayed in CIE xyY color space by conversion from CIE L*a*b* values. Skin color changed from green-yellow at 70 DAFB to reddish-yellow at 110–120 DAFB (Fig. 1C). Following CK treatment, skin color exhibited increased L* values (lightness), and thus exhibited higher chroma compared to the GA treatment and control groups. In addition, a decrease in a* values and increase in b* values indicated a green-yellowish color in the CK treatment group (Table 1). The effects of GA treatment on skin color change were small; however, chroma became slightly lower than those of controls (Fig. 1C; Table 1). Skin browning occurred after 90 DAFB in all treatments. The browning degree of whole grape clusters was lower in the GA and CK treatment groups than in controls (Fig. 1D). The browning degree of individual berries was also generally lower following GA/CK treatment than in controls; however, some berries randomly sampled from GA/CK treatments showed more severe browning than controls (Table 1).
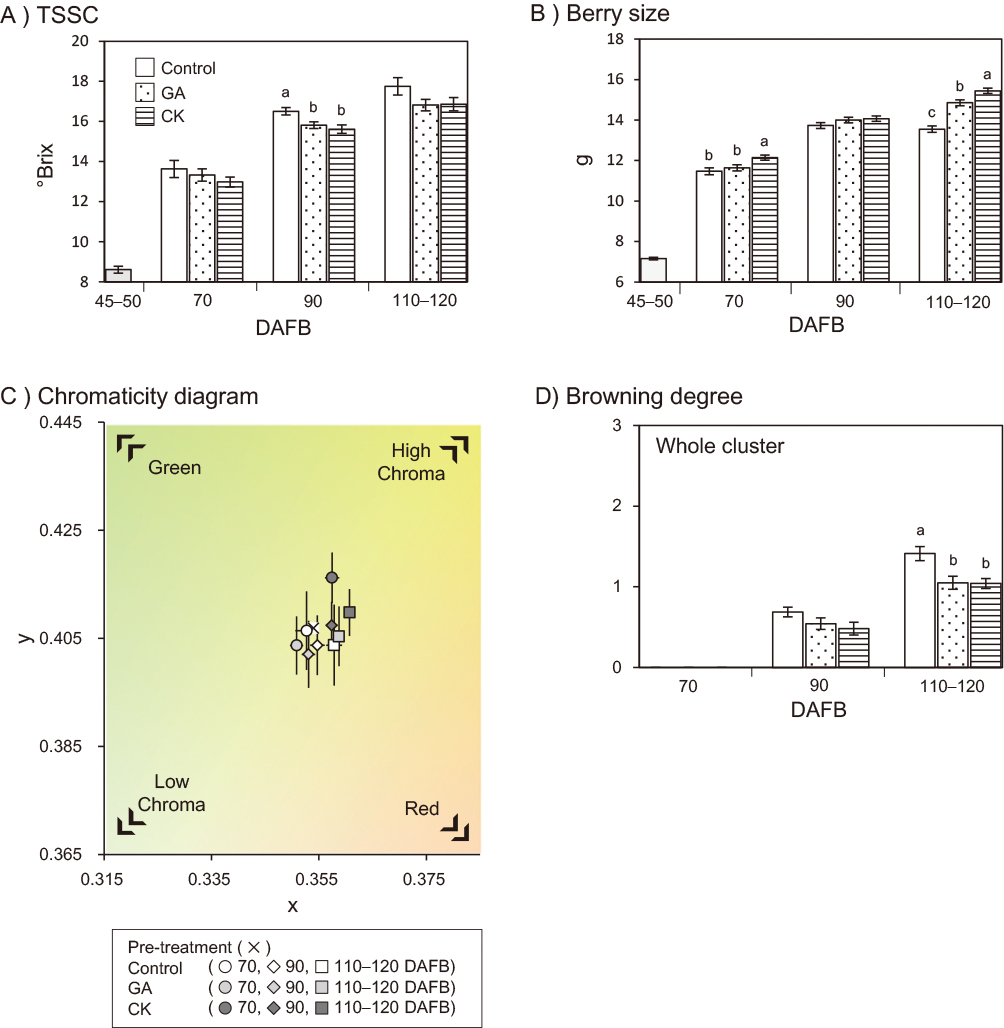
Effects of GA/CK treatment on TSSC, berry size, color, and degree of browning. Mean values of TSSC (n = 15–35), berry size (n = 132–355), color (n = 132–355), and browning degree (clusters; n = 53–69) for two years (i.e., 2013 and 2014). TSSC (A). Berry size (B). Skin color changes depicted in a CIE xyY color space chromaticity diagram (C). Degree of browning in clusters (D). The result of 45–50 DAFB indicates pre-treatment data (A and B). Different letters indicate significant differences between treatment groups within the same period according to Tukey’s HSD test at P < 0.05. Values are mean ± SE.

