2019 Volume 88 Issue 3 Pages 401-409
2019 Volume 88 Issue 3 Pages 401-409
The branching habit and stalk proportion of heading type broccoli (Brassica oleracea L. var. italica) differs based on the cultivar, but the extent of the effects of these factors on the apical head weight is unknown. The main objectives of this study were to elucidate the relationships between the apical head weight and the branching habit or the stalk proportion. Firstly, the shoots of broccoli from six cultivars were divided into four parts (apical head, leaves on the main stem, lateral branches, and the rest of the main stem), and the weight proportions of each part were investigated. The results showed that the cultivars showing higher branching tended to produce smaller apical heads. Furthermore, apical head weights showed a positive relationship with the area of the leaves on the main stem, but a negative relationship with those on the branches. Secondly, four cultivars were grown with lateral bud nipping. This significantly increased the apical head weight in the cultivars showing higher lateral branching, but did not significantly change the weight of the whole shoot. These results suggest competitive biomass allocation between the main shoot and lateral branches. The difference in the weight of lateral branches depended on the probability of axillary bud presence rather than on the number of internodes or weight per branch. Finally, a strong correlation between the apical head weights and the square of the stalk diameter was demonstrated by generalized linear models (R2 = 0.95). These findings will contribute to the knowledge base on diverse methods of broccoli cultivation.
Plants respond to environmental changes by distributing biomass among various plant organs to optimize vegetative growth for resource capture and reproductive growth for seed production (Bazzaz et al., 1987; McCarthy and Enquist, 2007; McConnaughay and Coleman, 1999; Poorter and Nagel, 2000). Since the biomass that can be produced by a plant is limited, allocation of biomass must be carefully modulated in response to environmental factors. In intact plants showing vigorous apical shoot growth, lateral buds are generally not essential to the life cycle; excessive branching is likely to be costly (Dun et al., 2006). Therefore, elongation of lateral buds is suppressed by apical buds, making them dormant; this phenomenon is known as apical dominance (Cline, 1991; Horvath et al., 2003). In addition, several factors such as the stage of whole plant development, the node and age of the bud, as well as endogenous (hormone and gene expression) and environmental factors (light, temperature, and photoperiod) are intricately involved in the retention of lateral bud dormancy (Beveridge et al., 2003; Dun et al., 2006; Luo et al., 2019). However, lateral buds are likely to emerge and develop under photoassimilation surplus conditions, and cease development and senesce under photoassimilation shortage conditions (Lafarge and Hammer, 2002). Meanwhile, the outgrowth of lateral buds often competes with that of the apical shoots, which affects the yield in an agricultural field setting. For example, in a previous study, overproduced tillers (branches) triggered foliar N deficiency and were considered one of the reasons for the lower harvest index in direct-seeded rice (Oryza sativa L.) (Schnier et al., 1990). Wheat (Triticum aestivum L.) lines containing the tiller inhibition (tin) gene, which exhibits a reduction in tillers by 40% compared to its free-tillering counterpart, produced a higher yield than all other lines studied (Duggan et al., 2005a, b). The development of a single large flower head entails nipping the axillary buds in a floricultural field setting (Adjei-Frimpong et al., 2011; Cockshull, 1982). The number of branches is usually restricted to increase the yields and quality of fruits and vegetables (De Swart et al., 2006; Georgiev, 1991; Maghfoer et al., 2015).
There are various methods of broccoli (Brassica oleracea L. var. italica) cultivation based on the use of lateral branches. Broccoli is roughly classified as a “sprouting” or “heading” type, depending on the degree of branching exhibited and the size of the inflorescences (Martin and Sideman, 2012). In the sprouting type, multiple smaller secondary heads are harvested instead of a single large head (Martin and Sideman, 2012; Reilly et al., 2014). In the heading type, only the apical (central) large head is usually harvested (Erdem et al., 2010; Jett et al., 1995; Tremblay, 1989), but sometimes, lateral heads (small secondary heads) are subsequently harvested, enabling an increase in total yield (Bouquet, 1950; Singh et al., 2011; Stephens, 1994; Yoldas et al., 2008). In some studies, techniques for harvesting two large heads from one plant by growing a lateral branch after the apical head harvest (Pornsuriya and Teeraskulchon, 1997; Sato, 2015; Takahashi et al., 2018a) or by pinching the apical bud and growing two lateral branches (Kodera, 1988; Takahashi et al., 2019), were examined or proposed. Each cultivar is bred and produced for a particular purpose, resulting in the development of a branching habit in each variety. It is known that a cultivar with a large apical head tends to have fewer branches empirically, while one with a small apical head tends to have more branches (Bouquet, 1950; Le Strange et al., 1996). However, the biomass trade-off between the main shoot and lateral branches has not been quantified yet. Elucidating the biomass allocation to lateral branches and the influence of lateral branches on the yield (apical head weights) will be beneficial for selecting and breeding a suitable broccoli cultivar. In addition, as we demonstrated that two large heads could be harvested by nipping extra branches (Takahashi et al., 2018a, 2019), elucidating the effect of nipping branches on head growth will also be beneficial to understanding and improving methods for more stable production.
