2020 Volume 89 Issue 1 Pages 22-29
2020 Volume 89 Issue 1 Pages 22-29
The translocation of calcium (Ca) within the tomato plant and the causes of Ca deficiency, a factor associated with blossom-end rot (BER) in fruit, are still a matter of conjecture. The objective of this study was to determine the effect of defoliation on BER incidence and Ca transport into different size tomato fruit cultivars. Four experiments were conducted. The start and end dates for each experiment were; 14 March–2 May, 22 July–23 August, 30 August–7 October 2017, and 20 May–25 June 2018, for experiments 1, 2, 3, and 4, respectively. Five tomato cultivars including one large (‘Momotaro fight (MF)’, ≥ 200 g), three medium (‘Lui 60 (L60)’, ‘Tio cook (TC)’, and ‘Cindy sweet (CS)’, 30–80 g), and one small (‘Pepe (PP)’, ≤ 20 g) fruit cultivars, respectively, were grown under moderate water stress controlled by a combination of root zone restriction and solar mediated fertigation. Leaf area of plants was reduced by 20–30% by removing alternate leaflets on all leaves. Defoliation significantly reduced BER in all experiments. In experiment 4, no BER was observed in defoliated plants of L60 and PP, and in MF and TC, BER incidence decreased to a quarter of the control. Defoliation increased the fruit growth rate (FGR) in experiment 1, in which the temperature was the lowest, by a ratio of 1.42 and by 1.39 in experiment 4, in which the radiation was strongest and day length longest. Defoliation increased the rate of daily Ca transport into fruit (CTR) in MF, L60, TC, CS, and PP by average ratios of 1.64, 1.55, 1.35, 1.30, and 1.13, respectively. The increase in CTR in defoliated plants was highest in experiment 4 with a ratio of 1.68 followed by 1.37, 1.33, and 1.28 in experiments 1, 3, and 2, respectively. Defoliation increased both FGR and CTR and there were significant linear relationships between them. However, the degree of increase was larger in CTR than that in FGR, especially in the BER-sensitive large fruit cultivar MF, and defoliation increased the total Ca concentration in fruit accordingly. We conclude that under moderate water stress by root zone restriction and certain other BER inductive conditions, defoliation could be a promising approach to reduce BER incidence by improving Ca nutrition in susceptible large fruit cultivars.
Ca plays an essential role in processes that preserve the structural and functional integrity of plant membranes, stabilize cell wall structures, regulate ion transport and selectivity, and control ion-exchange behavior, as well as cell wall enzyme activities (Marschner, 1995; Rengel, 1992). However, these functions can be seriously impaired due to reduced Ca availability, as Ca can be readily displaced from its membrane binding sites by other cations leading to development of physiological disorders in some fruit. Blossom-end rot (BER) is a physiological disorder in tomato (Solanum lycopersicum L.) that occurs under low apoplastic Ca2+ conditions. The plasma membrane can become leaky, leading to cell plasmolysis and eventually death (De Freitas et al., 2011; Suzuki et al., 2003). BER symptoms thus appear as a black sunken decay on the blossom-end of the tomato fruit.
Ca transport in the xylem occurs by mass flow of Ca2+ and some organically complexed Ca, and by chromatographic movement along Ca-exchange sites in the xylem walls. Competition between sinks is intensified when Ca2+ in the xylem is low and transpiration is great (Collier, 1983). As tissues grow, they provide sinks in the xylem exchange column to which Ca migrates. Clarkson (1984) previously demonstrated that under certain environmental conditions, buds, developing leaves and fruit provide major sinks for Ca delivery by the xylem. Plants continuously regulate transpiration by controlling the aperture of the stomatal pores on the surfaces of leaves. The principal atmospheric determinant of stomatal aperture is the humidity of the air, which can be expressed as the vapor pressure deficit (VPD) (McAdam, 2015).
BER has been shown to occur in plants with an adequate Ca supply when grown in environmental conditions that reduce Ca transport to rapidly growing distal fruit tissues (Ho and White, 2005; Saure, 2001). Other studies have shown that the fruit growth rate can play a role in inducing BER. Large fruit cultivars have a lower total Ca concentration than small fruit cultivars, resulting in severe and frequent BER incidence (Ho and White, 2005; Vinh et al., 2018). The authors have found a significant relationship between the concentration of water-soluble Ca in the distal portion of tomato and BER incidence under inductive conditions, such as root zone restriction (Ooyama et al., 2016; Yoshida et al., 2014) or an extended photosynthetic light period (Ooyama et al., 2017). Therefore, the promotive effects of irradiance and ambient temperature on fruit growth and/or perturbation in Ca uptake and distribution within the fruit may trigger BER development (Adams and Ho, 1993; De Freitas and Mitcham, 2012; Ho and White, 2005). Clarkson (1984) noted that Ca deficiency seldom arises because of a failure of Ca supply to plant roots and is more frequently explained by problems arising from its internal distribution and its allocation in mature and growing regions of the plant.