Effects of GA/CK treatments on TSSC, berry size, color, and the severity of browning in individual vines.
Although we detected no significant difference, GA and CK treatments both increased the berry skin chlorophyll content (Fig. 2). CK treatment resulted in higher chlorophyll a and b contents (by 1.4 times greater than control) than those measured in the GA treatment group (Table 1).

Effects of GA or CK treatment on chlorophyll content at 110–120 DAFB in 2013 and 2014. Values are mean ± SE (n = 12). Scale bar indicate 10 cm.
Gene expression analysis data for two experimental years (2013, 2014) are shown in Figures 3 and 4, respectively, because samples with and without skin browning were analyzed separately in 2014. All data are shown relative to values for 45–50 DAFB (pre-treatment), which was defined as 1.0.

Expression of genes related to phytohormone biosynthesis and signaling in the 2013 experiment. Relative gene expression levels of VvNCED1 (A), VvNCED2 (B), VvPP2Cs (C), VvACO1 (D), VvACO2 (E), VvACO3 (F), VvEIN3 (G), VvGAI1 (H), VvGID1 (I), VvCHKs (J), VvYUC1 (K), and VvARF1 (L) were analyzed using qRT-PCR. All data were normalized by VvEF1 and VvUbq expression levels and are shown as values relative to 45 DAFB (pre-treatment), which was set at 1.0. GA treatment was GA3 (Kyowa Hakko Bio). CK treatment was Flumet (Kyowa Hakko Bio). Control was water. Different letters indicate significant differences among treatments at each sampling date by Tukey’s HSD test at P < 0.05. Values are mean ± SE (n = 6–12).
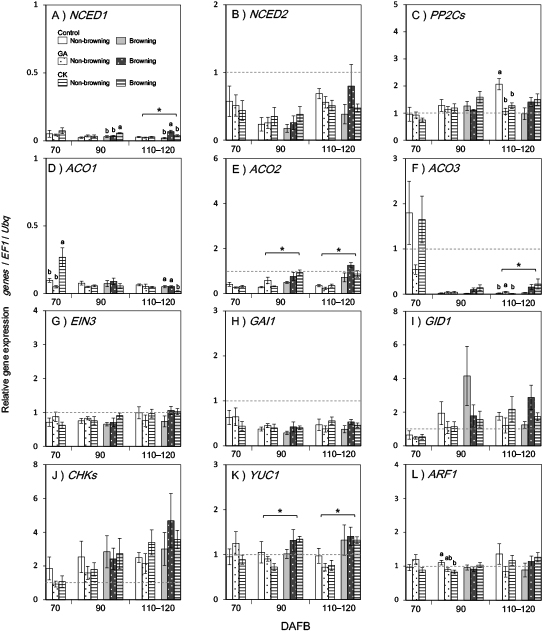
Expression of genes related to phytohormone biosynthesis and signaling in berry skin with/without browning in 2014. Relative gene expression levels of VvNCED1 (A), VvNCED2 (B), VvPP2Cs (C), VvACO1 (D), VvACO2 (E), VvACO3 (F), VvEIN3 (G), VvGAI1 (H), VvGID1 (I), VvCHKs (J), VvYUC1 (K), and VvARF1 (L) were analyzed by qRT-PCR. Berries with and without brown skin were analyzed separately at 90 DAFB and 110–120 DAFB. All data were normalized by VvEF1 and VvUbq expression levels and are shown as values relative to 50 DAFB (pre-treatment), which was set at 1.0. GA treatment was GA3 (TCI). CK treatment was CPPU (TCI). The control was water. Asterisks indicate significant differences between non-skin-browning and skin browning in t-tests at P < 0.05. Different letters indicate significant differences among treatments at each sampling date in Tukey’s HSD tests at P < 0.05. Values are mean ± SE (n = 6–36).
In the ABA biosynthesis pathway, VvNCED1 and 2 expressions decreased from 45 DAFB as the berries matured (Figs. 3A, B and 4A, B). VvNCED1 expression at 90 DAFB only increased in the CK treatment group (Figs. 3A and 4A). Subsequently, at 110–120 DAFB, VvNCED1 expression significantly increased in browning skin (Fig. 4A). VvNCED2 expression was slightly lower in the GA/CK treatment at 70 DAFB and 110–120 DAFB (Figs. 3B and 4B).
