2020 Volume 89 Issue 1 Pages 45-53
2020 Volume 89 Issue 1 Pages 45-53
In this study, we performed intergeneric crosses between Argyranthemum frutescens (L.) Sch. Bip. and seven perennial species of closely related genera [Artemisia absinthium L., Chrysanthemum lavandulifolium (Fisch. ex Trautv.) Makino × Chrysanthemum × morifolium Ramat., Dimorphotheca sinuata DC., Osteospermum ecklonis (de Candolle) Norlindh, Pericallis hybrida B. Nord., Rhodanthemum gayanum (Cross. & Durieu) B.H. Wilcox, K. Bremer & Humphries., and Rhodanthemum hosmariense (Ball) B.H. Wilcox, K. Bremer & Humphries.]. Using an embryo culture technique to generate intergeneric hybrids, we produced two putative hybrids from the crosses between A. frutescens and R. gayanum. In the putative hybrid derived from the cross between A. frutescens ‘Brilliant rouge’ × R. gayanum ‘Elf pink’, its ligulate flower color was similar to the ‘Brilliant rouge’ seed parent while the composition and total amount of anthocyanidins and/or leucoanthocyanidins were different. In the putative hybrid derived from the cross between A. frutescens ‘Sunday ripple’ and R. gayanum ‘African eyes’, its ligulate flower color differed from those of the parents. The ligulate flowers of the parents were white and produced no anthocyanidins, whereas the putative hybrids had light pink ligulate flowers and produced three anthocyanidins pigments [pelargonidins (including pelargonidin and/or leucopelargonidin), cyanidins (including cyanidin and/or leucocyanidin, peonidin), and delphinidins (including delphinidin and/or leucodelphinidin, malvidin)]. In addition, the cleaved amplified polymorphic sequence (CAPS) markers (Afl II) developed in this study confirmed that the putative hybrids were intergeneric hybrids of A. frutescens × R. gayanum. Therefore, these CAPS selection markers can be used to determine whether plants resulting from crosses are hybrids.
Argyranthemum frutescens (L.) Sch. Bip. is a perennial Asteraceae species that originated in the Canary Islands (Spain) and Madeira Islands (Portugal) (Bramwell and Bramwell, 2001; Press and Short, 1994). Plants of the genus Argyranthemum, of which there are 24 wild species, were introduced to Japan sometime between 1860 and 1880 (Bremer, 1994; Kitamura, 1988). To date, more than 200 cultivars of A. frutescens have been registered, many of which are bred for the cut and potted flower trade in Japan.
The two primary methods for breeding plants are cross-breeding (intraspecific, interspecific, and intergeneric hybridization) and mutation breeding including via irradiation such as X-ray, γ-ray, and heavy ion-beam, chemical processing and transposon mutagenesis (Hayes et al., 1955; Luan et al., 2007; Luo et al., 1991; Sasaki et al., 2008). To generate plants with novel traits such as those relating to flower color, flower size, and stress resistance, intergeneric hybridization is superior to the other two cross-breeding methods. Typically, however, few seeds are obtained from intergeneric crosses because their embryos generally die before maturing. To prevent embryo mortality, embryo- and ovule-culturing techniques are often utilized, which involve artificially cultivating embryos (or ovules) that have been removed from the plant before embryo mortality occurs (Sharma et al., 1996).
Embryo culture techniques have been used to produce intergeneric hybrids between species from many genera, including Chrysanthemum L., Rhododendron L., and Lilium L. (Deng et al., 2011; Eeckhaut et al., 2007; Van Tuyl et al., 1991). For example, these techniques have been used to create a hybrid between A. frutescens and Glebionis coronaria (L.) Cass. ex Spach., the latter of which belongs to a genus closely related to Argyranthemum (Furusato, 1977, 1978). The intergeneric hybrids of A. frutescens × Glebionis carinata (Schousb.) Tzvelev, such as ‘Carnival queen’ and ‘Furenka’, have flower colors and fragrances that differ from those of conventional A. frutescens cultivars (Inaba et al., 2008; Iwazaki and Inaba, 2008; Ohtsuka and Inaba, 2008). However, problems are often encountered when cultivating such hybrids, including poor plant vigor, sparse branching, and poor growth after flowering because Glebionis species are annuals. In addition, a reduction in the amount of heating energy is required for their cultivation due to increasing fuel prices. Therefore, new intergeneric hybrids with cultivation characteristics that are superior to those of the current hybrids are needed. However, although there are many perennial species of Asteraceae plants, reports of crosses between A. frutescens and perennial species belonging to genera closely related to Argyranthemum are limited.
