2020 Volume 89 Issue 2 Pages 87-95
2020 Volume 89 Issue 2 Pages 87-95
In Japan, over 95% of the acreage is covered with plastic to force June-bearing (seasonal flowering, SF) strawberry cultivars to produce fruit from late fall to early summer. During the late 1960s, a forcing technique was developed that advanced flower bud initiation to late summer and prevented the transplants from becoming dormant during winter. This new forcing technique involved nitrogen starvation of nursery plants to induce floral initiation. Until about 1980, strawberry growers in Japan used runner plants produced in waiting beds, but most transplants are now produced in plastic pots under rain shelters to avoid soil-borne diseases. Recently, the use of tray plants produced from hanging runner cuttings has become popular. To induce early floral initiation, the following artificial low temperature (LT) treatments have been established: (1) “Yarei”, a combination of a short day with solar radiation and LT under darkness in cooling facilities (Yarei-ko); (2) “Kaburei”, continuous dark-LT with refrigeration facilities including industrial warehouses; and (3) “Kanketsu-reizo”, intermittent LT storage. An overview of the technologies applied to plant propagation and the control of floral initiation of Japanese SF cultivars is provided in this review.
In Japan, forcing greenhouse production of early June-bearing cultivars is the predominant production method for cultivated strawberries (Fragaria × ananassa Duch.). For successful forcing strawberry production, usually accompanied by a considerable yield in December, flower bud differentiation within September and flowering in November are required. Japanese strawberry cultivars produce qualified fruit with an excellent flavor; however, they are often softer than United States or European cultivars. Therefore, the fruit must be picked and packaged carefully and intensively cultivated. These reasons suggest why most strawberry farms are limited to family businesses. The total acreage and production of strawberries have been steadily decreasing for decades; however, table-top production systems introduced after 1995 have allowed total production to remain constant (Fig. 1). Breeding programs and physiological, ecological, and horticultural research have been conducted all over Japan (Yoshida, 2012). Among the objectives of research programs, genetic and technical improvements that ensure early flowering have been important to increase early yield and to meet the demand for strawberries during the Christmas holiday season (Fig. 2).

Annual strawberry production and acreage in total (including open field) and substrate (table-top) production during the period from 1980 to 2018 (Ministry of Agriculture, Forestry and Fisheries, Japan). Note the acreage of strawberry cultivation has constantly declined during this period and the production declined from 1990 to 2015, but the production comes to a standstill recently. Although recent values are not available for substrate production after 2012, improved productivity of substrate systems may have contributed to maintain a certain level of production. Scale of acreage is different in substrate from total acreage.

Changes in the monthly mean price (solid lines) and supply (dotted lines) of strawberries at the Tokyo Metropolitan Central Wholesale Market. Note the differences in price and supply values between May and December.
The flowering of a plant is the result of reproductive growth that consists of sequential differentiation and the development of floral organs in the shoot meristem. The floral transition of a plant is the change or shift in the developmental phase from vegetative to reproductive, which is induced by environmental and/or internal factors. This floral transition may include physiological floral initiation and subsequent morphological differentiation of flower buds. However, the terms related to early phase reproductive growth, such as floral transition, floral or flower bud initiation, and flower bud differentiation and development are confusing. Each term represents a range of definitions or phenomena overlapping each other, and the term flower bud may indicate an individual bud of a young flower and/or a whole young inflorescence. Here, we use these terms as follows: floral transition, a change in developmental phase from vegetative to reproductive; floral initiation, the first phase of reproductive growth without apparent morphological changes in the apical meristem; flower bud differentiation, the next phases represent an enlarged apical meristem, along with differentiation of new floral organs and individual flower buds; and flower bud development, the qualitative improvement or progress of floral organs and flower buds often accompanied by quantitative growth.