Increased planting density was reported to reduce the apical head weight and stalk (the stem part of the head) diameter (Kahn et al., 1991). In another study, increased planting density was not reported to affect the apical head diameter, but it did reduce the apical head weight (Francescangeli et al., 2006). These studies suggest that the stalk size easily fluctuates and influences the apical head weight. It can be assumed that the differences in the apical head weights among different cultivars are caused by the difference in the stalk size. Therefore, elucidating the relationship between the apical head weight and stalk size is important, considering its effect on broccoli yield.
Therefore, in this study experiments were conducted using six cultivars to elucidate the influence of lateral branches on biomass allocation within the whole shoot, the differences in the characteristics of lateral branches among cultivars, and the relationship between the apical head weight and stalk diameter.
The experiments were conducted at a field in NARO, Tsukuba City, Ibaraki Prefecture, Japan (36°01' N, 140°06' E). The soil was classified as Andisol, Typic Hapludands. The cultivars used in this study were ‘Pixel’, ‘Ryokurei’, ‘Grandome’ (Sakata Seed Co., Ltd., Yokohama, Japan), ‘Speed Dome’ (Mikado Kyowa Seed Co., Ltd., Chiba, Japan), ‘Madoka’ (Brolead Co., Ltd., Tsu, Japan), and ‘Yumehibiki’ (Nanto Seed Co., Ltd., Kashihara, Japan). Seeds were sown in cell trays (25 mL × 128 cells) filled with compost (NAPLA type S; YANMAR Co., Ltd., Osaka, Japan) on 4 Jan. 2017 and grown in a greenhouse. The temperature in the greenhouse was maintained above 10°C. Starting from two weeks after sowing, the seedlings were fertilized with 300 mg N, 160 mg P2O5, and 340 mg K2O, in the form of a liquid (OAT Agrio Co., Ltd., Tokyo, Japan), per cell tray. To avoid exposing the seedlings to the severely cold climate of the field immediately, they were acclimatized to low temperatures above 1°C for 3–5 days just before transplanting. The seedlings were transplanted on 15 Feb. 2017 into the study field, covered with black plastic mulch and placed in transparent low plastic tunnels (PVC film, 0.075 mm thickness) at a planting density of 1.6 plants·m−2 (160 cm between ridges and 40 cm between plants). The practical density for heading type broccoli is 3.0–3.6 plants·m−2 (Jett et al., 1995; Schellenberg et al., 2009), but we set approximately half of this density to accommodate the development of branches. The field was fertilized with N, P2O5, and K2O at rates of 4, 4, and 3 kg·a−1, respectively.
When apical heads reached a diameter of 12 cm, the whole shoot was divided into four parts: the apical head, leaves on the main stem, lateral branches, and the rest of the main stem. Then, the fresh weight (FW) of each part was measured. The stalk of the apical head was trimmed at 15 cm from the top of the dome to give a cut-off section based on the typical standard in Japan (Kodera, 1988). Leafstalks on the apical head were trimmed within the width of the head diameter (cf. Takahashi et al., 2018a). The diameter of the cut-off section was measured as the stalk diameter. Both axillary branches (originating from leaf axils) and adventitious branches (originating from the boundary part between the main stem and roots) were regarded as lateral branches as long as the thickness of the basal part was more than 5 mm. Leaf area (LA) including leafstalks was measured using an area meter (LI-3100; Meiwafosis Co., Ltd., Tokyo, Japan). Hereafter, the sum of LA of the leaves on the main stem is expressed as LAM, that of the lateral branches as LAB, and the sum of LAM and LAB (LA of the whole shoot) as LAW. Six plants of each cultivar were included per replicate; three replicates were studied. Three plants of each cultivar were dried at 80°C for one week, and dry matter was weighed. The dry matter concentration (%) of each part of each cultivar was calculated by dividing dry matter weights by FW.
Nipping lateral buds (Exp. 2)The cultivars used in this experiment were ‘Pixel’, ‘Ryokurei’, ‘Speed Dome’, and ‘Yumehibiki’. Their seeds were sown on 14 Feb. 2018 and they were transplanted on 9 March 2018. The conditions for seedling nursing and cultivation were the same as those in Exp. 1 except for the plant density, which was maintained at 3.2 plants·m−2 (160 cm between ridges, 60 cm between two columns of plants in one ridge, and 40 cm between plants). In nipping plots, lateral buds were removed as soon as they were generated. When apical heads reached a diameter of 12 cm, the whole shoot was divided and weighed as in Exp. 1. In both control and nipping plots, three plants of each cultivar were included per replicate; three replicates were studied.
