2020 Volume 89 Issue 3 Pages 328-336
2020 Volume 89 Issue 3 Pages 328-336
We aimed to assess the effect of longkong peel extract (LPE)-silver particle-alginate coating on the postharvest decay and browning of longkong fruit during storage at 13°C and 90–95% relative humidity (RH). Longkong was coated with 0, 0.45 or 0.90 mg·L−1 LPE in silver particle-alginate solution and then stored at 13°C and 90–95% RH for nine days. In the mixture consisting of the longkong peel extract and silver solution, the silver particle size was modified to yield fine particles. Electron micrography showed the presence of silver particle sizes ranging in size from approximately 71–625 nm. Fruit coated without LPE exhibited severe browning, weight loss and decay incidence during storage. Coating with 0.45 and 0.90 mg·L−1 LPE significantly inhibited the increase in fruit browning by mainly reducing peroxidase (POD) and polyphenol oxidase (PPO) activities. LPE coating effectively reduced the longkong weight loss and decay incidence. No fruit treatment showed a significant titratable acidity change or increased soluble solids at the end of storage. These results indicate that coating with LPE is a promising approach to inhibit decay and browning and to maintain the quality of longkong during low-temperature storage.
Longkong (Aglaia dookkoo Griff.), a member of the Meliaceae family, has recently become an economically valuable fruit for Thailand in export markets. The fruit originates from southern Thailand, and major longkong cultivation now currently occurs in the eastern regions. Longkong is a non-climacteric tropical fruit, with a high fresh market value. However, the fruit rapidly loses postharvest quality due principally to peel (pericarp) browning and postharvest disease infection, resulting in price reduction. The fruit is susceptible to decay caused by fungal and bacterial infections during storage (Venkatachalam, 2016). The most common types of fruit diseases include sooty mold (Meliola spp.) and fruit rot (Cylindrocladium spp.) (Suanphairoch et al., 2003). The biological browning process in plants has been reported to be mainly associated with the oxidizing action of PPO on polyphenols to produce quinones that are later responsible for polymerization of the developed colour (Chang, 2009; Constabel and Barbehenn, 2008; Queiroz et al., 2008). Although it is difficult to maintain the appearance of the fruit at room temperature without proper postharvest conditions, numerous methods can prevent oxidation, such as chemicals, controlled atmosphere environments and coating treatments. Fruit coatings have been extensively studied to extend the shelf life of many fresh fruits. In the present study, modified coating materials were used to maintain the postharvest quality of longkong.
In addition to the prevention of weight loss and control of gaseous permeability, fruit coatings are generally used as antimicrobials to reduce or inhibit the growth of microorganisms. Edible coatings on foods can prevent moisture loss, water vapor transfer between components in a heterogeneous food system, ice formation in frozen foods, and gaseous permeability of gases such as oxygen and carbon dioxide (Baldwin, 2007; Cutter and Summer, 2002; Ustunol, 2009). Coatings are not only used to coat products from animals or processed foods (Coma, 2008; Ustunol, 2009) but also fruits, vegetables, and minimally processed or fresh-cut produce (Rojas-Graü et al., 2009). Alginate is a natural polysaccharide extracted from brown seaweed belonging to the family Phaeophyceae (Valero et al., 2013). The colloidal properties of alginate enable it to thicken and stabilize insoluble polymers in a reaction with multivalent metal cations such as calcium (Montero-Calderón et al., 2008). The application of an alginate coating on whole fruits has maintained the quality of guavas (Nair et al., 2018), enhanced the shelf life of mandarin (Chen et al., 2016) and okra (Gundewadi et al., 2018) and delayed weight and acidity loss, softening and colour changes in plum cultivars (Valero et al., 2013).