In this study, in order to evaluate the cross result between A. frutescens and perennial plants of closely related genera and ultimately obtain robust hybrids of A. frutescens, we attempted a cross between A. frutescens and seven perennial species belonging to genera closely related to Argyranthemum. Then, we attempted to produce intergeneric hybrids from these crosses using an embryo culture technique. For the intergeneric hybrids produced in this study and their parents, we evaluated leaf and flower characteristics and flower color. Moreover, we attempted to develop genetic markers to identify the hybrids.
Four A. frutescens cultivars as seed parents (‘Brilliant rouge’, ‘Lovely friend’, ‘Moon light’, and ‘Sunday ripple’) and seven perennial species of Asteraceae plants as pollen parents [Artemisia absinthium L., Chrysanthemum lavandulifolium (Fisch. ex Trautv.) Makino × Chrysanthemum × morifolium Ramat., Dimorphotheca sinuata DC., Osteospermum ecklonis (de Candolle) Norlindh, Pericallis hybrida B. Nord. Rhodanthemum gayanum (Cross. & Durieu) B.H. Wilcox, K. Bremer & Humphries., and Rhodanthemum hosmariense (Ball) B.H. Wilcox, K. Bremer & Humphries] were selected. All cross combinations are shown in Table 1. Cross-breeding was performed in an environmentally controlled greenhouse at the Izu Agricultural Research Center of the Shizuoka Research Institute of Agriculture and Forestry (Japan). Pollen from freshly opened flowers was transferred to the seed parent using a brush during sunny mornings from March to May in 2015 and 2016. After being pollinated, female flowers were enclosed within a paper bag.

Cross combinations and results of intergeneric crosses using an embryo culture techunique.
In the cross between A. frutescens and Glebionis carinata (Schousb.) Tzvelev, hybrids were efficiently obtained by embryo rescue approximately 3 weeks after crossing (Ueda and Yamada, 2006). Based on this, approximately 3 weeks after pollination embryos were excised and cultured. Capitulums (female flowers) were sterilized in 1% sodium hypochlorite solution for 10 min and then rinsed two times in sterile distilled water. Ovary coats and integuments were aseptically removed under a dissecting microscope, and the mature embryos were immediately cultured in sterilized culture tubes containing 10 mL of modified Murashige and Skoog (MS) medium (pH = 5.8) (Murashige and Skoog, 1962), consisting of half-strength mineral salts supplemented with 30 g·L−1 sucrose and 3 g·L−1 gelatin gum, and then sterilized in an autoclave at 120°C for 20 min. The cultures were then placed in half-strength MS medium, and subsequently maintained at 23°C under a 16-h light/8-h dark photoperiod. Once the plants had produced roots, they were transferred to polyethylene pots (6 cm in diameter) filled with expanded vermiculite. After one month, the plants were transplanted to clay pots (18 cm in diameter) filled with commercial medium and cultivated in a greenhouse.
2. Morphological characteristics of putative hybrids 1) Measurement of morphological characteristicsFor each putative hybrid and its parents, we measured the following characteristics using six individuals of each plant type: plant height, crown width, flower neck length, flower diameter, number of first branchings, leaf length, leaf width, number of ligulate flowers, ligulate flower length, ligulate flower width and presence/absence of leaf trichomes.
2) Quantification of flower color characteristicsFor each putative hybrid and its parents, we quantified the color of ligulate flowers from six randomly selected plants using a colorimeter (CM-700d; KONICA MINOLTA INC., Tokyo, Japan). Only ligulate flowers that had opened completely were thus characterized.