Comparison of lateral branches among cultivars (Exp. 3)The number of internodes (NI) from the first internode directly under the first true leaf, to the last one directly under the first floret of the apical head, was counted for the plants in Exp. 1. The probability of axillary bud presence (PA) was derived by dividing the number of axillary buds per plant by the respective NI. We collected lateral branches with basal part thickness of more than 5 mm, and measured their FW, LA, basal part thickness, and length and diameter of the lateral head. The FW of one lateral branch is expressed as WB.
Analysis of the relationship between apical head weight and stalk diameterThe relationship between the FW of apical heads and stalk diameters obtained in Exp. 1 was examined using generalized linear models (GLM) (Crawley, 2005; Takahashi et al., 2018b). FW values of apical heads and stalk diameters were represented as A and S, respectively, in GLM and equations. Assuming that the observed variation in A follows a Gaussian distribution with a mean of Ai and standard deviation of σi, we set S as the explanatory variable in the linear predictor. Subsequently, the below log link function was applied:
The parameters in the above equation (β0, β1, and β2) and Akaike’s information criterion (AIC) value were estimated based on the following three patterns: including both terms of the first and second degree (Eq. #1), including only the term of the first degree (Eq. #2), and including only the term of the second degree (Eq. #3), using the ‘glm’ function of the R software package (R Core Team, http://www.R-project.org., 2015). The resulting equations were then expressed in the following form:
Finally, we confirmed the best model according to the significances of explanatory variables, the coefficient of determination (R2), and the AIC value.
Statistical analysisStatistical analysis was performed using R software. Multiple tests for proportion data (dry matter concentration and PA) were performed after inverse sine transformation.
Each cultivar needed 68–76 days after transplanting to reach the standard size for harvest (Table 1). The FW of apical heads varied depending on the cultivar; the cultivars are arranged from left to right in Figure 1 in decreasing order of apical head FWs. The highest proportion of apical head within the shoot was 31.4% in ‘Madoka’ (Fig. 2a). The proportions of apical heads were approximately 25% in ‘Grandome’, ‘Yumehibiki’, ‘Pixel’, and ‘Speed dome’. The lowest proportion of apical heads was 17.2% in ‘Ryokurei’.
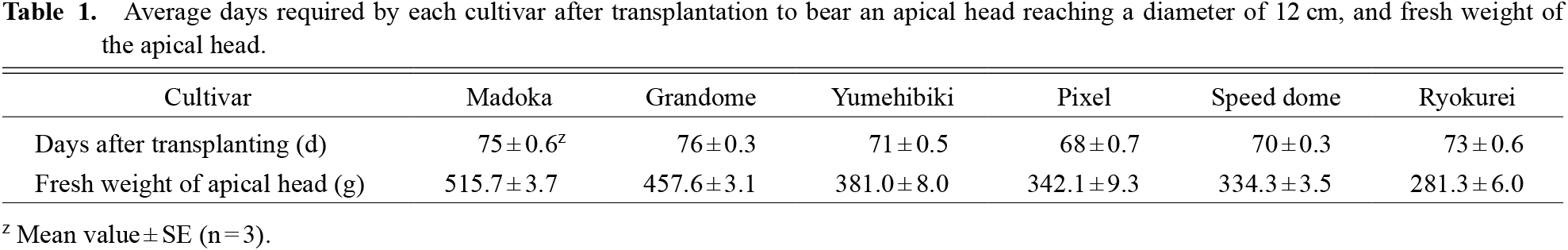
Average days required by each cultivar after transplantation to bear an apical head reaching a diameter of 12 cm, and fresh weight of the apical head.

Appearances of cultivars used in this study. Leaves were removed for easy recognition of the shapes. As plants were pulled out in a random manner, the roots in the picture do not reflect the whole roots.
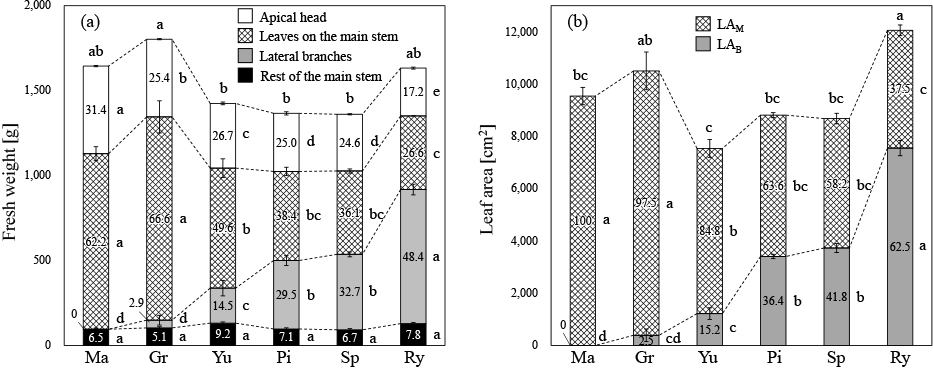
(a) Fresh weights and (b) leaf areas of each part according to the cultivar. Ma, Gr, Yu, Pi, Sp, and Ry indicate ‘Madoka’, ‘Grandome’, ‘Yumehibiki’, ‘Pixel’, ‘Speed dome’, and ‘Ryokurei’, respectively. LAM and LAB indicate the leaf areas of leaves on the main stem and lateral branches, respectively. The values in bars indicate the proportions (%) of the part within the whole shoots or leaf areas. Tukey-Kramer’s test was performed based on the values of fresh weights or leaf areas, and the same letters within the same parts indicate non-significant differences at P < 0.05. Error bars indicate SE (n = 3).