Cultivated strawberries can be roughly divided into two groups, June-bearers and everbearers, based on their flowering habits. June-bearing strawberries, accounting for more than 95% of the Japanese market, are classified as facultative or obligate short day (SD) plants (Eguchi, 1932; Sønsteby and Heide, 2006) and are often called SD or seasonal flowering (SF) cultivars. Generally, floral initiation is inhibited under warm conditions (e.g., above 25°C) or occurs under cool conditions (e.g., below 15°C) independent of the photoperiod (Heide et al., 2013; Ito and Saito, 1962). Anthesis occurs during the following spring under natural conditions because reproductive growth is promoted by long days (LD) and warm temperature (Eguchi, 1939; Fujimoto, 1971; Heide et al., 2013). In contrast, in everbearing (EB) cultivars, flowering occurs perpetually without SD or cold stimuli and is promoted by LD under excessive high temperatures (Heide et al., 2013; Nishiyama et al., 1999). In Japan, the application of artificial floral-inducing treatments (e.g., cooling and SD) for SF cultivars is performed before planting to realize early fruit production. Subsequent promotion (e.g., heating, supplemental CO2 supply, and LD treatments) for reproductive and vegetative growth and development is conducted in greenhouses (Yoshida, 2012). Under such conditions, most SF cultivars do not become dormant, exhibit pseudo-perpetual flowering habits, and produce qualified fruit throughout the winter and spring seasons. EB cultivars that exhibit perpetual flowering under natural conditions have been grown to produce fruits in summer and autumn, the changeover period for SF cultivars.
Recent molecular genetic studies have demonstrated that FT/TFL1-like genes are expressed in the leaves and shoot apical meristem (SAM) of F. vesca as floral promoters and/or repressors and it is thought that F. × ananassa may also involve similar flowering regulation mechanisms (Heide et al., 2013; Kurokura et al., 2013). FvFT1, a possible key floral repressor, was down-regulated under floral inductive conditions in SF F. vesca (Iwata et al., 2012; Koskela et al., 2012). Nakano et al. (2015) reported that in the SAM of an SF cultivar, ‘Nyoho’, the expression of FaTFL1 was also down-regulated under inductive conditions and consequently FaFT3, a possible promoter of F. × ananassa, and FaAP1, a floral meristem identity gene, were up-regulated. In many studies on the floral transition of cultivated strawberries, the apparent doming of the meristem tissues has been thought to indicate a typical floral transition and beginning of flower bud differentiation (Eguchi, 1932; Morishita and Honda, 1984; Yoshida, 1992). However, only down-regulation of FaTFL1 was observed in the meristems representing doming. Significant upregulation of floral promoting genes, including FaFT3 and FaAP1, could be detected only when most of the meristem tissue samples began to differentiate the floral meristem that developed into the primary flower of inflorescence. Further investigations on the expression of FT/TFL1-like genes during the floral transition of SF strawberries are required for a better understanding of flowering control.
From the 1970s to mid-1980s when forcing strawberry production in plastic housing became popular, ‘Hokowase’ (released from Hyogo Prefecture, 1957) was a leading cultivar for a long time. As this cultivar was selected for open field production, it requires 400–500 h of chilling to break dormancy, and achieve successful growth and yield in semi-forcing production. LD treatments, such as day-extension, night break, or intermittent lighting, as well as GA3 treatment, have become essential procedures to prevent the cultivar from becoming dormant during winter in forcing production. ‘Toyonoka’ (Veg. Ornam. Crop. Res. Sta. MAFF, 1984) and ‘Nyoho’ (Tochigi Prefecture, 1985) were released concurrently and became popular in the western and eastern regions of Japan, respectively, because their productivity, early harvest, fruit quality, and shelf-life were proven to be superior to ‘Hokowase’. ‘Tochiotome’ (Tochigi Prefecture, 1996) has since replaced ‘Nyoho’ and has been the leading strawberry cultivar in Japan this century, accounting for more than 50% of the Tokyo Metropolitan Central Wholesale Market (http://www.shijou.metro.tokyo.jp/torihiki/geppo/) during the latest season. Many public and private breeding programs were started in the 1990s. In approximately the last 20 years, these regional public breeding programs have released large-fruited productive cultivars, such as ‘Saga-honoka’ (Saga Prefecture, 2001), ‘Beni-hoppe’ (Shizuoka Prefecture, 2002), ‘Yumenoka’ (Aichi Prefecture, 2005), ‘Fukuoka-S6’ (Ama-oh®; Fukuoka Prefecture, 2005), and ‘Kotoka’ (Nara Prefecture, 2009). These SF cultivars are not as early compared as the recently released very early cultivars, such as ‘Kaorino’ (Mie Prefecture, 2015) and ‘Kirapika’ (Shizuoka Prefecture, 2017). Such cultivars can differentiate flower buds before September and be harvested in October without specific flower inductive treatment; however, they sometimes experience unexpected early flowering and shoot growth where the vegetative branch crown is lost through direct flowering or runnering of the axillary shoots (Kawata et al., 2016; Yoshida and Nakayama, 2008). These two cultivars and also ‘Beni-hoppe’ exhibit vigorous vegetative growth during the winter season without LD treatment. A breeding consortium consisting of Mie, Kagawa, Chiba Prefecture and NARO introduced an EB habit into SF genotypes and released the first Japanese seeds propagating EB cultivar ‘Yotsuboshi’ (2017).