As for antimicrobial properties, additional elements are mixed with the coating materials. In addition to using natural preservatives, noble-metal nanomaterials such as silver nanoparticles have received positive attention because of their desirable physicochemical properties. Silver (Ag2+), probably in different ionic forms, is extremely effective at damaging a vast range of microorganisms (Liau et al., 1997). Silver particles can be synthesized by various techniques, including physical, chemical, and biological methods (Nair and Laurencin, 2007; Sharma et al., 2009; Zhang et al., 2008). Over the past several years, there has been increasing interest in the applications of silver particles because of their antimicrobial activities (Choi et al., 2008), and they have been proposed as potential treatment agents (Rai et al., 2009). Kumar et al. (2015) developed a practical method to synthesize silver plates using peel rambutan extract that functioned as a stabilizing and reducing agent. The main constituents of rambutan peel are anthocyanins and other phenolic compounds; the inclusion of rambutan peel is beneficial for coating reactions because of its natural antioxidants. Consequently, rambutan peel extract can be used as an alternative agent to develop technology-based treatments (Lim, 2013; Kumar et al., 2015). In the present study, we were interested in using longkong peel as an agricultural waste for silver-particle production. Longkong peel contains many phytochemicals, such as antioxidants, phenolic compounds, scopoletin, rutin, and chlorogenic acid (Klungsupya et al., 2015), which can be finely incorporated with silver particles. Because size reduction of suitable material sources is the main purpose of making the particles (Meyers et al., 2006), Kumar et al. (2015) demonstrated a reaction to produce silver particles by the addition of a natural plant extract. Ag2+ particles were highly induced to silver particles in both trigonal and hexagonal forms by mixing rambutan peel extract. It is therefore reasonable to suppose that longkong peel extract (LPE) biomolecules could play a role as a mild reducing agent for Ag2+ ions and as a capping agent for Ag particles. Ag2+ ions can interact with O–H or CHO groups of the phytochemicals in the extract, subsequently leading to oxidation to the –C = O and −COOH forms and size reduction of Ag2+ to silver particles (Cao et al., 2008; Kumar et al., 2015).
No information is available regarding the use of longkong peel extract to improve silver particles in alginate coating solutions or use of a modified coating to maintain the quality of fresh produce. The objective of this work was to assess the postharvest quality, decay, and browning of longkong fruit coated by longkong peel extract-silver particle-alginate during storage at 13°C and 90–95% RH.
In the preparation of longkong peel extract (LPE) powder, fresh longkong peels (120 fruits) were collected, washed, and boiled in distilled water (DW) for 30 min at 90°C. The boiled peels (100 g) were crushed in 100 mL of DW. The peel homogenate was filtered through cheesecloth. The filtrate was then treated by using an equal volume of chilled acetone. The precipitate was collected after centrifuging the mixture at 1,000 rpm for 5 min. The pellet was air-dried to obtain an extract powder for further experimental use.
To prepare the alginate solution, 0.5% (w/v) sodium alginate powder in DW was heated to 70°C, and the solution was stirred until it became clear. Glycerol as a plasticizer at 1.5% (w/v) was added to the alginate solution. N-Acetylcysteine (1% w/v) and calcium chloride (2% w/v) were added to the solution to make crosslinks of the carbohydrate polymers. Sunflower oil at 0.025% (w/v) was then added to emulsify the film-forming solution. The mixture was homogenized for 3 min at 12,500 rpm using an Ultra Turrax T25 system (IKA Werke GMBH, Staufen, Germany) and then degassed under a vacuum. The formulation was chosen in accordance with the previous research of Rojas-Graü et al. (2006, 2007, 2008).
In the preparation of LPE-based silver particles, silver nitrate (AgNO3) was first dissolved in DW at a concentration of 1.0 M to produce an AgNO3 solution; LPE powder was dissolved in the AgNO3 solution (0, 1.0 and 2.0 mg·L−1) (Rojas-Graü et al., 2007). The LPE mixtures were incubated at 80°C in a water bath for 3 min at pH 3 to induce the formation of smaller particles. Different concentrations of the mixtures were then incorporated into a 0.5% alginate coating solution. The final applied silver particles with longkong peel extract and alginate were used at concentrations of 0, 0.45 and 0.90 mg·L−1 LPE in a silver particle-alginate solution for coating. Treatment without LPE (0 mg·L−1 LPE) represented the control treatment.