The proportion of leaves on the main stem was significantly higher in ‘Madoka’ (62.2%) and ‘Grandome’ (66.6%), than that in other cultivars (Fig. 2a). However, the lowest proportion of leaves on the main stem was 26.6% in ‘Ryokurei’.
There were no lateral branches in ‘Madoka’ (Fig. 1). It was difficult to classify the lateral branches of ‘Grandome’ as axillary or adventitious buds. They seemed to be adventitious buds because they grew from the boundary part between the stem and roots (Fig. 1), but they were robust and few for adventitious buds. They could be axillary buds from the low-order leaf axils buried in the soil by ridging. In either case, only 22.2% of the plants showed lateral branches, resulting in a very low lateral branch proportion (2.9%) in ‘Grandome’ (Fig. 2a). The rest of the cultivars had a certain amount of lateral branches, all of which originated from axillary buds (Fig. 1); the proportions of the lateral branches were 14.5% in ‘Yumehibiki’, 29.5% in ‘Pixel’, 32.7% in ‘Speed dome’, and 48.4% in ‘Ryokurei’ (Fig. 2a).
There were no significant differences in the remaining parts of the main stem among cultivars; their proportions ranged between 5.1–9.2% (Fig. 2a). Additionally, there were no significant differences in dry matter concentrations, all of which were approximately 9%, regardless of the part or cultivar (Table S1).
The plant ratios of LAM and LAB are shown in Figure 2b. The FW of the whole shoot showed a positive relationship with LAW, while the FW of the apical head showed a positive relationship with LAM and a negative relationship with LAB. The FW of lateral branches was the opposite of the above results at r > 0.7 of correlation coefficients and P < 0.001 level of significance for regression coefficients (Table 2).
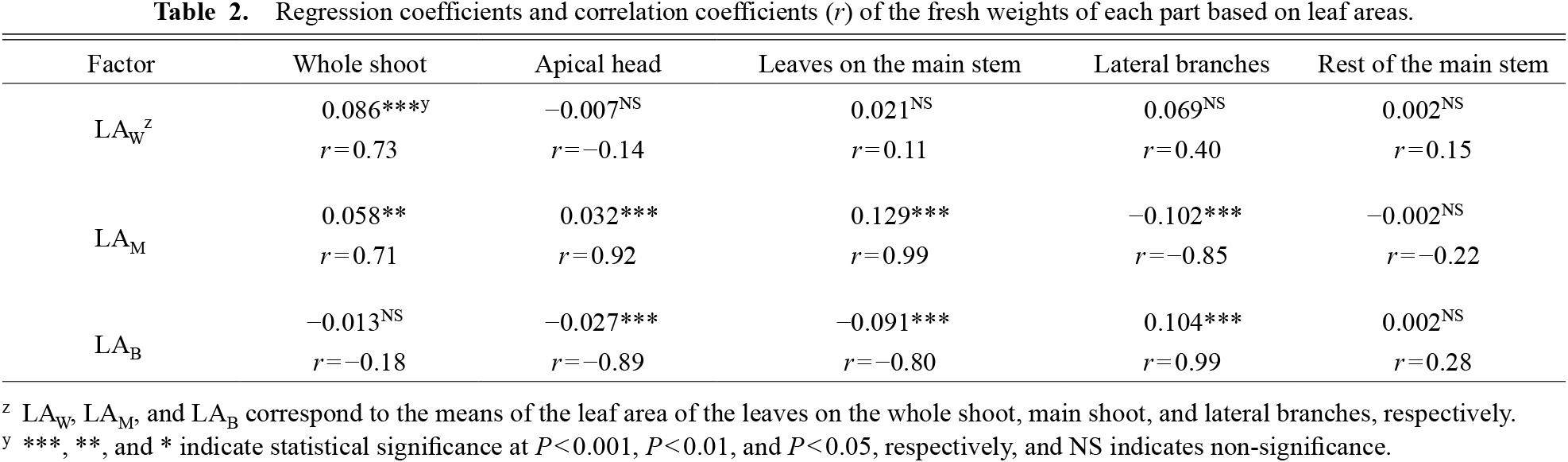
Regression coefficients and correlation coefficients (r) of the fresh weights of each part based on leaf areas.