Although genetic improvements for early flowering and harvesting are advancing, controlling the floral initiation of existing SF cultivars that have attractive characteristics and are popular in the market has been important to ensure early yield. In Japan, flower controlling technologies have been developed specifically to meet the peak demand during the Christmas weeks. The present study provides an overview of the technologies applied to plant propagation and control of floral initiation in forcing strawberry production of Japanese SF cultivars.
Before the 1970s, strawberry plants were usually propagated in 2–3 m wide propagation beds and transplanted onto waiting beds with ca. 20 × 20 cm between and within row spacing before mid-July (the end of the rainy season). ‘Hokowase’ plants for semi-forcing production were also raised like this and were finally transplanted onto raised beds in October. During the mid-1960s, an experienced grower in Nara found that a considerable number of plants unexpectedly flowered in November when propagated runner plants were transplanted to poor soil at the end of August or the beginning of September. He focused on this phenomenon and discussed the results with a researcher from the Nara Agricultural Experiment Station, where they revealed that partial root pruning in the waiting bed, known as “Dankon-zurashi”, can lead to advanced flowering (Fujimoto and Kimura, 1970). Root pruning suppresses nitrogen (N) absorption and NO3–N in petiole tissue significantly decreases; consequently, advanced floral initiation is achieved. Before that, “Yama-age” i.e., growing the transplants of early cultivars at high elevation sites, was the only practical procedure to advance floral initiation.
Although root pruning enabled advanced flowering and fruiting and this method was used across the country, it caused increased frequency of fungal infection and resulted in an epidemic of Fusarium wilt in the susceptible cultivar ‘Hokowase’ within a few years. This disease was controlled by adopting soil solarization practices (Kodama and Fukui, 1979) and propagating transplants in polyethylene pots (Arai et al., 1980). These practices increased labor costs, but were found to be quite reliable against the Fusarium infection and other soil-borne pathogens. Furthermore, N nutrition and subsequent flower bud initiation of pot-raised transplants can be easily controlled. After the release of ‘Toyonoka’ and ‘Nyoho’, rain shelters were used to propagate pot plants to reduce anthracnose infection due to splashing rainwater because the two new cultivars are highly susceptible to the pathogen. In general, over-wintered mother plants are planted in a large planter containing 5–10 L/plant of peat moss-based medium under a rain shelter. Then, 30 to 50 runner plants developed from a mother plant are fixed with a pin and directly rooted on a 7.5–10.5 cm polyethylene pot from May to July.
Recently, the use of tray plants grown from runner cuttings has become increasingly popular, leading to an increase in the production of strawberries in table-top substrate culture systems. These systems have advantages including low cost and easy disease control, and easier plant management and handling compared to conventional pot plants or waiting-bed plants (Yoshida and Motomura, 2011). Runner plants hanging from table-top substrates with 2 to 4 expanded leaves are excised from the mother plants, planted in cell trays, and frequently irrigated automatically until they root. For practical reasons, growers in Kagawa prepare 100 to 200 disease-free mother plants to produce 10%–20% of the total runner cuttings required for 10 a of production using greenhouses that contain 8,000 to 10,000 transplants. The rest of the cuttings, 80%–90% of the transplants, are propagated from the fruit-harvested plants propagated from the disease-free mother plants in the previous year. By applying such a propagating procedure, the labor cost for transplant production can be decreased to less than 50% compared to conventional pot plant production that requires 400 to 500 h/10 a (Yoshida, 2012).
One practice, “Yama-age”, was established during the mid-1950s to grow transplants at high elevation sites located on hillsides or on the outskirts of Mt. Fuji where cooler temperatures prevail during the summer (Ninomiya, 1954). The effect of the elevation of the growing site on flower bud differentiation in the waiting-bed plants of ‘Fukuba’ is shown in Figure 3.

Effect of the elevation of transplant growing site on flower bud differentiation in strawberry ‘Fukuba’ (Ninomiya, 1954).