3) Quantitative analysis of three primary anthocyanidinsIn order to investigate the pigment composition of each flower, simple and rough quantification using high-performance liquid chromatography (HPLC) was conducted. This method detected not only the anthocyanidin content, but also the leucoanthocyanidin content. Extracts from fresh ligulate flowers (0.1 g) of each putative hybrid and its parents were obtained using MAW (MeOH:CH3COOH:H2O = 9:1:10) and they were hydrolyzed for 1.5 h with 6 N HCl. Three primary anthocyanidins pigments [pelargonidins (including pelargonidin and/or leucopelargonidin), cyanidins (including cyanidin and/or leucocyanidin, peonidin) and delphinidins (including delphinidin and/or leucodelphinidin, malvidin)] from each extract were quantitatively analyzed using a HPLC [SHIMADZU CORPORATION., Kyoto, Japan. Prominence series (LC-20AB, SIL-20A, CBM-20A, SPD-M20A, and CTO-20A)], equipped with a diode array detector (Shimadzu SPD-M10A), on a Shodax C18M 4E (4.6 × 250 mm, 5 μm) column set at 40°C with a flow rate of 1 mL·min−1 and monitored at 530 nm. We used a solvent system for 40 min consisting of a linear gradient elution ranging from 20% to 85% of solvent B (1.5% H3PO4, 20% CH3CN, 25% CH3COOH in H2O) in solvent A (1.5% H3PO4 in H2O).
3. Discrimination of putative hybrids using genetic markers 1) DNA extraction and amplification of targeted regionsA NucleoSpin Plant II DNA extraction kit (TAKARA BIO INC., Shiga, Japan) was used to extract total genomic DNA from 0.2 g of young leaves collected from putative hybrids and their parents. The ITS region of nuclear ribosomal DNA was amplified using the primer pair 5'-AGAAATCGTAACAAGGTTTCCGTAGG-3' (Zhao et al., 2010) and ITS4 5'-TCCTCCGCTTATTGATATGC-3' (White et al., 1990). The PCR mixture (20 μL) contained 1 μL of template genomic DNA (100 ng·μL−1), 1 μL of each primer (10 μM), 4 μL of 5 × KapaTaq Extra buffer (NIPPON Genetics Co., Ltd., Tokyo, Japan), 1.4 μL of MgCl2 (25 mM), 0.6 μL of dNTP Mixture (10 mM), 0.1 μL of KapaTaq Extra DNA Polymerase (5 U·μL−1), and 10.9 μL of sterilized water.
PCR amplifications were performed in a VeritiTM Thermal Cycler (Thermo Fisher Scientific Inc., Waltham, MA, USA) with an initial denaturation at 95°C for 2 min, followed by 35 cycles at 95°C for 20 s, 55°C for 15 s, and 68°C for 2 min, with a final extension at 68°C for 2 min.
2) Preparation of genetic markersWe developed a set of cleaved amplified polymorphic sequence (CAPS) markers based on the Takara Cut-Site Navigator (http://www.takara-bio.co.jp/enzyme/enzyme_search.php) using genetic sequences of the ITS region for A. frutescens (EF577287) and R. gayanum (AF155312, AF155275, AB359793, AB359707, and L77777) obtained from GenBank. We selected the restriction enzyme Afl II, which appeared to cleave the PCR amplification product of A. frutescens, but not that of R. gayanum.
The restriction enzyme reaction mixture (10 μL) contained 3 μL of PCR amplification products, 0.5 μL of restriction enzyme Afl II, 1 μL of 10 × M buffer, 1 μL of 0.1% BSA, and 4.5 μL of sterilized water. The restriction enzyme reaction was performed at 37°C for 3 h. Mixtures containing 8 μL of enzyme-restricted reaction products and 2 μL Bromophenol blue were resolved by electrophoresis (100 v for 40 min) on 1.5% (w·v−1) agarose gels in 1 × TAE buffer and stained with ethidium bromide.