Despite the many studies on the relationship between Ca deficiency and BER development, the translocation of Ca within the plant and the causes of Ca deficiency in fruit are still a matter of conjecture (Saure, 2005). Several studies have suggested strategies that can be used to control BER such as reducing excessive gibberellin levels (Saure, 2005), whole plant and fruit specific abscisic acid spray treatments (Barickman et al., 2014; De Freitas et al., 2014) and control of potential BER-inductive factors, especially the environment (Yoshida et al., 2014). However, few studies have investigated cultural practices that can reduce BER incidence in tomatoes, such as defoliation. Despite the major role of leaves in plant transpiration and the importance of the leaf area index, which is commonly recognized for its role photosynthesis, few studies have reported on the effect of defoliation on Ca transport and development of BER in tomato. Sato et al. (2004) showed that defoliation could reduce BER incidence in hydroponically grown tomatoes; however, they did not determine Ca in fruit tissue and no further work has been done to ascertain the Ca transport and concentration in fruit of defoliated plants. Secondly, since previous studies have shown that large fruit cultivars in tomato have a low total Ca concentration and are more susceptible to BER (Vinh et al., 2018; Yoshida et al., 2014), an understanding of the Ca transport potential of different size tomato fruit cultivars in relation to environmental changes is required. We were able to repeatedly induce BER incidence by growing tomato plants with a combination of restricted root zone volume and solar mediated fertigation control. Here, we report the effect of defoliation on Ca transport into different size tomato fruit as influenced by environmental conditions under moderate water stress provided by root zone restriction.
As shown in Table 1, four experiments were carried out in a plastic house (6 m wide × 19 m long × 4 m high) at the Faculty of Agriculture, Okayama University from January 2017 to June 2018. The temperature in the plastic house was maintained above 12°C by a warm-air heater and adequate ventilation was applied with a fan and windows when the temperature exceeded 28°C. Daily environmental data were recorded for each experimental period and means were calculated from anthesis to sampling for each analyzed fruit.

Mean growing conditions of five tomato cultivars from the beginning of anthesis to the end of sampling for four experimental periods (2017–2018).
Five tomato cultivars including one large (‘Momotaro fight (MF)’, ≥ 200 g), three medium (‘Lui 60 (L60)’, 40–80 g; ‘Tio cook (TC)’, 40–80 g; and ‘Cindy sweet (CS)’, 30–80 g), and one small (‘Pepe (PP)’, ≤ 20 g) fruit cultivars, respectively, were grown under moderate water stress controlled by a combination of root zone restriction and solar mediated fertigation. The seeds were sourced from Takii Co., Ltd., Kyoto, Japan (MF, TC, L60, and PP) and Sakata Seed Corp., Yokohama, Japan (CS). The seeds were then sown on vermiculite moistened with water in trays and placed in a growth chamber set at 25°C and with a 12 h day length. At the 3rd true leaf stage, the seedlings were transplanted into plastic pots (12 cm in diameter) filled with tomato growing medium. The seedlings were then moved to the plastic house and fertigated daily with half strength Enshi solution with an EC of 120–130 mS·m−1. At the growth stage in which flower buds on the plants were visible, the plants were transferred to a solar mediated fertigation system within the plastic house as previously described (Yoshida et al., 2007, 2014). The supplied amount of nutrient solution and fertigation frequency were adjusted to 33–240 mL per 1–4 MJ·m−2 to ensure that 10–20% of the supplied solution was discharged in the MF control plant.
Six plants were maintained for each cultivar in each experiment. All lateral shoots were removed regularly throughout the growing period. Half the plants were used as untreated controls and the other half were used for the treatment. The treatment involved removal of alternate leaflets on all three leaves above a flowering truss (Fig. S1), henceforth referred to as “defoliation” in this study. This treatment was applied to all leaves between the 1st and the 5th truss to ensure the 1st fruit on the 3rd truss had reached the sampling stage. The terminal leaflet on each leaf was not removed. Defoliation treatment commenced at anthesis of the 1st flower on the truss for three leaves just above each truss. Six leaves below the 1st truss were retained on all the plants. A day after the end of sampling the 1st fruit on the 3rd truss, and leaf area of all leaves (including the midrib) above the 1st and below the 4th truss were measured using an area meter (Model LI-3100; Li-COR, Inc. Lincoln, NE, USA).