After the findings of Fujimoto and Kimura (1970) that revealed that the control of N nutrition can be a practical controlling means of floral initiation in ‘Hokowase’, many studies have been conducted on the N nutrition of strawberry transplants. In general, N is a major component of chlorophyll and the most important nutrient for plant growth. However, it is well known that excessive N causes over-vigorous vegetative growth of crop plants and often results in the failed development of floral organs and poor crop yield. Compared to total-N in the leaf blade, the concentration of NO3–N in the petiole increases later, but it is more sensitive and has a large range (Fig. 4). Absorbed N in a deficient plant is quickly assimilated into chlorophyll or other proteins, but excess N in the plant is usually pooled in vacuoles as NO3− (Petrovic et al., 2009).

Differences in the total- and NO3-N concentration in strawberry tissues as affected by the concentration of NO3-N in supplied solutions. Solutions prepared with Ca(NO3)2 were sub-irrigated daily (Inoue et al., 1994).
Table 1 shows the effect of N nutrition expressed as NO3–N concentration of the petiole on the flower bud differentiation of ‘Toyonoka’. Supra-optimal N nutrition, over 0.4%DW (≒400–500 mg·L−1 in petiole sap) of NO3–N repressed strawberry floral initiation and resulted in delayed and non-uniform flowering. However, when SD-low temperature (LT) was applied from the end of August, floral initiation was triggered in the plants with a high NO3-N concentration, which repressed the initiation of plants that had SD-LT applied earlier or dark-LT simultaneously (Inoue et al., 1994). Therefore, the repressive effect of excessive N on floral initiation may be expressed only when the repressive stimulus of day length and/or temperature is moderate or critical. In other words, N nutrition may change or shift the critical day length and/or temperature, repressing floral initiation.

Effect of N nutrition of pot grown nursery plants on flower bud differentiation and development during artificial flower induction treatments for strawberry ‘Toyonoka’ (Inoue et al., 1994).
If N nutrition is removed for a long time and NO3–N in the petiole decreases to a trace level, whole-plant growth and the development of differentiated flower buds are suppressed (Yoshida et al., 1992). Such extreme N control was often applied during the 1980s; however, Maegawa and Minegishi (1991) demonstrated that appropriate N supply during SD-LT treatment enabled advanced floral initiation and subsequent flower bud differentiation and development (Table 2). Until pot plant production became popular, controlling N nutrition of waiting-bed plants could be realized only via partial root pruning known as “Dankon-zurashi”. The effect of pruning was not stable, as several factors including variable soil fertility, rainfall after pruning, and intensity of pruning affected the effectiveness of this rough treatment. Most growers, officers in extension services, and researchers could not identify appropriate procedures to achieve suboptimal nutritional control. Consequently, growers generally continued to apply excessive N starvation to pot grown transplants. N nutrition has usually been determined by measuring NO3–N in the squeezed petiole sap of the 3rd newly expanded leaf, as the differences among mature leaves are small (Table 3). The concentration can be accurately measured using a reflectometer and test strips.

Effect of N fertilization during flower induction treatment of SD-LT on flower bud differentiation, subsequent development, and number of un-emerged leaf primordia in strawberry cultivars (Maegawa and Minegishi, 1991).

Concentration of NO3-N in squeezed sap of strawberry petioles as affected by the leaf age (Kawasato and Nakatsune, 1977).
By following the breeding of two early and productive new cultivars, ‘Nyoho’ (Akagi et al., 1985) and ‘Toyonoka’ (Honda et al., 1985), two artificial flower-inducing treatments were established: (1) night cooling, i.e., approximately 16 h of LT treatment under conditions of darkness with an 8-h period of solar radiation and ambient temperature, and (2) continuous LT storage under darkness. In both these treatments, an LT of approximately 15°C has been recommended (Fushihara et al., 1989; Kumakura and Shishido, 1993; Yoshida and Ozaki, 2011).
1) SD-LD treatment, “Yarei” (night cooling)In 1977, a researcher in Chiba successfully achieved advanced floral initiation of ‘Reiko’ and ‘Hokowase’ by placing transplants into a refrigerator every day and providing SD (long night) and cool conditions (Narukawa, 1986). Six years later, a facility called “Yarei-ko” (Yoshida, 2012) was established to operate in Aichi and was utilized for commercial strawberry production. Its initial and running costs were expensive, but labor costs were low. This facility enabled sufficiently advanced flowering and provided a notable income by increasing the early yield in December.