Longkong fruit was collected from a commercial orchard located in ‘Chanthaburi’ province, eastern Thailand. Individual longkong fruit were submerged in 200 ppm of sodium hypochloride solution for 15 min at room temperature and briefly air-dried. The treatments were as follows: (1) control treatment (0 mg·L−1 LPE), (2) 0.45 mg·L−1 LPE and (3) 0.90 mg·L−1 LPE. The longkong fruits (660 fruits) were divided into three groups (1, 2, and 3), and the coating was formed by immersing the fruit in the emulsion for 1 min per fruit. The treated samples were dried at room temperature (25°C, 60–70% RH) for 1 h and then immediately transferred to refrigerated storage at 13°C with 90–95% relative humidity for nine days.
Scanning electron microscopy (SEM)One drop of the reaction suspension was spread on a microfilter membrane and vacuum-dried at room temperature. The sample was placed on a stub, coated with a gold layer, and then observed under an SEM (SU-5000; Hitachi High-Technologies Corporation, Tokyo, Japan).
Decay incidenceThe postharvest decay of fruits was determined as a percentage. The incidence of decay was calculated for decayed fruits from 120 fruits in total in each treatment. Whole-fruit decay originating from quiescent infections was subjectively evaluated by the modified method of Fallik et al. (1993).
Weight lossLongkong fruit from each treatment were individually weighed. The percentage of fruit weight loss during storage was calculated using the following equation:
Peel (pericarp) browning was estimated as a browning score by measuring the extent of the total browning area on each fruit surface based on the following scores: 1 = no browning, 2 = < 20% of the peel surface, 3 = 20–40% of the peel surface, and 4 = 40–60% of the peel surface and 5 ≥ 60% of the peel surface. The non-marketability of longkong fruit was indicated by a peel browning score ≥ 4.
Total phenolic contentThe total phenolic content was quantified using the method proposed by Singleton et al. (1999). Extractions were prepared from the centre of the longkong peel. Two grams of longkong peel were homogenized with 20 mL of 80% ethanol for 1 min. The extract was filtered and centrifuged at 10,000 rpm for 15 min. The supernatant (1 mL) was mixed with 1 mL of Folin–Ciocalteu reagent (Sigma Aldrich, Buchs, Switzerland) and 10 mL of 7% sodium carbonate. The final volume was adjusted to 25 mL by DW, and the sample was incubated at the room temperature for 1 h. The absorbance was read at 760 nm by using a spectrophotometer (UV-1601; Shimadzu Corporation, Kyoto, Japan), and the units were expressed as milligrams of gallic acid equivalents per gram fresh weight of longkong peel.
Extraction and assays of PPO and POD activitiesThe pericarp tissues (2 g) from 20 fruits were homogenized in 20 mL of 0.05 M phosphate buffer (pH 7) and 0.2 g of polyvinyl pyrrolidone (insoluble) at 4°C. The extract was collected by filtration through cheesecloth and then centrifuged for 20 min at 12,500 rpm at 4°C. The supernatant was collected as the crude enzyme extract.
PPO activity was assayed by measuring the substrate oxidation of 4-methylcatechol at 410 nm using a spectrophotometer (UV-1601; Shimadzu Corporation) according to the method of Jiang (2000). One unit of PPO activity was defined as a change of 0.001 in the absorbance per minute.
POD activity was assayed according to the method of Zhang et al. (2005). The 3-mL reaction mixture included 20 μL of enzyme extract, 2.78 mL of 0.05 M phosphate buffer (pH 7.0), 0.1 mL of 20 mM H2O2 and 0.1 mL of 20 mM guaiacol. An increase in absorbance at 470 nm was recorded for 2 min. One unit of enzyme activity was defined as the amount of enzyme that caused a change of 0.01 in the absorbance per minute.
The protein contents were determined from crude enzyme extracts by using the Bradford method (Bradford, 1976) with bovine serum albumin as a standard.
Total titratable acidityTotal titratable acidity (TA) was measured as follows: 1 mL of extracted juice was diluted with 9 mL of DW, and 0.1 M NaOH was added by using an automatic titrator to a final pH of 8.1 was reached (AUT-501; DKK-TOA Corporation, Tokyo, Japan). Total titratable acidity was expressed as a percentage of citric acid. All of the measurements were carried out according to AOAC procedures (Horwitz, 2000; Nunes et al., 2009).
Total soluble solid contentTotal soluble solids (TSSs) from fruit juice (the aril part) were measured by using a digital refractometer (PAL-1; ATAGO CO., LTD., Tokyo, Japan). The TSS results were expressed as percentages.