Nine mature ovules were germinated from 60 crosses between A. frutescens and R. gayanum, from which two plants were eventually obtained (Table 1). In contrast, no germinating plants were obtained from crosses between A. frutescens and the other six pollen parents: A. absinthium, C. lavandulifolium × Chrysanthemum × morifolium, D. sinuate, O. ecklonis, P. hybrida, and R. hosmariense (Table 1).
2. Morphological characteristics of the putative hybridsLigulate flowers of the putative hybrid A. frutescens ‘Brilliant rouge’ × R. gayanum ‘Elf pink’ were dark salmon in color and similar to those of ‘Brilliant rouge’ (Fig. 1). Tubular flowers of the putative hybrid were dark brown and produced no pollen. In addition, the putative hybrid differed from its parents with regards to the following morphological characteristics (Table 2). Plants of the putative hybrid (61.3 cm) were shorter than ‘Brilliant rouge’ (77.0 cm), but taller than ‘Elf pink’ (39.5 cm). Although the crown width of the putative hybrid (36.7 cm) did not differ from that of either of its parents, the flower diameter of the putative hybrid (48.4 mm) was wider than that of ‘Elf pink’ (40.0 mm) and similar to that of ‘Brilliant rouge’ (50.5 mm). The number of growth branches at first branching of the putative hybrid (3.3) was lower than that of ‘Elf pink’ (13.0) and similar to that of ‘Brilliant rouge’ (4.7). Also, the number of ligulate flowers produced by the putative hybrid (24.3) was higher than that of ‘Brilliant rouge’ (20.7) and ‘Elf pink’ (20.5).

Flowers and leaves of the putative hybrid Argyranthemum frutescens ‘Brilliant rouge’ × Rhodanthemum gayanum ‘Elf pink’ and its parents (Upper left: A. frutescens ‘Brilliant rouge’, Upper right: R. gayanum ‘Elf pink’, Lower: Putative hybrid A. frutescens ‘Brilliant rouge’ × R. gayanum ‘Elf pink’).
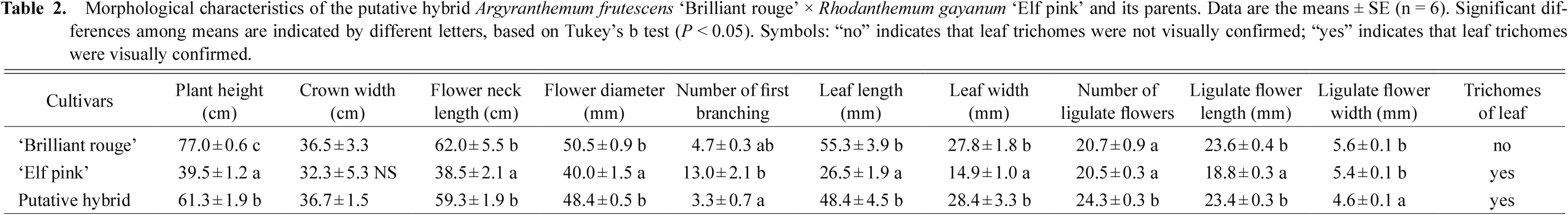
Morphological characteristics of the putative hybrid Argyranthemum frutescens ‘Brilliant rouge’ × Rhodanthemum gayanum ‘Elf pink’ and its parents. Data are the means ± SE (n = 6). Significant differences among means are indicated by different letters, based on Tukey’s b test (P < 0.05). Symbols: “no” indicates that leaf trichomes were not visually confirmed; “yes” indicates that leaf trichomes were visually confirmed.
Ligulate flowers of the putative hybrid A. frutescens ‘Sunday ripple’ × R gayanum ‘African eyes’ were light pink in color and differed from those of its parents, both of which had white flowers (Fig. 2). Tubular flowers of the putative hybrid were dark brown and produced no pollen. In addition, the putative hybrid differed from its parents with regards to the following morphological characteristics (Table 3). Plants of the putative hybrid (44.0 cm) were taller than ‘Sunday ripple’ (31.3 cm) and similar to ‘African eyes’ (42.3 cm). The crown width of the putative hybrid (39.2 cm) was wider than that of ‘African eyes’ (28.3 cm) and similar to that of ‘Sunday ripple’ (36.9 cm). Likewise, the flower diameter of the putative hybrid (45.5 mm) was wider than that of ‘African eyes’ (35.4 mm) and similar to that of ‘Sunday ripple’ (46.6 mm). The number of growth branches at first branching of the putative hybrid (6) was lower than that of ‘African eyes’ (20.7) and similar to that of ‘Sunday ripple’ (8.5).