The date of anthesis of the 1st fruit on each truss was recorded. Sampling was conducted for Ca analysis at 14 (large fruit cultivar), 18 (medium size fruit cultivars), and 20 (small fruit cultivar) days after anthesis, respectively, to obtain enough fruit tissue for Ca extraction. Flowers on each truss were thinned to retain only four, eight and 20 fruits for the large, medium and small fruit cultivars, respectively. A minimum of four and maximum of nine well-developed and BER-unaffected 1st fruits of the 1st–3rd trusses were sampled for Ca and growth analysis.
At sampling, fruit fresh weight without the calyx was measured to determine the fruit growth rate. The fruit was then divided equatorially into two portions, proximal and distal, and prepared for Ca extraction. Sequential extraction to water and hydrochloric acid (HCl)-soluble Ca fractions that served as representatives for (1) apoplastic and cytoplasmic Ca2+, loosely wall-bound Ca; (2) residual insoluble Ca, was performed as described in Yoshida et al. (2014). Ca concentration was determined using atomic absorption spectrometry (SPCA-6210; Shimadzu, Kyoto, Japan) and described as μmol·g−1 FW. BER incidence was recorded when observed.
The fruit growth rate was calculated as fresh weight divided by the number of days after anthesis. The daily Ca transport rate was determined by dividing the Ca amount in fruit by the number of days after anthesis. The incidence of BER in the 1st–3rd trusses was recorded until leaf area measurement and calculated as a percentage of BER-affected fruits of the total number of fruits on each truss, excluding sampled fruit and small, young fruit on the truss at the end of sampling that had not reached the sampling stage (under 1 cm and approx. less than 3 g); this was common for the small fruit cultivar, PP (Table S1). Microsoft Excel spreadsheets were used for data analysis and multiple comparisons of means were done using Tukey’s test (P < 0.05). A summary of the statistical analysis results is shown in Table 2.
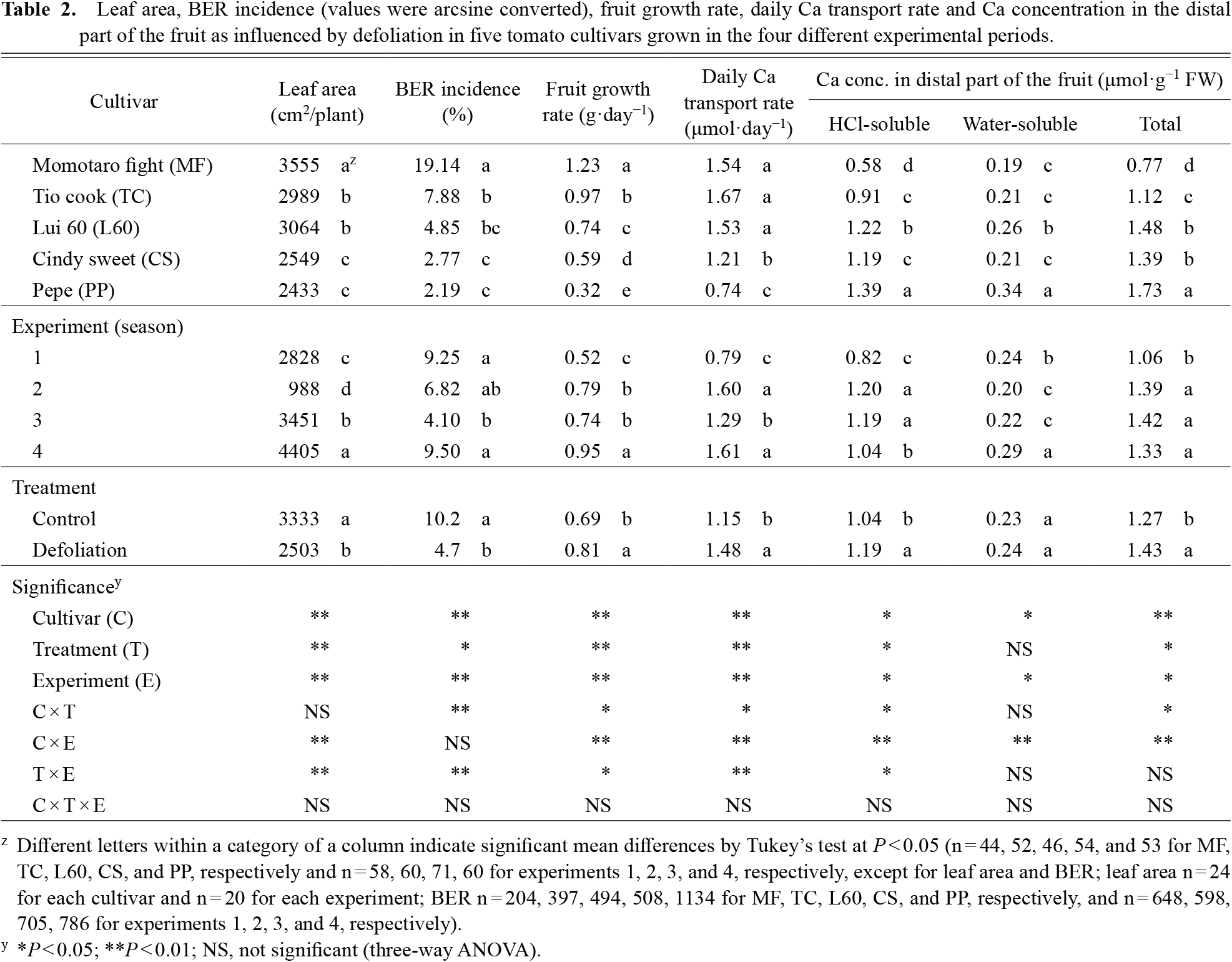
Leaf area, BER incidence (values were arcsine converted), fruit growth rate, daily Ca transport rate and Ca concentration in the distal part of the fruit as influenced by defoliation in five tomato cultivars grown in the four different experimental periods.