Based on two years of replicated data, the expression patterns of VvPP2Cs, VvGID1, and VvCHKs were similar, although there were several significant differences among treatment groups (Figs. 3C, I, L and 4C, I, L). Despite some exceptions, the expression levels of VvPP2Cs, VvGID1, and VvCHKs were lower after GA/CK treatment. In the late-maturation stage (110–120 DAFB), VvPP2Cs expression in non-browning skin was significantly lower in GA/CK treatment groups than in controls (Fig. 4C). In contrast, VvGID1 and VvCHKs expression in the CK treatment group appeared to increase (Figs. 3I, L and 4I, L).
Among ethylene biosynthesis pathway genes, VvACO1 expression decreased from 45 DAFB in all treatment groups. VvACO1 expression in the CK treatment group at 110–120 DAFB was suppressed in both 2013 and 2014 (Figs. 3D and 4D). VvACO2 expression significantly increased in browning skin as early as 90 DAFB, regardless of treatment (Fig. 4E). Subsequently, VvACO3 expression increased in browning skin 110–120 DAFB (Fig. 4F).
The expression patterns of VvEIN3, VvGAI1, VvYUC1, and VvARF1 genes were somewhat similar. Although there were no significant differences in VvEIN3, VvGAI1, and VvYUC1 expression after GA/CK treatment (Figs. 3G, H, M and 4G, H, M), VvYUC1 expression was significantly higher in browning skin in 2014 (Fig. 4M). The expression of VvYUC1 and VvARF1, which are respectively involved in auxin biosynthesis and signaling, tended to be lower after CK treatment than in controls (Figs. 3M, N and 4M, N). The expression of GA oxidase genes (VvGA2ox, VvGA3ox, and VvGA20ox), which are involved in GA biosynthesis, were also examined; however, they were not detected (data not shown).
Figure 5 shows a heat map representing gene expression levels relative to those of the control group at 70 DAFB, before skin browning starts. Generally, gene expression was lower in the GA/CK treatment group than in controls. GA/CK treatment reduced the expression levels of VvGID1, VvCHKs, VvNCED2, VvPP2Cs, VvACO2, and VvACO3 in both years. The degree of skin browning at 90 DAFB was more severe in 2014 than in 2013, especially in the CK treatment. The expression levels of VvEIN3, VvYUC1, and VvARF1 were correlated with the severity of berry skin browning in the GA treatment group, and VvNCED1 and VvACO1 expression in the CK treatment group were higher than in the control group. These higher expression levels were observed 70 DAFB in the CK treatment group, which contributed to a higher degree of skin browning 90 DAFB in that group.

Gene expression relative to that of the control treatment at 70 DAFB and skin browning at 90 DAFB. Heat map representation of values relative to control for gene expression (A) and browning degree (B). (A) Gene expression levels were calculated as relative values, with an average of 70 DAFB in 2013 and 2014, which were set as 1.0 in the control. (B) Browning degree was calculated as a relative value, with an average of 90 DAFB in 2013 and 2014, which was set as 1.0 in the control.
GA and CK treatment both suppressed the increase in sugar content in grapes (Fig. 1A), but increased berry size (Fig. 1B). The increase in berry volume was likely caused by an increase in water content. Conversely, TSSC may have been decreased by this diluting effect. These effects have been observed in other grape cultivars (Hirano et al., 1996; Peppi and Fidelibus, 2008; Reynolds et al., 1992; Zabadal and Bukovac, 2006; Zoffoli et al., 2009). Cytokinin affects cell division in the early stage of fruit development (Werner et al., 2001). CK treatment at bloom time can increase the final size of the ‘Shine Muscat’ berry (Mochida et al., 2013). In ‘Cabernet Sauvignon’ grape berry cells, GA3 regulates hexokinase gene expression, thereby altering intracellular glucose metabolism and cell proliferation (Zhang et al., 2014). GA also suppresses the expression of some sugar transporter genes in ‘Malbec’ berries (Murcia et al., 2016). Sugar accumulation is considered negatively affected by GA and CPPU. In this study, CPPU treatment was more effective than GA3 treatment at the veraison stage, although the application concentrations were different. Berry maturation was delayed by GA/CK treatment and this was the opposite result of ABA/ethephon treatment (Suehiro et al., 2019). Brassinosteroid and jasmonic acid have also been reported to promote grape berry maturation and ripening (Jia et al., 2016; Symons et al., 2006). GA and CK are retardants of berry maturation.