Nipping lateral branches did not significantly change the FW of the whole shoot in any cultivar (Fig. 3). In ‘Yumehibiki’ and ‘Pixel’, nipping did not significantly change the FW of the apical head, leaves on the main stem or the rest of stem (Fig. 3a, b). Meanwhile, nipping significantly increased the FW of leaves on the main stem of ‘Speed dome’ and the FW of the apical head and leaves on the main stem of ‘Ryokurei’ (Fig. 3c, d).
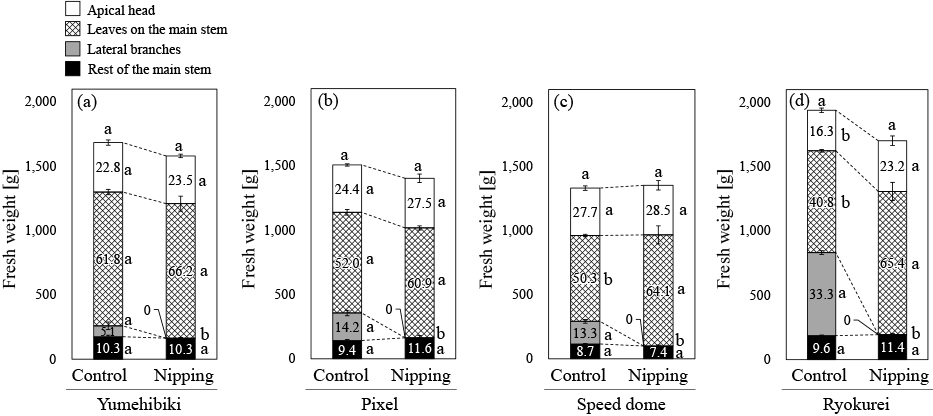
Fresh weights of each part of each cultivar; (a) ‘Yumehibiki’, (b) ‘Pixel’, (c) ‘Speed dome’, and (d) ‘Ryokurei’. The values in bars indicate the fresh weight proportions (%) of the parts within the whole shoots. The same letters within the same parts indicate non-significant difference at P < 0.05 (Tukey-Kramer’s test). Error bars indicate SE (n = 3).
The NI of ‘Madoka’ and ‘Grandome’ ranged from 21.2–22.0, which was significantly higher than that of the other four cultivars, at 15.4–17.0 (Table 3). The PA varied significantly depending on the cultivar. The PA of ‘Madoka’ was 0% (Table 3). Although it was undetermined if lateral branches of ‘Grandome’ were axillary or adventitious buds, the PA of ‘Grandome’ was less than 1.8%. The PA of ‘Yumehibiki’, ‘Pixel’, ‘Speed dome’, and ‘Ryokurei’ were 23.4%, 50.1%, 55.6%, and 85.3%, respectively. The lateral branches of ‘Ryokurei’ had distinctive