Flowers and leaves of the putative hybrid Argyranthemum frutescens ‘Sunday ripple’ × Rhodanthemum gayanum ‘African eyes’ and its parents (Upper left: A. frutescens ‘Sunday ripple’, Upper right: R. gayanum ‘African eyes’, Lower: Putative hybrid A. frutescens ‘Sunday ripple’ × R. gayanum ‘African eyes’).

Morphological characteristics of the putative hybrid Argyranthemum frutescens ‘Sunday ripple’ × Rhodanthemum gayanum ‘African eyes’ and its parents. Data are the means ± SE (n = 6). Significant differences among means are indicated by different letters, based on Tukey’s b test (P < 0.05). Symbols: “no” indicates that leaf trichomes were not visually confirmed. “yes” indicates that leaf trichomes were visually confirmed.
Color values of the putative hybrid A. frutescens ‘Brilliant rouge’ × R. gayanum ‘Elf pink’ and those of its parents are shown in Table 4. For the putative hybrid, the intensity of redness (a*) and chroma (C*) were similar to the color values for ‘Brilliant rouge’ (its seed parent). The intensity of yellow (b*) in the putative hybrid did not differ significantly from that of ‘Elf pink’ (its pollen parent), whereas the luminosity (L*) of the putative hybrid was intermediate between that of its parents.

Color values of the putative hybrid Argyranthemum frutescens ‘Brilliant rouge’ × Rhodanthemum gayanum ‘Elf pink’ and its parents. Data are the means ± SE (n = 6). Significant differences among means are indicated by different letters, based on Tukey’s b test (P < 0.05).
Color values of the putative hybrid A. frutescens ‘Sunday ripple’ × R. gayanum ‘African eyes’ and those of its parents are shown in Table 5. Both luminosity (L*) and the intensity of yellow (b*) of the putative hybrid were lower than those of its parents, whereas the intensity of redness (a*) and chroma (C*) of the putative hybrid were significantly higher than those of its parents.

Color values of the putative hybrid Argyranthemum frutescens ‘Sunday ripple’ × Rhodanthemum gayanum ‘African eyes’ and its parents. Data are the means ± SE (n = 6). Significant differences among means are indicated by different letters, based on Tukey’s b test (P < 0.05).
Three anthocyanidins pigments [pelargonidins (including pelargonidin and/or leucopelargonidin), cyanidins (including cyanidin and/or leucocyanidin), and delphinidins (including delphinidin and/or leucodelphinidin, malvidin)] were detected in the flowers of the putative intergeneric hybrid A. frutescens ‘Brilliant rouge’ × R. gayanum ‘Elf pink’ and its parents (Table 6). Total anthocyanidins and/or leucoanthocyanidins contents in the putative hybrid were at an intermediate amount compared to the parents. The ratio of cyanidins composition of the putative hybrid (approximately 63%) was lower than the ratio of this composition of both parents [approximately 94% (seed parent), 91% (pollen parent)]. The ratio of pelargonidins composition of the putative hybrid (approximately 34%) was considerably higher than the ratio of this composition of both parents (approximately 2%). The ratio of delphinidins composition of the putative hybrid (approximately 4%) was similar to the ratio of this composition of both parents [approximately 4% (seed parent), 7% (pollen parent)].