Mean values of the environmental conditions and changes in daily mean temperature for the four experiments from the beginning of anthesis to the end of sampling are shown in Table 1 and Figure S2, respectively. In experiment 1, the lowest and highest daily mean temperatures during the experimental period were 12.7°C and 23.1°C, respectively. Solar radiation and VPD were at medium levels and day length was shorter than in experiment 2 and 4. In experiment 2, temperatures were high throughout the period with the lowest and highest daily means of 25.9°C and 33.6°C, respectively. Solar radiation and VPD were high in this experiment and day length was long. In experiment 3, daily mean temperature steadily decreased from 29.2°C to 18°C. However, solar radiation was low and day length was short. In experiment 4, a steady increase in temperature from 18.7°C to 27.6°C was recorded. Solar radiation and VPD were the highest, and day length was long.
Defoliation consistently reduced leaf area by 20–30% in all the cultivars in all the experimental periods (Table 2; Fig. S3). Leaf area of control plants was largest in experiment 4 in which the temperature was a medium value and solar radiation and day length were maximum, and was smallest in experiment 2 in which temperature and VPD were highest. Large and medium fruit cultivars had larger leaf areas than the small cultivar PP in all experimental periods.
Effect of defoliation on BER incidenceBER incidence was significantly different between cultivars, experimental periods and treatments as shown in Table 2. The large fruit cultivar MF, consistently had the highest BER incidence and the small fruit cultivar PP had the lowest incidence in all experiments (Fig. S4). The lowest BER incidence in our experiments was observed in experiment 3 with all the cultivars showing incidence below 10%, except for MF with 19%.
Defoliation corresponded to a reduction in incidence of BER in each of the experiments. However, defoliation had the smallest and largest effects on BER in experiments 3 and 4. In the latter experiment, no BER was observed in defoliated plants of L60 and PP and BER incidence was reduced to a quarter of the control in the BER-susceptible large fruit cultivar MF and medium fruit cultivar TC.
Effect of defoliation on the fruit growth rateThe fruit growth rate was higher in defoliated plants compared to the control plants throughout the experiments (Table 2; Fig. S5). The lowest fruit growth rate was observed in experiment 1 and differences among the other 3 experiments were small. However, it increased considerably by defoliation in experiment 1 and 4 by ratios of 1.42 and 1.39, in which temperature was the lowest in the former and radiation and day length were largest and longest in the latter, respectively. In experiment 3, which had low radiation and short day length, defoliation increased the fruit growth rate by the lowest ratio of 1.07. On average, defoliation increased the fruit growth rate in MF, TC, L60, CS, and PP by ratios of 1.31, 1.29, 1.22, 1.21, and 1.19, respectively. The large and medium size fruit cultivars consistently had a higher fruit growth rate than the small fruit cultivar PP.
Effect of defoliation on the daily Ca transport rateDaily Ca transport into fruits increased in defoliated plants in all the cultivars and in all experimental periods (Table 2; Fig. S6). In controls, the Ca transport rate was highest in experiment 4 and 2, and lowest in experiment 1. The increase in the Ca transport rate in defoliated plants was highest in experiment 4 by a ratio of 1.68, followed by 1.37, 1.33, and 1.28 in experiments 1, 3, and 2, respectively. The increase in the Ca transport rate in defoliated plants was higher in large and medium fruit cultivars than in the small fruit cultivar. Defoliation increased the daily Ca transport rate in MF, L60, TC, CS, and PP by average ratios of 1.64, 1.55, 1.35, 1.30, 1.13, respectively.