features. For example, WB and LA were significantly larger but the thickness was significantly thinner than that of any other cultivar (Table 3). The branches of ‘Pixel’ and ‘Ryokurei’ were significantly longer than those of other cultivars (Table 3). The largest lateral head diameter was observed in ‘Pixel’, followed by ‘Speed dome’; ‘Yumehibiki’ and ‘Rykurei’ had the smallest diameters (Table 3).
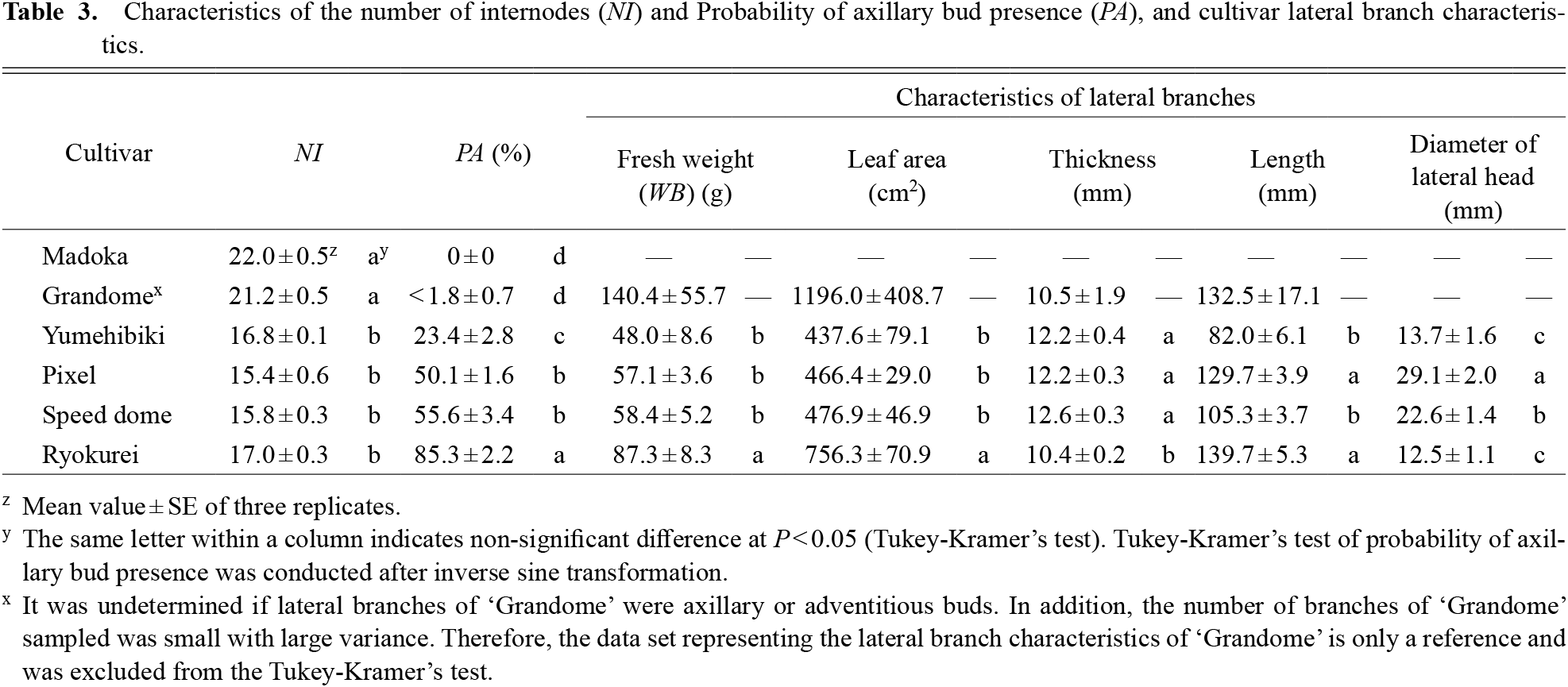
Characteristics of the number of internodes (NI) and Probability of axillary bud presence (PA), and cultivar lateral branch characteristics.
Regression analysis between A and S was conducted using the data from all the plots of the six cultivars included in Exp. 1 by GLM, considering the three candidate equations #1, #2, and #3 (Table 4). We confirmed Eq. #3 to be the best model because all the explanatory variables were significantly different from zero, and the equation showed the lowest AIC value (Table 4; Fig. 4). The coefficient of determination (R2) of Eq. #3 was also higher than that of any of the other equations.
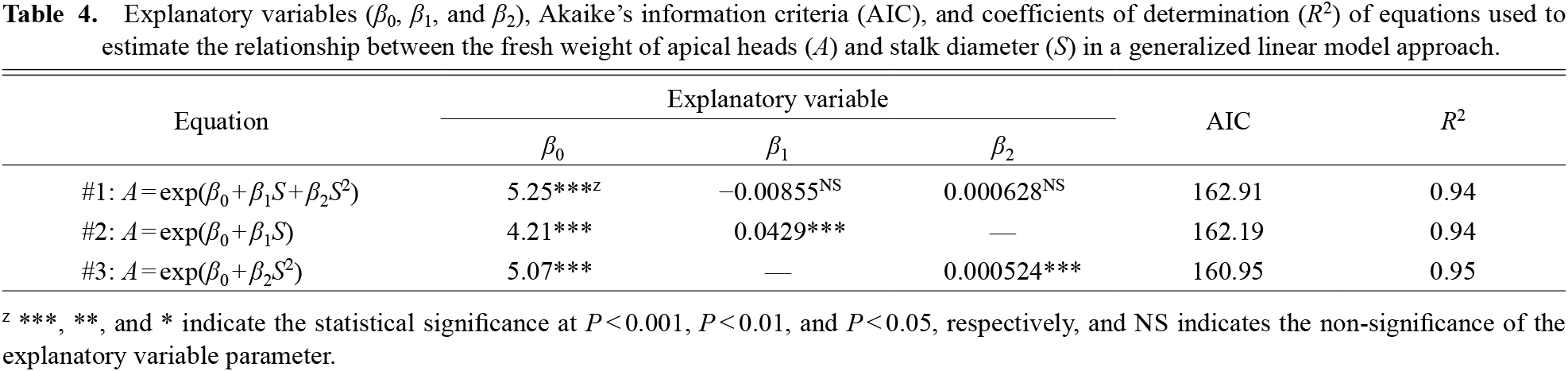
Explanatory variables (β0, β1, and β2), Akaike’s information criteria (AIC), and coefficients of determination (R2) of equations used to estimate the relationship between the fresh weight of apical heads (A) and stalk diameter (S) in a generalized linear model approach.