The ratio of Anthocyanidins composition of the hybrid Argyranthemum frutescens ‘Brilliant rouge’ × Rhodanthemum gayanum ‘Elf pink’ and those of its parents. Data are the means (n = 6). Pelargonidins (including pelargonidin and/or leucopelargonidin), cyanidins (including cyanidin and/or leucocyanidin) and delphinidins (including delphinidin and/or leucodelphinidin, malvidin). Petunidin and peonidin were not detected in the cultivars. Significant differences among means are indicated by different letters, based on Tukey’s b test (P < 0.05) after arc-sine transformation.
Three anthocyanidins pigments [pelargonidins (including pelargonidin and/or leucopelargonidin), cyanidins (including cyanidin and/or leucocyanidin, peonidin), and delphinidins (including delphinidin and/or leucodelphinidin, malvidin)] were detected in the flowers of the putative intergeneric hybrid A. frutescens ‘Sunday ripple’ × R. gayanum ‘African eyes’ (Table 7). We found that the ratio of cyanidins composition accounted for more than 96%. In contrast, no anthocyanidins were detected in the flowers of either of the putative hybrid’s parents.

The ratio of Anthocyanidins composition of the hybrids Argyranthemum frutescens ‘Sunday ripple’ × Rhodanthemum gayanum ‘African eyes’ and those of its parents. Data are the means (n = 6). ND: Not Detected. Pelargonidins (including pelargonidin and/or leucopelargonidin), cyanidins (including cyanidin and/or leucocyanidin, peonidin) and delphinidins (including delphinidin and/or leucodelphinidin, malvidin). Petunidin was not detected in the cultivars.
We were able to identify the putative hybrids based on differences detected in the number of expressed CAPS marker bands (Fig. 3). A. frutescens was distinguished by the presence of two specific bands (approximately 300 and 800 bp), whereas R. gayanum could be identified by the presence of a single band (approximately 1100 bp). We detected three bands (approximately 300, 800, and 1100 bp) in the putative hybrids A. frutescens × R. gayanum, indicating that A. frutescens × R. gayanum were its parents.

CAPS band pattern of A. frutescens, R. gayanum, and the putative hybrids (A. frutescens × R. gayanum) using the primer restriction enzyme Afl II.
Although we used an embryo culture technique to obtain hybrid embryos, no germinating plants could be obtained by crossing A. frutescens with the six Asteraceae species A. absinthium, C. lavandulifolium × Chrysanthemum × morifolium, D. sinuata, O. ecklonis, P. hybrid, and R. hosmariense. In contrast, by crossing A. frutescens with R. gayanum, we were able to obtain nine mature ovules that germinated and two plants (using an embryo culture technique) (Table 1). It is well known that germination rates are influenced by the type of medium used, the medium composition, sucrose concentration, phytohormone concentration/combinations, and the timing of embryo excision after crossing (Cisneros and Tel-Zur, 2010; Fratini and Ruiz, 2006; Pellegrineschi et al., 1997; Yang et al., 2007). Accordingly, germination rates in the crossing A. frutescens with R. gayanum can be improved by optimizing the characteristics of the growth medium, phytohormone concentrations, and the timing of embryo removal.
The commercially important leaf and flower characteristics (crown width, flower diameter, and the number of first branchings) of the two hybrids of A. frutescens × R. gayanum were not different from those of the seed parents (A. frutescens) (Tables 2 and 3). In contrast, the number of ligulate flowers of the hybrid A. frutescens ‘Brilliant rouge’ × R. gayanum ‘Elf pink’ was significantly higher than those of the parents (Table 2). Thus, from the standpoint of trade, crossing A. frutescens with R. gayanum may produce hybrids with advantageous morphological characteristics.
It is empirically known that the intergeneric hybrids of A. frutescens × G. carinata are sensitive to cold, and their growth after flowering and productivity are poor because Glebionis species are annuals. In contrast, it has been reported that hybrids with cold resistance can be produced by crossing Chrysanthemum with closely related species that have excellent cold resistance (Cheng et al., 2010; Deng et al., 2011). Thus, the hybrids of A. frutescens × R. gayanum in this study may also have high cold resistance and productivity because Rhodanthemum species are perennial and have excellent cold resistance. Therefore, this experiment showed the potential to add novel traits in A. frutescens cultivars by using an embryo culture technique. Future studies will involve detailed analysis of the cultivation characteristics, including robustness, perennial characteristics, and cold resistance of the hybrids of A. frutescens and R. gayanum.