Statistical analysisAll of the experiments were performed using a completely randomized design. Three replications were carried out to examine the analytical parameters of decay incidence, browning assessment, weight loss, titratable acidity and soluble solid contents. To determine the total phenol compounds and activities of PPO and POD, three replicates were used. The treatment means were separated using the least significant difference method. The significance level was set as P < 0.05. The data are presented as the means ± standard error (SE).
Figure 1 shows that LPE-silver particles formed in the suspension (A: 0 mg of LPE, B: 1 mg of LPE; and C, D: 2 mg of LPE). These micrographs demonstrated the presence of fine particles of silver with a mean size ranging from 71–254 nm (Fig. 1A). In Figure 1B, it was revealed that at 1 mg of LPE, the silver solution cyclically formed silver particles with an average particle size of approximately 448–625 nm. Similarly, Babu and Prabu (2011) synthesized 150- to 1000-nm silver particles using a flower extract of Calotropisprocera. Figure 1D shows silver (130 nm) in the centre of LPE and distributed in solution. The particles were approximately 1.58 μm in size. The silver ions could be changed to particles using the peel extract of longkong (Aglaia dookkoo Griff.).
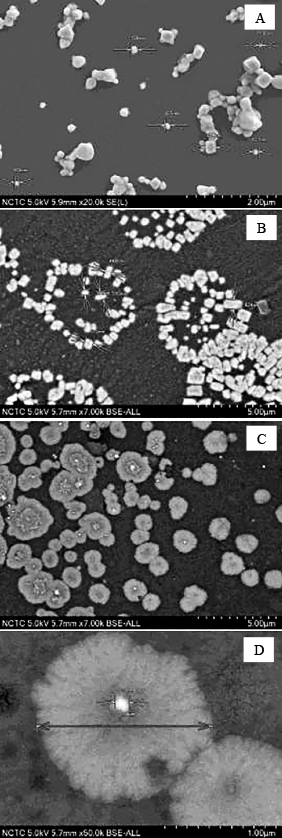
Scanning electron micrographs of silver particles synthesized with LPE powder at 0 mg (A), 1 mg (B), and 2 mg (C and D). Silver nitrate (1.0 mM) was incubated at 80°C for 3 min at pH 3.0 (magnification, 19,000 ×).
Longkong fruit coated with 0.90 mg·L−1 LPE in silver particle-alginate displayed different effects in terms of the reduction in decay incidence (Fig. 2A). At day 6, control fruits showed a 13% decay incidence, while fruits treated with 0.45 and 0.90 mg·L−1 LPE showed decay incidences of approximately 6% and 0%, respectively. Following storage, all longkong fruits coated with 0.45 and 0.90 mg·L−1of LPE showed a lower decay incidence (0–10%) than 20% of control fruits coated without LPE. Farunsang et al. (2017) indicated that Colletotrichum spp. and Lasiodiplodia sp. were significant pathogens that caused the postharvest fruit rot of longkong. The peel of longkong fruit exhibited higher O2-bullet and OH-bullet scavenging activities. Phytochemical analysis of longkong peel showed the presence of various phenolic substances, mainly scopoletin, rutin, and chlorogenic acid. As reported previously, phenolic compounds from mango peel extract, such as trans-cinnamic acid, inhibit the mycelial growth of Colletotrichum falcatum and Phytophthora capsici (Kim et al., 2012). Nychas et al. (2003) reported the effect of bioactive plant compounds on inhibiting the growth of bacterial cells on minimally processed foods, such as degradation of the cell wall, damage to cytoplasmic membrane proteins of Staphylococcus aureus and Pseudomonas aeruginosa (Lambert et al., 2001), and leakage and coagulation of the cytoplasmic contents of Candida spp. (Burt, 2004). In fact, silver particles can attach to cell membranes and penetrate bacteria, while silver ions can disrupt major biological processes, leading to cell death. The particles release silver ions (Ag2+) within bacterial cells, enhancing the bactericidal activity of the silver particles (Feng et al., 2000; Rai et al., 2009). Meanwhile, alginate could reduce moisture migration, gaseous exchange, and oxidative reactions and suppress pathogen growth and physiological disorders (Gundewadi et al., 2018; Salvia-Trujillo et al., 2015; Sessa et al., 2015).