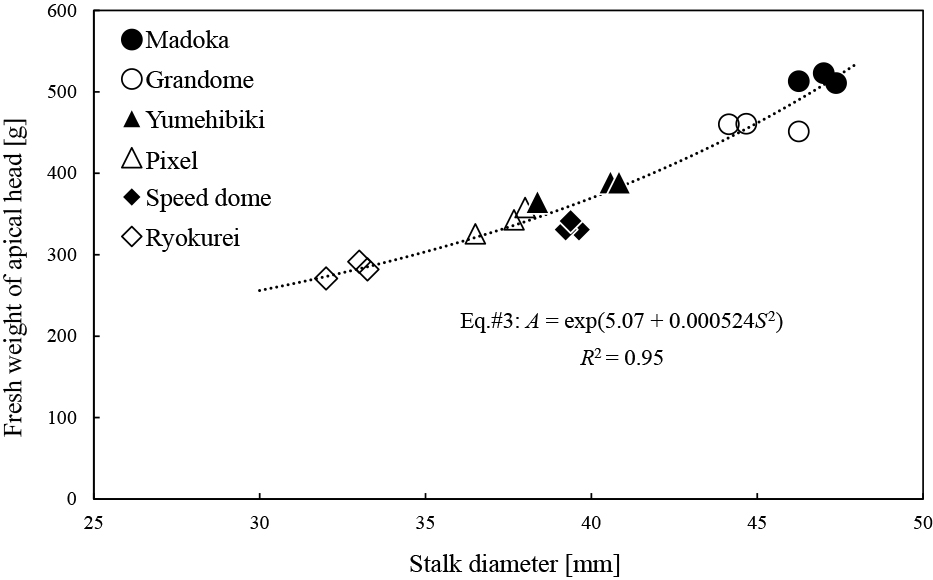
Regression curve of Eq. #3 between fresh weights of the apical heads and stalk diameters. A and S in the equation indicate fresh weights of apical heads and stalk diameters, respectively.
Although various comparisons were conducted based on FW in this study, it is possible to discuss the biomass allocation to each part of the broccoli shoot because there was no significant difference in dry matter concentration among parts and cultivars (Table S1). As biomass allocation and bud outgrowth are strongly affected by environmental conditions (Dun et al., 2006; McConnaughay and Coleman, 1999), the proportions of certain parts of certain cultivars were not constant. Furthermore, the breakdown of shoots of the same cultivar were different between Exp. 1 and 2, which is in agreement with previous studies showing that the proportion of lateral branches decreases with an increase in planting density (Chung, 1982; Cutcliffe, 1975). However, since the branching habit of cultivars showed the same tendency in Exp. 1 and 2, it is possible to consider the biomass allocation to lateral branches based on these experiments.
Based on a comparison of the proportion of each part among six cultivars, it was revealed that the proportion of the main shoot (apical head and leaves on the main stem) decreased with increases in that of the lateral branches (Fig. 2a). If photoassimilates are always distributed to the main shoot as a priority, and only the amount of photoassimilates exceeding the sink limitation of the main shoot are used for the development of other parts, the whole shoot weight would decrease by just the weight of the part removed and the weight of the main shoot would remain constant (Barbier et al., 2015; Egli and Bruening, 2001; Reynolds et al., 2005). However, the FW of the whole shoot did not significantly change despite nipping the branches, while the FW of the main shoot increased in Exp. 2 (Fig. 3). In addition, this tendency was marked when the proportion of lateral branches was high, as in the case of ‘Ryokurei’. This means that the total quantity of photoassimilates that a broccoli plant can produce for a certain period is almost constant regardless of the bud outgrowth and proportion of total photoassimilates distributed to lateral branches. Therefore, we quantitatively demonstrated a competitive relationship between apical head development and lateral bud outgrowth, which was already empirically known (Bouquet, 1950; Le Strange et al., 1996). Pornsuriya and Teeraskulchon (1997) reported that nipping all the lateral buds tended to produce the highest yield. Although the reason for the increasing yield was not mentioned, it is probable that the FW of apical heads increased, and this is consistent with our result.
From the perspective of light interception, plant growth and LA are closely related (Charles-Edwards, 1982; Francescangeli et al., 2006). In accordance with former studies, the FW of the whole shoot tended to be high in cultivars with high LAW in this study (Fig. 2a, b; Table 2). However, the FW of apical heads was not correlated with LAW, but was positively correlated with LAM and negatively with LAB (Table 2). Furthermore, the FW of lateral branches showed an inverse relationship with that of the apical heads (Table 2). These results imply that the leaves on the main stem supply photoassimilates mainly to the main shoot, while those on branches supply to branches. Further studies are needed to elucidate the role of light interception, although it is likely that the main shoot competes with branches for light interception; in other words, the main shoot fails to receive as much light as the branches do, which correlates with the proportion of total photoassimilates distributed to the branches.