Generally, plant tissue colors are determined by levels of chlorophyll, carotenoids, and anthocyanin pigments (Lancaster et al., 1997). Chlorophyll breakdown is an important catabolic process in ripening fruit (Barry et al., 2008; Hörtensteiner and Kräutler, 2011). In colored grapes, a high chlorophyll concentration is maintained in berry skin during pre-maturing stages, and then berry coloration progresses through chlorophyll decomposition and an increase in anthocyanins at the maturation stage (Gény et al., 2004; Olivares et al., 2017). In yellow-green skinned grapes, the color tone of the berry skin is mainly influenced by chlorophyll and carotenoids, with chlorophyll having the greater influence (Lancaster et al., 1997). CK treatment increases the size and number of chloroplasts in berry skin, leading to an increase in chlorophyll content (Okazaki et al., 2009; Werner et al., 2001). In Arabidopsis, CK treatment can increase chlorophyll content in leaves by altering reception and signaling gene expression (Riefler et al., 2006), thereby changing the tissue color (Olivares et al., 2017). In addition to the increase in chlorophyll content in leaves, CK can also delay leaf senescence (Danilova et al., 2017; Werner et al., 2001). In ‘Shine Muscat’ berry skin, chlorophyll was less decomposed following GA or CK treatment (Fig. 2); thus, skin color remained greenish during the maturation stage (Fig. 1C; Table 1). A previous study demonstrated that ‘Shine Muscat’ browning clusters display weaker greenness than non-browning clusters throughout the maturation stage (Kanazawa and Takahashi, 2011). Therefore, this phenomenon may be an anti-senescence process.
Effects of GA/CK treatment on skin browningThe application of a high-concentration CPPU (>5 ppm) treatment at the full bloom stage can reduce skin browning at the maturation stage in ‘Shine Muscat’ (Mochida et al., 2013). The current study demonstrated that CK treatment at the veraison stage can also reduce skin browning during maturation (Fig. 1D; Table 1). CK can suppress skin browning regardless of the timing of its application. Thus, both GA and CK treatments at the veraison stage also reduced skin browning (Fig. 1D; Table 1). Although the degree of browning after GA/CK treatment was reduced, and lower than that of controls, VvPPO2 gene expression in browning skin tissue was upregulated even in the GA/CK treatment (data not shown), as shown in a previous study (Suehiro et al., 2014). In peaches, exogenous GA application treatment decreased PPO activity and inhibited tissue browning in fruit (Knapp et al., 1970). The induction or prevention of plant tissue browning has been widely examined through the application of phytohormone or chemical treatments. For example, 2,4-dichlorophenoxyacetic acid (2,4-D), a herbicide with auxin-like activity, can decrease browning in the potato, artichoke, and tomato and reduce the activities of phenol oxidases to prevent tissue browning in the lettuce (Matheis, 1983; Tomás-Barberán et al., 1997). Therefore, in addition to CK and GA, auxin may be another important phytohormone involved in plant tissue browning reactions. In the current study, VvNCED1, VvACO2, VvACO3, and VvYUC1 were upregulated in browning grape berry skin (Fig. 4). The activation of ABA, ethylene, and auxin biosynthesis pathways was also inferred to be involved in browning.
Effects of GA/CK treatment on phytohormone-related gene expressionThe expression of the VvNCED1 and 2 genes decreased toward the maturation stage, regardless of treatment (Figs. 3A, B and 4A, B). In particular, VvNCED1 expression after 70 DAFB remained low compared to that during the veraison stage (45–50 DAFB) (Figs. 3A and 4A). The ABA biosynthesis pathway is thought to be more active in immature berries. Weak effects of exogenous CK or GA treatment on VvNCEDs expression were observed in browning skin. PP2C is a major regulator of the ABA signaling mechanism (Umezawa et al., 2009). The lower expression level of the VvPP2Cs gene after GA/CK treatment, which was notable in non-browning skin at 110–120 DAFB in 2014 (Fig. 4C), implies that ABA signaling is suppressed in berry skin (Figs. 3C and 4C). ABA signaling is probably weakened in the anti-senescence process by GA/CK treatment.