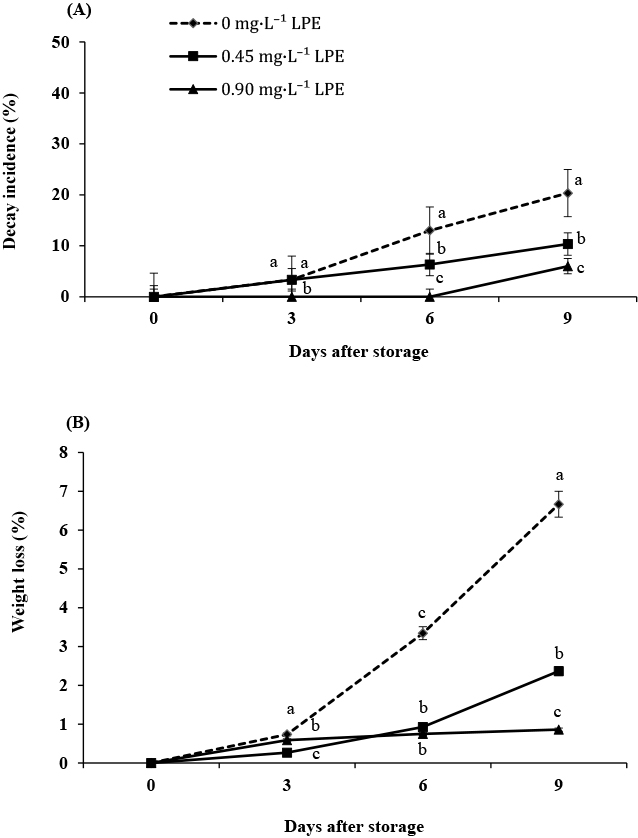
Decay incidence (A) and weight loss (B) of coated longkong treated with solutions of 0 (control), 0.45 and 0.90 mg·L−1 LPE during storage for nine days at 13°C and 90–95% RH. Different letters indicate significant differences among the samples after storage at different LPEs at P < 0.05 by Duncan’s multiple-range test. The values in the figure are expressed as means ± SE of three replicates.
Weight loss suggests fruit dehydration caused by transpiration and involves water transfer from cells to the surrounding atmosphere; this enables the evaluation of coating efficiency for quality preservation (Pérez-Gago et al., 2010). In this study, an alginate coating was used to form a film, acting as a gas exchange barrier that did not modify water transport. LPE was introduced into the coating to create a lipophilic environment capable of acting as a barrier against water, as demonstrated in the samples with 0.45 and 0.90 mg·L−1 LPE. The results showed less weight loss in all of the LPE-coated fruits than in the control fruits during storage (Fig. 2B).
The browning of the longkong peel increased gradually over the nine days of storage with all treatments (Fig. 3A). The browning of the control fruits was greater than that of fruits coated with silver particle-alginate containing 0.45 and 0.90 mg·L−1 LPE after nine days of storage. The phenolic content (tannic acid), as a strong cross-linker, reduced the water vapor permeability and caused thicker film formation (Menezes et al., 2019). Longkong coated with silver particle-alginate containing LPE at 0.45 and 0.90 mg·L−1 showed delayed longkong browning after six days of storage. This delay occurred because of increased cross linking in the presence of LPE, leading to an improvement in the coating properties. Figure 1A shows the presence of small silver particles without LPE that increased with the addition of LPE at 0.45 and 0.90 mg·L−1 compared with the other particle groups (Fig. 1B, C, respectively). The browning score of longkong coated with silver particle-alginate plus LPE at 0.45 mg·L−1 was 3, which was significantly lower than that of the control on the ninth day of storage. The better result obtained with thicker coatings in the presence of LPE may be due to the coating acting as a barrier against oxygen, which is important for the progression of browning reactions.