Influence of defoliation on the relationship between the daily Ca transport rate and fruit growth rateThere were significant linear relationships between fruit growth rate and the daily Ca transport rate into fruits and the trends differed among cultivars (Fig. 1). MF had the lowest slope value at 0.74 in the control and 1.19 in the defoliated plants and the effect of defoliation on the Ca transport rate was larger than that on the fruit growth rate. The slope values of PP were larger than MF, but the effect of defoliation on the value was smaller than MF. The slope value of TC, one of the medium size fruit cultivars, was the highest at 1.87 in the control and 1.79 in the defoliated plants. The effect of defoliation on the slope value was small, as the effect on the fruit growth rate was larger compared with that in MF or PP. The other two medium size fruit cultivars showed a similar trend. The slope ratio (defoliated/control) of 1.61 in MF was the highest, followed by PP, L60, TC, and CS at 1.31, 1.15, 0.94, and 0.82, respectively.
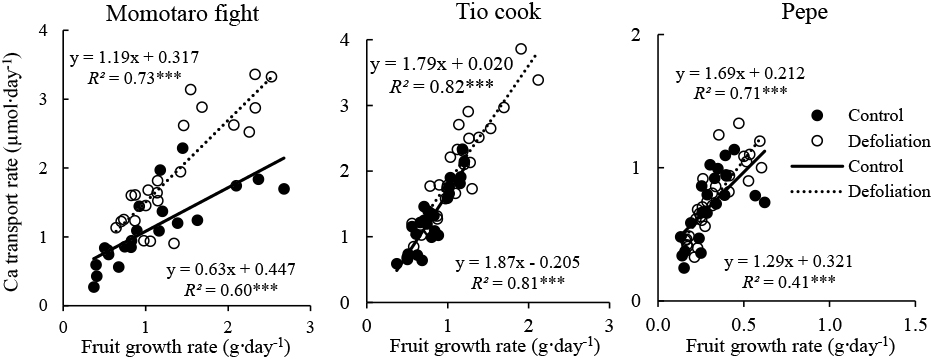
Comparison of influence of defoliation on the relationship between the rate of Ca transport into fruit and fruit growth in large (‘Momotaro fight’), medium (‘Tio cook’), and small (‘Pepe’) size tomato fruit cultivars. *** indicate significance at P < 0.001.
The total Ca concentration in the distal part of the fruit significantly differed among cultivars, treatments and experiments (Table 2). As shown in Figure S7, MF, a large fruit cultivar, had the lowest Ca concentration in the distal part of the fruit with 0.62, 0.88, 0.57, and 0.75 μmol·g−1FW, in experiments 1, 2, 3, and 4 in the control plants. PP, a small fruit cultivar, had the highest Ca concentration in the distal part of the fruit among the cultivars with 1.08, 1.45, 2.06, and 1.78 μmol·g−1FW, in experiments 1, 2, 3, and 4, respectively. Among medium fruit cultivars, L60 representing the high Ca transport rate and medium level fruit growth rate exhibited the highest Ca concentration in the distal part of the fruit. The water-soluble Ca concentration was also the highest in the small fruit cultivar PP in all experiments and the lowest in MF in the control, except for experiment 2 (Table 2).
The objective of this study was to determine the effect of defoliation on BER incidence and Ca transport into different size tomato fruit cultivars under moderate water stress. Many studies have established the relationship between Ca deficiency and BER in fruit (Adams and Ho, 1993; De Freitas et al., 2014; Ooyama et al., 2016, 2017; Vinh et al., 2018; Yoshida et al., 2014). Development of BER in tomato fruit has been known to occur mostly under conditions of dryness or an inadequate supply of Ca in the root zone (De Freitas and Mitcham, 2012; Ho and White, 2005; Saure, 2001, 2005; Yoshida et al., 2014). In this study, we used a combination of restricted root zone volume and solar-mediated fertigation control. Under this system, moderate water stress levels were maintained continuously and BER symptoms were observed in all seasons and in all cultivars at varying severity levels (Table 2; Fig. S4). These results show that this system is an effective tool in BER studies as previously reported (Yoshida et al., 2007, 2014).
Leaf area analysis revealed that alternate leaflet removal reduced the leaf area of tomato plants by 20–30% and cultivars varied in their responses to defoliation (Fig. S3). Large and medium fruit cultivars had larger leaves than the small fruit cultivar in this study. Interestingly, alternate leaflet removal did not reduce the leaf area more than 31% irrespective of the cultivar. The reduction far smaller than 50% was probably due to the midrib, terminal leaflet, and the compensatory expansion of retained leaflets on the leaf. Sato et al. (2004) also applied similar treatment by removing half the number of leaflets from a leaf, but they did not measure leaf area. The compensatory expansion of leaflets varies depending on the cultivar and growing season, and finally results in differences in leaf area reduction.