The similarity of expression patterns among VvPP2Cs, VvGID1, and VvCHKs may indicate phytohormonal crosstalk and a common expressional regulation mechanism (Figs. 3C, I, L and 4C, I, L). In general, several phytohormones act cooperatively, affecting each other (Wang and Irving, 2011). In GA homeostasis, the expression of GA biosynthesis- and GA signaling-related genes involving GID1 is regulated by a feedback mechanism (Griffiths et al., 2006; Sun and Gubler, 2004). We also found that exogenous GA treatment decreased VvGID1 expression (Figs. 3I and 4I). An interaction between CK and GA is thus suggested; for example, exogenous CK treatment affects the expression of GA oxidases in grape berries (Lu et al., 2016) and vice versa; feedback control of CK signaling by exogenous GA has also been observed (Greenboim-Wainberg et al., 2005). CK-related gene expression is also controlled by a feedback mechanism after CK treatment (Heyl and Schmülling, 2003). The reduced expression levels of VvCHKs observed at 70 and 90 DAFB may have been due to temporary feedback control by CK or interaction control by GA.
GA/CK treatment significantly affected VvACO1 expression at the berry maturation stage. However, the effect size and manner differed according to the sampling date and experimental year (Figs. 3D and 4D). The effects of VvACO1 expression remain completely obscure. We observed significant upregulation of its expression at 70 DAFB of CK treatment in 2014 and at 90 DAFB of GA treatment in 2013. In contrast, significant downregulation was observed at 110–120 DAFB of CK treatment in both 2013 and 2014 (Figs. 3D, 4D, and 5).
Meanwhile, the expression of VvEIN3 and VvGAI1 was not affected by the treatments (Figs. 3G, H and 4G, H). These genes appear to have been regulated independently of effects from other phytohormones.
Auxin and CK are thought to be important phytohormones for fruit maturation (McAtee et al., 2013). Grape berry auxin and CK content decrease before maturation (Böttcher et al., 2011). Although in our study the effect size was small, the expression of VvYUC1 and VvARF1 decreased after CK treatment (Figs. 3M, N and 4M, N). This indicates the suppression of biosynthesis and signaling of auxin by CK, and an interaction between auxin and CK (Jones et al., 2010).
Relationship between phytohormone-related gene expression at 70 DAFB and degree of skin browning at the subsequent maturation stageCK/GA treatment at the veraison stage (45–50 DAFB) had a negative effect on the expression of phytohormone-related genes at 70 DAFB in berry skin (Fig. 5). In particular, GA/CK treatment reduced the expression of the VvGID1 and VvCHKs genes, which are respectively involved in GA and CK signaling. This indicates negative feedback regulation in the GA and CK pathways, which may slow berry maturation by reducing the senescence of fruit cells (Danilova et al., 2017). Furthermore, the expression levels of VvNCED2, VvPP2Cs, VvACO2, and VvACO3 decreased. The ABA and ethylene pathways were also affected by these treatments. In 2014, because the anti-senescence effect by CK treatment was weak, more severe skin browning occurred in the CK treatment group at 90 DAFB, in a manner correlated with the expression of VvNCED1 and VvACO1 genes, which had already been significantly upregulated at 70 DAFB. ABA and ethylene biosynthesis may trigger browning. Moreover, the effects of CK treatment on the expression of these genes may be volatile or limited compared to GA treatment (Fig. 5), although the impact of CK treatment on berry size and sugar accumulation was larger than that of GA treatment (Fig. 1). The degree of skin browning at 90 DAFB after GA treatment in 2014 was higher than that in 2013, perhaps because the auxin and ethylene pathways were already activated at 70 DAFB in the GA treatment in 2014. GA/CK treatment at the veraison stage can affect the phytohormone biosynthesis and signaling pathways broadly in the subsequent developmental stages; however, the effect size depends greatly on the experimental conditions, such as year and plant.
ConclusionsIn the yellow-green skinned grape ‘Shine Muscat’, GA/CK treatment at the veraison stage significantly altered berry appearance at harvest and reduced the occurrence of skin browning. Berry maturation was delayed by GA/CK treatment. In particular, CK treatment increased chlorophyll content in berry skin and maintained a greenish color. GA/CK treatment resulted in larger berries. However, negative effects were also observed; these treatments retarded sugar accumulation and chlorophyll degradation. GA/CK treatment at the veraison stage lowered the expression levels of VvPP2Cs, VvGID1, and VvCHKs in subsequent developmental stages, which indicates that signal transduction of ABA, GA, and CK were affected by GA/CK. Hence, negative feedback regulation of the GA and CK pathways may have occurred. The reduced expression of VvPP2Cs after GA/CK treatment was associated with reduced occurrence of skin browning. In contrast, VvACO2 and VvYUC1 expression was significantly increased in browning skin. We conclude that activation of the auxin and ethylene biosynthesis pathways is involved in skin browning.