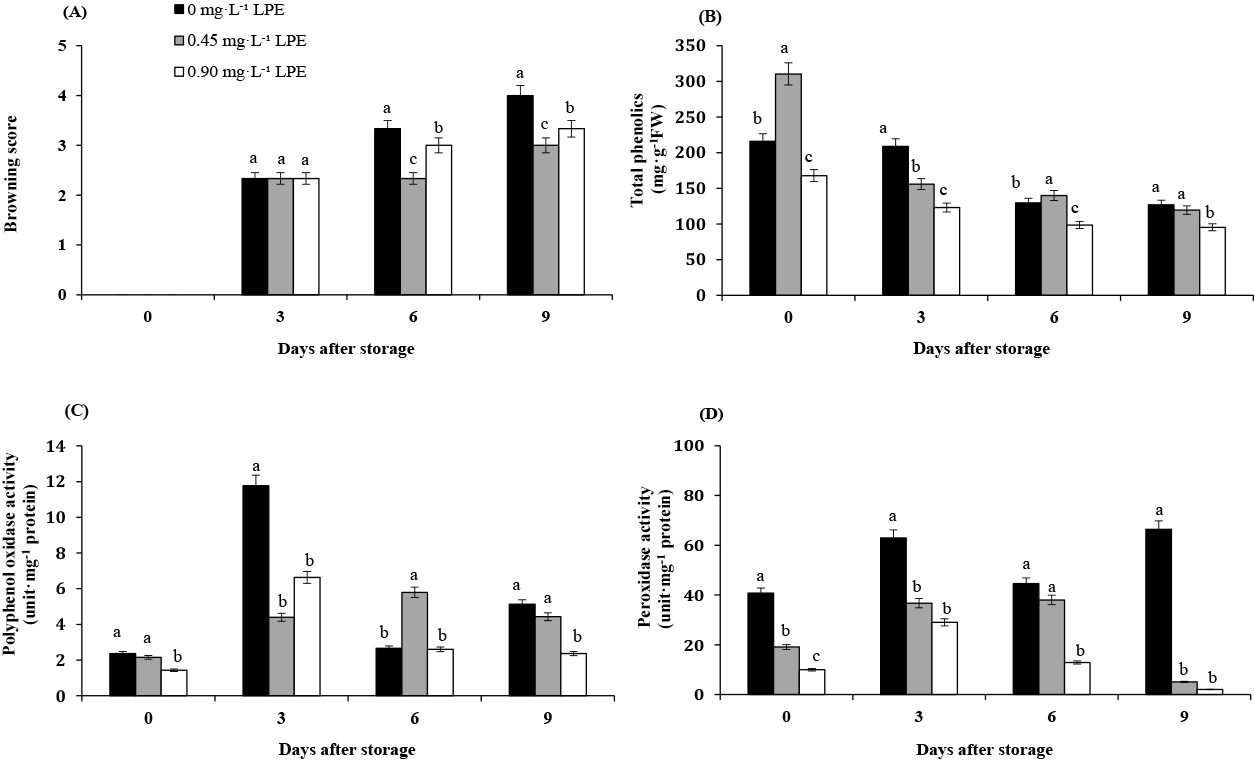
Browning score (A), total phenolic compounds (B), polyphenol oxidase activity (C), and peroxidase activity (D) of coated longkong treated with solutions of 0 (control), 0.45 and 0.90 mg·L−1 LPE during storage for nine days at 13°C and 90–95% RH. The dashed line represents the non-marketability level of longkong fruit. Different letters indicate significant differences among the samples after storage at different LPE concentrations at P < 0.05 by Duncan’s multiple-range test. The values in the figure are expressed as means ± SE of three replicates.