The ligulate flower color of the hybrid A. frutescens ‘Brilliant rouge’ × R. gayanum ‘Elf pink’ was similar to that of the ‘Brilliant rouge’ seed parent, and the color values [intensity of redness (a*) and chroma (C*)] of the hybrid were similar to those of ‘Brilliant rouge’. However, the total anthocyanidins and/or leucoanthocyanidins content of the hybrid was lower than that of ‘Brilliant rouge’ (Fig. 1; Tables 4 and 6). While the ratio of cyanidins composition in the hybrid was lower than the ratio of this composition of the both parents, the ratio of pelargonidins composition in the hybrid was significantly higher than that of both parents. As a result, the pigment composition was different between the two parents and the hybrid (Table 6).
The ligulate flowers of the hybrid A. frutescens ‘Sunday ripple’ × R. gayanum ‘African eyes’ were light pink in color, different from its parents, and also the color values [intensity of redness (a*) and chroma (C*)] of the hybrid were significantly higher than those of its parents. (Fig. 2; Table 5). This was mainly caused by the accumulation of anthocyanidins in the ligulate flowers of the hybrid, although no anthocyanidins were detected the ligulate flowers of the parents (Table 7). In this study, anthocyanidin content was detected by a simple HPLC analysis method. Using this method, leucoanthocyanidin may also be detected and based on these findings, detailed analysis should be conducted in the future. This study showed that the flower color and pigment composition of the hybrids A. frutescens × R. gayanum differ from those of their parents, indicating the possibility of producing hybrids with new flower colors.
For the hybrid derived from the cross between A. frutescens ‘Sunday ripple’ and R. gayanum ‘African eyes’, its ligulate flower color was light pink, while both parents were white. The white flower causative gene has been reported in many plants including mutations in anthocyanin biosynthetic genes [Gentian (Nishihara et al., 2006), Antirrhinum majus (Martin et al., 1991)], regulatory genes for anthocyanin [Antirrhinum majus (Martin et al., 1991), Ipomoea nil (Morita et al., 2006), Petunia hybrid (Albert et al., 2011)], and anthocyanin transporter genes [Petunia hybrid (Mueller et al., 2000)]. These findings and our results suggest that the white flowers of both parents are derived from mutations of different recessive genes. The hybrid’s ligulate flowers would have been colored by complementing those of the parent’s causative genes. In addition, it was suggested that the white flower causative gene of the parents was not anthocyanidin synthase (ANS) because leucoanthocyanidins were not detected in the parents. In the future, the expression of these genes needs to be investigated in more detail, and this may clarify the cause of the hybrid’s flower color (light pink) and the white flower color of each parent.
The number of bands cleaved by the enzyme Afl II showed that the putative hybrids were intergeneric hybrids of A. frutescens × R. gayanum (Fig. 3). Therefore, this study elucidated that A. frutescens is compatible with perennial species of R. gayanum. In addition, given that both plants parents-specific bands could be detected, these results will enable us to use the developed selection markers to promote intergeneric hybrid breeding and improve breeding efficiency.
Other genetic markers can be used to discriminate putative hybrids. For example, intergeneric hybrids between A. frutescens and Glebionis carinata have been discriminated using Random Amplified Polymorphic DNA (RAPD) markers (Morikawa et al., 2014). Likewise, hybrids between Rosa damascena and R. bourboniana have been discriminated using RAPD and Simple Sequence Repeat (SSR) markers (Kaul et al., 2009). Furthermore, sex determination in Eucommia ulmoides and tolerance determination in wheat powdery mildew have been determined using Sequence-characterized amplified region (SCAR) markers (Liu et al., 1999; Xu et al., 2004). In the present study, we developed CAPS markers that can be used to discriminate intergeneric hybrids between A. frutescens and R. gayanum, and we also plan to develop SCAR markers (or SSR markers) that will enable us to determine the hybridity of the resulting progeny.