In this study, defoliation significantly reduced BER in all the experiments. This result is similar to that of Sato et al. (2004). Competition for Ca between the leaves and fruits with Ca moving preferentially to the leaves rather than to the fruit under rapid transpiration (Ho, 1989) may cause inadequate distribution of Ca in the fruit at periods of critical demand, leading to a Ca deficiency associated with BER disorder (Adams and Ho, 1993). The reduced BER incidence following defoliation may have been a result of the increased Ca transport rate into fruit, leading to an increased Ca concentration in the fruit. BER decreased considerably in the large and medium fruit cultivars that had the largest increase in Ca concentration due to defoliation (Fig. S7); this probably became available for structural functions in the fruit at a point before BER was triggered, unlike in the control plants. Cultivars differed in their susceptibility to BER. Large and medium fruit cultivars were more susceptible than the small fruit cultivar. This result agrees with several other studies that have associated differences in susceptibility to BER among cultivars to genetic characteristics regulating the potential size and growth rate of fruit (Adams and Ho, 1992; Ho and White, 2005; Marcelis and Ho, 1999).
Fruit growth rate and potential fruit size are important parameters in understanding Ca transport into tomato fruit. Previous studies have suggested that rapid fruit expansion may result in a lag or reduction in Ca transport to rapidly growing distal fruit tissue, along with an increase in Ca2+ demand by fruit tissue (De Freitas and Mitcham, 2012; Ho and White, 2005; Saure, 2005). In this study, defoliation increased the fruit growth rate (Fig. S5) more in the large fruit cultivars than in the small fruit cultivars under conditions of low temperature, high solar radiation and long day length. This result differs from that of Sato et al. (2004), whose study found no significant effect on fruit growth. However, they conducted their experiment only in one season and also with a hydroponics system. Therefore, our result implies that under root zone restriction, that is, moderate water stress, defoliation probably improved water conditions within the fruit. Even though the fruit growth rate was increased by defoliation and therefore the fruit were expected to be more susceptible to BER, the simultaneous increase in Ca transport rate and Ca concentration in the fruit may have resulted in the lower BER observed in defoliated plants.
It is widely known that Ca cannot be readily remobilized once unloaded from the xylem, and in particular, to points downstream of transpiration flow. Since Ca is transported via the transpiration stream, leaves, as highly transpiring organs, usually have higher Ca levels than the low transpiring organs such as fruit. Higher transpiration and growth rates can reduce water potential and increase tissue strength as sinks for xylem Ca2+ (Conn and Giliham, 2010; White and Broadley, 2003). Under conditions which favor high transpiration of leaves within a greenhouse, competition for Ca between leaves and fruit can occur leading to reduced Ca concentration in fruit at critical times resulting in development of BER. In our study, the observed increase in Ca transport rate and Ca concentration in defoliated plants (Figs. S6 and S7) was probably a result of reduced transpiration in the leaves. This led to less competition for water between the leaves and fruits causing more Ca to be distributed into the fruits, especially in the large and medium fruit cultivars.
Both physiological and molecular mechanisms are involved in the uptake and transport of Ca that affect the distribution and accumulation of Ca in plant tissues. Ca accumulation in tomato fruit has been shown to be dependent on rates of xylem sap flow that is influenced by transpiration and growth rates; however, cultivar differences in these parameters have also been reported (De Freitas et al., 2014; Ho et al., 1993; Riboldi et al., 2018; Vinh et al., 2018). Our study showed a significant linear relationship between Ca transport into tomato fruit and fruit growth rate (Fig. 1) in all the different size fruit cultivars, with the large and medium fruit cultivars showing a higher Ca transport rate than the smaller fruit cultivar. When comparing the large and medium size fruit cultivars, the difference in fruit growth rate ranged from 0.59 to 1.23 g·day−1 and was larger than that of the Ca transport rate that ranged from 1.21 to 1.67 μmol·day−1. Therefore, rapid fruit expansion of large fruit cultivars may be the underlying cause of BER sensitivity. Defoliation had a larger effect on the Ca transport rate in the large and medium size fruit cultivars than in the small fruit cultivar (Fig. S6). This implies that the Ca transporting potential into tomato fruit is proportional to fruit expansion and that defoliation may have caused a compensatory physiological effect enabling the fruit to draw in more water and thus increase the Ca transport rate into the fruit.
However, in addition to the fruit growth rate, multiple regression analysis showed that environmental factors affecting BER development including temperature, radiation, VPD and day length (Adams and Ho, 1993; De Freitas and Mitcham, 2012; Ho and White, 2005; Ooyama et al., 2016; Yoshida et al., 2014) play an important role in explaining Ca transport (Table 3; Fig. S2). As a whole, temperature and day length are positively, and radiation negatively, related to Ca transport. For BER development, these environmental factors also play a significant role, except for day length (Table 4). In contrast to Ca transport, temperature is negatively, and radiation positively, related to BER incidence. Although an extended photoperiod significantly increased BER development in our previous study (Ooyama et al., 2017), day length showed no significant effect on BER incidence in this study. Length of photoperiod and/or dark period may be less important compared to cumulative solar radiation.