The phenolic content in the peels of all treatments decreased during the storage period (Fig. 3B). Browning is mainly caused by the oxidation of phenolic compounds to o-quinones; this reaction is catalysed by oxidative enzymes, including PPO and POD (Garcia and Barrett, 2002). Assuming a reduction in phenolic compounds, treatments that inhibit the reactions in which oxidative enzymes are involved (i.e., PPO and POD) may accumulate higher levels of phenols (Capotorto et al., 2018). The initial total phenolic content of the longkong fruit coated with silver particle-alginate containing 0.45 mg·L−1 LPE was significantly higher than that of control fruits and longkong fruit treated with silver particle-alginate containing 0.90 mg·L−1 LPE. The total phenolic content of longkong was significantly increased with the continuous increase in the weeks of the maturation period from 13 weeks (42.65 mg/100 g) to 16 weeks (58.71 mg/100 g) (Venkatachalam and Meenune, 2012). Lichanporn et al. (2009) reported that the total phenolic content in all parts (lower, middle, and top) of the fruit was significantly increased during storage, while the total phenolic content of the lower part accumulated faster than that in the middle or top parts. The higher level of phenolic content in longkong fruit is also a reason for its enzymatic browning. The postharvest browning of fruits and vegetables is related to the cellular synthesis of phenolic compounds, which are oxidized into quinines and polymerized into brown polymers (Martinez and Whitaker, 1995). As a key enzyme in the early steps of phenolic biosynthesis, PAL plays an important role in the production and accumulation of phenols in fruits and vegetables (Ke and Saltveit, 1989) and is genetically induced by various stress conditions (Dixon and Paiva, 1995). As shown in Figure 3B, the initial total phenolic content of longkong fruit coated with silver particle-alginate containing 0.90 mg·L−1 LPE was considerably lower than that in the control (P < 0.05); after nine days of storage, the fruits coated with 0.90 mg·L−1 of LPE had the lowest total phenolic content (95.35 mg·g−1 FW). As a potential coating material to delay the ripening process, the effect of alginate is enhanced upon the incorporation of any antioxidant or antimicrobial agent (Nair et al., 2018). Although LPE is a rich source of phenols, the inhibitory role of phenolic compounds, particularly cinnamic acid, against PPO is due to its homologue structure with endogenous phenolic substrates (Shi et al., 2005).
The PPO activity was highest in all treatments on day 3, except for treatment with 0.45 mg·L−1 LPE. The PPO activity in the treatments with 0.45 and 0.90 mg·L−1 LPE was lower than that in the control samples (Fig. 3C). The latent form of PPO is often activated during ripening, senescence or stress conditions when the membrane permeability is dysfunctional, resulting in increased PPO activity (Mayer, 1987). Although the PPO activity in longkong fruit increased during the first three days of storage, at the end of storage the PPO activities in the treatments with 0.45 and 0.90 mg·L−1 LPE were lower than those of the control at 4.42 and 2.37 unit·mg−1 protein, respectively. POD activity increased rapidly in the treatments with control fruit in the first three days, followed by a rapid decrease (Fig. 3D). Generally, enzymatic browning in fruits and vegetables is associated with a reaction between polyphenol substrates and oxygen with PPO and POD (Sapers and Hicks, 1989). Although PPO can catalyse the hydroxylation of monophenols to o-diphenols and the oxidation of o-diphenols to their corresponding o-quinones (Richard and Gauillard, 1997), diphenols may function as reducing substrates in this reaction (Chisari et al., 2007). The activity of POD, another oxidoreductase enzyme involved in enzymatic browning, in the treatments with 0.45 and 0.90 mg·L−1 LPE increased progressively during the first three days of storage. However, the POD of fruit coated with silver particle-alginate containing all concentrations of LPE was significantly lower than that of the control samples after nine days of storage. The concentration in the treatment with 0.90 mg·L−1 LPE was the lowest at 2.05 units·mg−1 protein (Fig. 3D). However, the PPO and POD activities increased rapidly in all treatments in the first three days. These results may have been caused by the increased longkong pericarp PPO, POD, and PAL enzyme activities during on-tree maturation, inducing peel redness during ripening. Furthermore, the longkong exocarp (outer peel) contains many trichomes that could be susceptible to environmental stresses, and this exocarp acts as a potent stimulator of PPO and POD activities (Venkatachalam and Meenune, 2012). However, the POD and PPO activities of fruit coated with silver particle-alginate containing 0.45 and 0.90 mg·L−1 LPE were lower than those of the control samples because LPE exogenous phenolics possess antioxidant activity by capturing free radicals produced during oxidative stress (Peretto et al., 2017). Moreover, LPE can promote auto-oxidation and metal chelation and modulated the activity of some enzymes (Howard et al., 2003).