A stable flower-inducing effect can be expected using this procedure, as the duration of the treatment can be extended until flower bud differentiation is confirmed by microscopic observation. For SD-LT, restricting N nutrition is not necessary. When the N supply is severely restricted, negative effects on the differentiation and further development of flower buds often cancel out the promoting effect on floral initiation (Table 2).
2) LT storage (dark-LT), “Ankoku-reizo” often abbreviated as “Kaburei”Ito and Saito (1962) reported that some LT-treated plants, 9°C in darkness, had differentiated flower buds. Trials for artificial flower induction of ‘Toyonoka’ were conducted (Furuya et al., 1988; Fushihara and Takao, 1987) and successful flower bud differentiation was achieved in a dark refrigerator using pot-grown, aged, large, and N-starved transplants (Tables 1 and 4). Not only have prefabricated cold rooms been used for the precooling of picked strawberries, but large industrial refrigeration warehouses can also be used to apply the treatment on a large scale. The costs associated with this treatment are fairly low, but sometimes the inductive stimulus is insufficient for a significant portion of treated plants. Unlike SD-LT, prolongation of storage is more likely to repress rather than promote floral initiation in non-responder plants (Table 5). Depletion of the limited pool of photosynthate may cause this reversion of flowering (Nishizawa et al., 1997; Tooke et al., 2005). Although the dark-LT treated plants obtained increased the early yield in December, total yield was usually less than that of the non-treated plants (Fushihara et al., 1989). Treated plants with etiolated leaves thereafter presented with poor vegetative and reproductive growth and development. These adverse effects may have resulted in the decreased yield. Some procedures, such as the insertion of an 8-h light period into the continuous storage or modifying the temperature regime, have been proposed to improve the effectiveness of this method, but the obtained benefit other than early flowering is still inadequate.
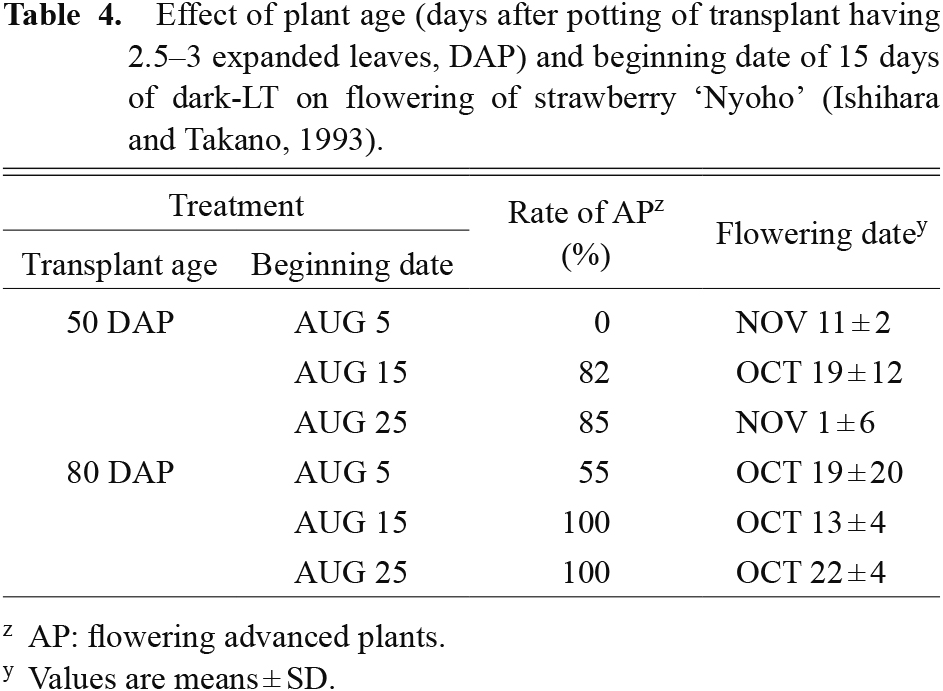
Effect of plant age (days after potting of transplant having 2.5–3 expanded leaves, DAP) and beginning date of 15 days of dark-LT on flowering of strawberry ‘Nyoho’ (Ishihara and Takano, 1993).