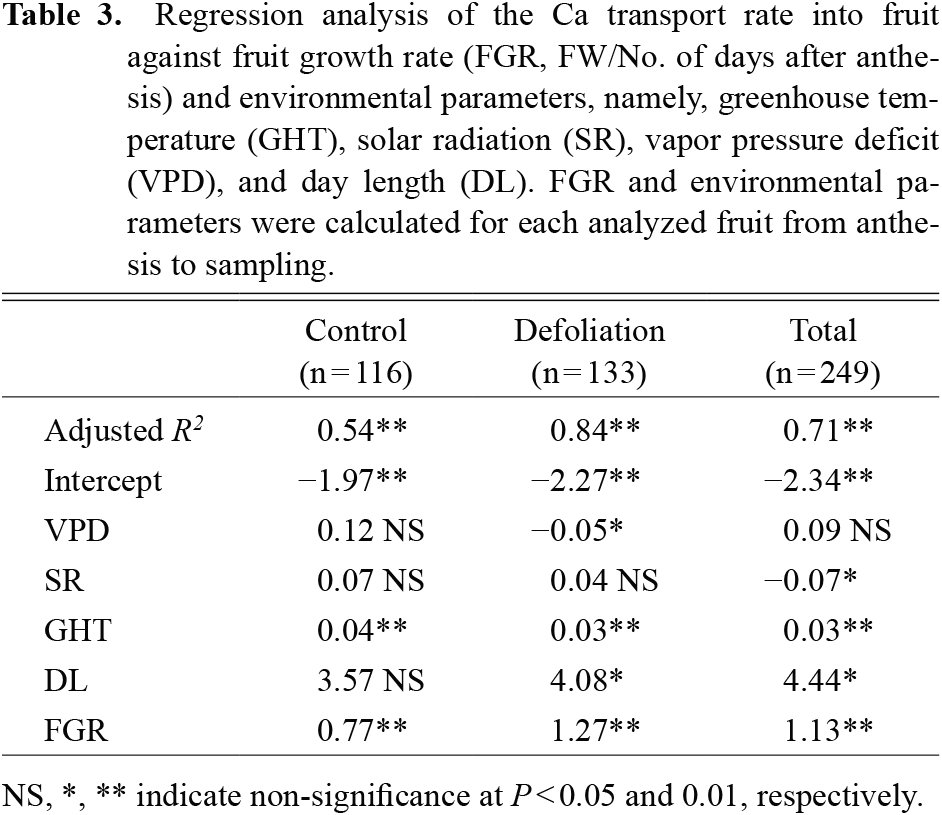
Regression analysis of the Ca transport rate into fruit against fruit growth rate (FGR, FW/No. of days after anthesis) and environmental parameters, namely, greenhouse temperature (GHT), solar radiation (SR), vapor pressure deficit (VPD), and day length (DL). FGR and environmental parameters were calculated for each analyzed fruit from anthesis to sampling.
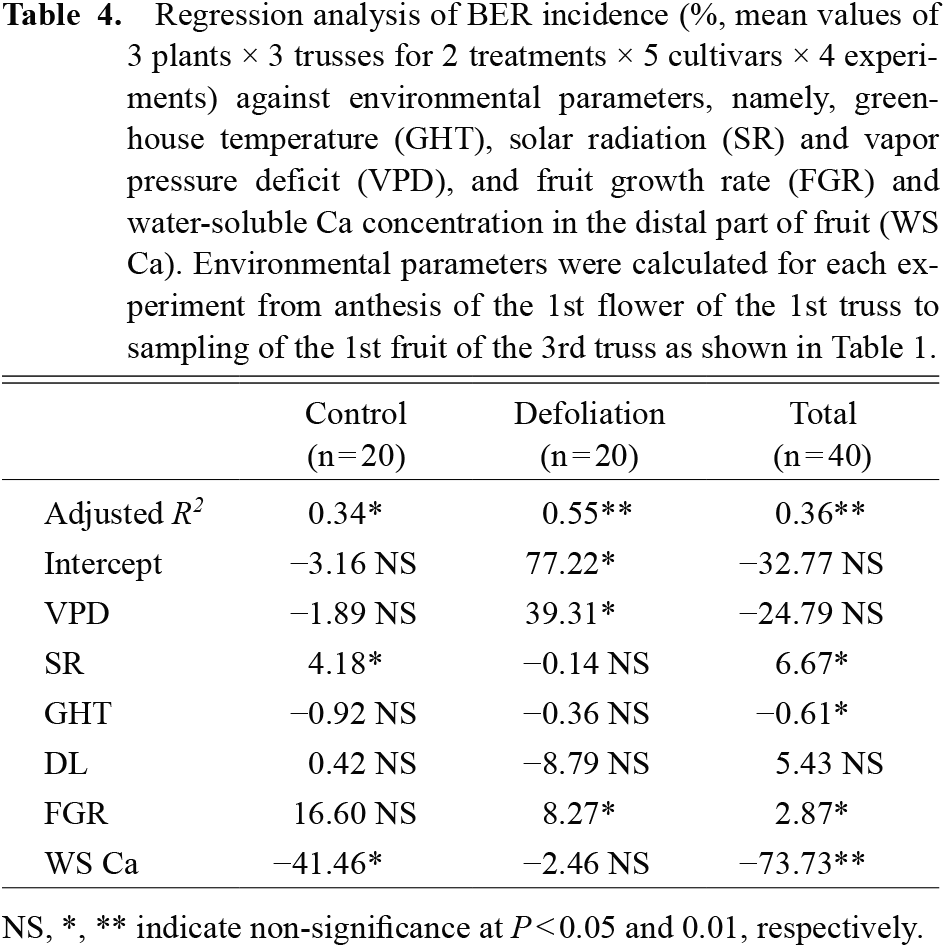
Regression analysis of BER incidence (%, mean values of 3 plants × 3 trusses for 2 treatments × 5 cultivars × 4 experiments) against environmental parameters, namely, greenhouse temperature (GHT), solar radiation (SR) and vapor pressure deficit (VPD), and fruit growth rate (FGR) and water-soluble Ca concentration in the distal part of fruit (WS Ca). Environmental parameters were calculated for each experiment from anthesis of the 1st flower of the 1st truss to sampling of the 1st fruit of the 3rd truss as shown in Table 1.
When control and defoliated plants were analyzed separately, only VPD showed an adverse effect on defoliated plants compared to controls, and the effect was significant only in defoliated plants in both Ca transport and BER. Solar radiation and day length were occasionally statistically significant; however, temperature, which is closely correlated to the fruit growth rate, was regularly significant in explaining the Ca transport rate into fruit. This result may indicate that rapid fruit growth may be the dominant factor affecting Ca transport rather than environmental factors, except for temperature. Therefore, environmental factors directly affecting leaf transpiration may be not effective for control plants subjected to moderate water stress, but occasionally effective for less stressed defoliated plants in explaining the Ca transport rate in tomato. As for BER incidence, environmental factors and also fruit growth rate and water-soluble Ca concentration in the distal part of the fruit that were highly significant parameters in our previous studies (Ooyama et al., 2016; Vinh et al., 2018; Yoshida et al., 2014) were only occasionally effective parameters in this study. Here, we did not take BER-affected fruit samples, and the number of samples fluctuated, especially for control plants. Therefore, fruit rapidly growing and/or containing very low water-soluble Ca may have developed BER and been omitted from fruit samples. Nonetheless, even though the effect of defoliation on the water-soluble Ca concentration was not significant, the P value was less than 0.1.