Pattern of branch generation and branch characteristics (Exp. 3)Even though all six cultivars used in this study were classified as heading type broccoli, the number of lateral branches was significantly different among them (Fig. 2a). It is possible to express the number of lateral branches on a plant as the product of NI and PA (NI × PA), and the FW of lateral branches on a plant as the product of NI, PA, and WB (NI × PA × WB). Contrary to our expectation that these three factors individually differ according to cultivars, only PA varied across a wide range (PA = 0–85%) in this study. There were only two grades of NI: NI = 21–22 (‘Madoka’ and ‘Grandome’) and NI = 15–17 (the other four cultivars), and two grades of WB: WB = 87 g (‘Ryokurei’) and WB = 48–58 g (the other three cultivars). This indicated that PA is likely to be the determining factor of the branching habit among cultivars, but further research comparing numerous cultivars is necessary to confirm its importance.
Additionally, the characteristics of lateral branches were different among cultivars. Particularly, the branches of ‘Ryokurei’ were distinctive. While its FW and LA per bud were significantly higher than those of the other cultivars (Table 3), it had the longest and thinnest lateral branches, reflecting its slender shape. Stalk diameters of ‘Ryokurei’ apical heads were also the smallest among the cultivars (Fig. 4); therefore, such a main shoot characteristic may be reflected in lateral shoots as a genetic trait of this cultivar. It is assumed that the yield of ‘Ryokurei’ was not very high because of the low FW of the apical head. However, the high branching habit is suitable for harvesting both apical and lateral heads (Bouquet, 1950; Singh et al., 2011; Stephens, 1994; Yoldas et al., 2008). ‘Madoka’ clearly produced the largest apical heads (Table 1), but lateral heads cannot be expected at all because it does not produce any branches. As ‘Ryokurei’ showed the same FW level of whole shoots as ‘Madoka’ (Fig. 2a), their total yields, including lateral heads, may be comparable. Therefore, it is quite important to understand the branching habits and select an appropriate cultivar in accordance with cultivation objectives.
Analysis of the relationship between apical head weight and stalk diameterFrancescangeli et al. (2006) suggested that differences in the stalks affect the change in FW of apical heads based on the planting density because the head diameter, floret number, and weight did not change. This was evidenced by the strong relationship between A and S in this study (Table 4; Fig. 4). It was possible to select a regression curve (Eq. #1) or a line (Eq. #2) as in typical regression analysis due to the high R2 value; however, we found a more suitable curve (Eq. #3) by GLM. An apical head can be divided into two parts, the flower bud (florets) dome, and stalk. Although the shapes of the domes were slightly different depending on the cultivar (Fig. 1), the influence on the FW seemed limited because every head was harvested uniformly with the same dome diameter (12 cm). On the contrary, stalk thicknesses were not uniform. As the stalk can be regarded as a column of standard length (15 cm), its weight should be proportional to the cross-sectional area, i.e., the square of the stalk (semi) diameter. Hence, Eq. #3, which explains the FW of apical heads using the square of stalk diameters, appears reasonable (Table 4).
In Exp. 2, the average FW values (± SE, n = 3) of the apical heads and stalk diameters of ‘Ryokurei’ in the control plots were 315.7 ± 16.7 g and 35.9 ± 0.6 mm, respectively. They increased to 394.8 ± 11.8 g and 42.2 ± 0.5 mm, respectively, in the nipping plots. When stalk diameters are 35.9 mm (control plot) and 42.2 mm (nipping plot), Eq. #3 estimates the FW of apical heads as 314.0 g and 406.4 g, respectively, showing that the means estimated by Eq. #3 for the FW of apical heads are accurate with relative errors of less than 3% ([315.7 − 314.0]/314.0 = 0.54%; [394.8 − 406.4]/406.4 = −2.9%). Therefore, Eq. #3 is the best equation derived from different cultivars to explain the relationship between the FW of apical heads and stalk diameters. It may also be applicable to explain the changes in the FW of apical heads and stalk diameter caused by different cultivation conditions in the same cultivar.
Because of the multidimensional approach used to evaluate the lateral branches of broccoli, we could elucidate the proportion of lateral branches, the competitive relationship between the main shoot and lateral branches, plasticity of head size based on the presence of lateral branches, differences in branch characteristics (generation pattern and shape) among cultivars, and the correlation between the apical head weight and stalk diameter. Based on these findings, it is clearly suggested that using a cultivar with a weaker branching habit or suppressing lateral bud outgrowth by taking some countermeasures would be effective to increase the yield when aiming to harvest a single apical head. In the methods we have reported before to harvest two large heads (Takahashi et al., 2018a, 2019), in which we recommended nipping extra branches, the effect of nipping extra branches is also supported by the logic of avoiding biomass competition among branches. Therefore, the findings in this study will contribute to increase knowledge regarding these diverse methods and improve the stable production of broccoli.
We thank Mr. Kuriyama, Ms. Tsutsui, Mr. Sakamoto, and Ms. Onishi of the Institute of Vegetable and Floriculture Science, NARO, for growing the plants and supporting the experiments.