Effects of temperature and duration of continuous dark at low temperature on flowering of strawberry cultivars. All treatments were began on 21 July for 12 cm pot grown plants and transplanted just after the end of the treatments. Control plants were planted on 15 August (Shishido et al., 1990).
As described previously, tray plant propagation using hanging runner plants is becoming popular. These plants are usually small and easy to handle, but they are less sensitive to flower-inducing stimuli compared to conventional pot-grown plants (Kondo and Matsuzaki, 2011). Recently, ILTS was proposed as a new low-cost and useful flower-inducing procedure (Sano et al., 2013; Yoshida et al., 2012) in which tray plants were alternately placed in a refrigerator (15°C, in the dark) and outdoor ambient conditions for three to four days each, with the cycle repeated two or three times (Kinjo et al., 2018; Yamazaki et al., 2016; Yano et al., 2015).
Intermittently cold-stored plants flowered significantly earlier than untreated controls by six to 10 days and more than the continuously treated plants by four to 15 days (Fig. 5). The three to four days under outdoor conditions imposed in the LT storage induced uniform flower initiation by improving carbohydrate nutrition in strawberries. Such an improvement in combination with the accumulated stimulus of LT can overcome the partly negative or neutral effect of ambient high temperature and critical day length for flower induction. When the natural temperature and day length are not strictly inhibitive to flower initiation, the repeated combination of a few days of inductive low temperature in the dark and the same duration of non-inhibitive natural conditions may effectively induce floral transition and subsequent differentiation of flower buds in June-bearing strawberries (Yoshida et al., 2012). The efficient use of a refrigerator could be achieved by alternately subjecting two groups of plants to the same LT duration in dark and outdoor conditions.

Effect of intermittent low temperature storage (ILTS, 4D-LT/4D-ambient/4D-LT, from 31 August) on flowering of tray grown strawberry ‘Nyoho’ as compared with 12D of continuous dark-LT (from 31 August) and non-treated control. Plants were transplanted on 13 September, 2008 on to peat bags (Yoshida et al., 2012).
The latent heat of vaporization has been used to induce advanced floral initiation by decreasing the medium temperature using water permeable pots made of paper mold, unwoven fabric, or meshed plastics (Araki et al., 2005; Ikari, 2010). However, the inductive effect on floral initiation depends on the moisture content of the medium. The water content of the medium must be controlled by the sub-soil or frequent overhead irrigation, and a well-ventilated condition is also necessary (Azuma and Araki, 2006).
Shading in July to August can reduce the temperature during the daytime and induce advanced floral initiation (Kano and Kato, 1997). In ILTS, however, excessive shading or rainy weather in the ambient environment (not cold-stored period) have been shown to reduce the inductive effect of the treatment (Inazumi et al., 2013).
LD has been shown to be an intensively repressing factor of floral initiation under moderate temperature conditions (Ito and Saito, 1962). Although SD treatment has been recognized as an important flower-inducing method, it is not effective during the hot summer season without some type of LT treatment. Yamasaki et al. (2005), however, demonstrated that SD treatment in June and July enabled September flowering and resulted in a substantial yield in October in a northern area where cooler temperatures prevail in the summer. Six weeks of SD treatment from 10 June was applied to propagating runner plants that were connected to the mother plant, just fixed on plastic pots, and beginning to root. The young transplants that were cut and planted in a greenhouse just after the end of SD treatment were similarly, or often more, productive than well established and old transplants at the beginning of SD.
The advanced flowering of transplants has been practiced as described above; however, the differentiation of subsequent inflorescences on axillary shoots is often considerably delayed (Fig. 5). Direct and partial temperature control of crown tissue, including the apical meristem, can be an effective measure against the delayed flowering of axillary inflorescences (Sone et al., 2007). Plastic tubes in which cooled or underground water is running are set to contact with crown tissue to maintain a tissue temperature of about 20°C. Such a device can also be applied for heating during the winter season.
Several breeding programs are being used in Japan and more than 10 new genotypes of Fragaria have been released each year that are available for registration by public and private organizations. Each new genotype should be superior to the others in one or more aspects; however, only a few of the new genotypes are popular. Genetic improvements in several important characteristics is in progress and early new cultivars such as ‘Akihime’, ‘Sagahonoka’, and ‘Kaorino’ have been released and achieved a considerable market share. However, not so early or sometimes old cultivars also maintain significant market share. Further technological and genetic improvements to control flowering are still important challenges in strawberry research.