Several studies have reported that Ca allocation among tissues, between symplastic and apoplastic spaces, or among various cell compartments determines the availability of Ca for its structural function (De Freitas and Mitcham, 2012; Vinh et al., 2018; Yoshida et al., 2014). Vinh et al. (2018) suggested that water-soluble Ca, including the apoplastic and cytoplasmic Ca2+ that are more closely related to cell physiological processes compared to cell wall bound or insoluble Ca fractions in the distal part of young tomato fruit, can be useful predictors of BER incidence risk. In this study, defoliation increased the Ca concentration in the distal part of the fruit in all cultivars (Table 2; Fig. S7). This increase is probably why there was decreased BER incidence in the defoliated plants compared to the controls. Most likely, in defoliated plants, sufficient water-soluble Ca accumulated in time within the distal part of the fruit, thus maintaining the Ca homeostasis within the fruit.
In conclusion, alternate leaflet removal applied to moderately water stressed plants significantly increased the fruit growth rate, increased Ca transport into tomato fruit and reduced BER in susceptible large fruit cultivars (Table 2). Compared to small fruit, the higher Ca transport rate into fruit in the large fruit cultivar was probably due to a faster fruit growth rate. However, in the large fruit cultivar, the Ca level often could not meet demand, resulting in BER incidence. We therefore hypothesize that defoliation probably reduced the competition for water between the leaves and fruits due to reduced transpiration, causing sufficient Ca to be transported into the fruits at a critical time before BER was triggered. We would therefore recommend defoliation as a simple cultural practice that can be used to mitigate against BER development in large fruit cultivars not only under root-restricted conditions, but also under other BER-inductive conditions. However, the defoliation technique used for this study can be labor intensive. Further research with the aim of establishing a practical number of whole leaves that can be removed to increase Ca within the fruit without consequently stressing the plant and/or compromising fruit quality is proposed.