Figure 3A–D show the effect of LPE on the browning score, phenolic content, and PPO and POD activities. All of the treatments with no browning at zero time correlated better with the PPO activity. The control sample showed increased PPO activity on day 3, and the phenolic content was reduced, indicating that enzymatic browning of fruit is the key property of phenolic oxidation by PPO (Jiang et al., 2004). Additionally, peroxidase reacts with phenolic compounds using H2O2 as a co-substrate (Nicolas et al., 1994), and the POD activity of control fruit showed high activity during storage. The increase in POD activity was involved in stress conditions such as membrane damage (Fig. 3A), weight loss (Fig. 2A) and disease (Fig. 2B). The longkong fruit coating with silver particle-alginate containing 0.45 mg·L−1 LPE showed increased PPO and POD activities on the 3rd and 6th days and then decreased PPO and POD activities until the end of storage that were related to the browning score, but these were lower than those of control fruit. These differences may be due to the fact that the coating acted as a semi-permeable barrier against oxygen, water loss and oxidation reaction rates (Arowora et al., 2013), and fruit coated with silver particle-alginate containing LPE promoted restricted gas exchange on the surface of longkong.
The total titratable acidity of all fruits increased within six days and then decreased slowly until day 9 (Fig. 4A). The coating treatment with 0.90 mg·L−1 LPE showed lower titratable acidity than any other treatments after three days of storage. The decrease in acidity after six days of storage may have been due to the rapid utilization of acids in respiratory pathways following maturity, demonstrating that the decrease depends on many factors, including the LPE concentration on the coating’s surface (Jain et al., 2003; Mercado-Silva et al., 1998; Singh and Pal, 2008). The total soluble solid content of chemically-treated fruits changed slightly during the nine days of storage (Fig. 4B). Application of an alginate coating was the most effective to stop the conversion of acids present in the fruit into sugars (Bassetto et al., 2005) because they created a new environment on the surface of longkong fruit.
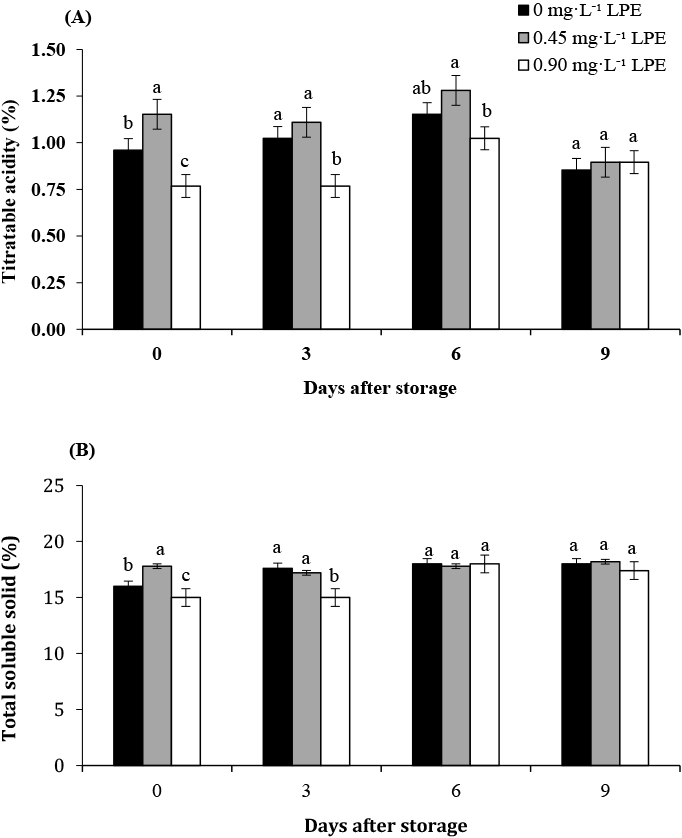
Titratable acidity (A) and total soluble solid (B) of coated longkong treated with solutions of 0 (control), 0.45 and 0.90 mg·L−1 LPE during storage for nine days at 13°C and 90–95% RH. Different letters indicate significant differences among the samples after storage at different LPE concentrations at P < 0.05 by Duncan’s multiple-range test. The values in the figure are expressed as the means ± SE of three replicates.
In conclusion, longkong fruit coated with a silver particle-alginate containing 0.45 and 0.90 mg·L−1 LPE demonstrated delayed browning and decreased weight loss, decay incidence and POD and PPO activities during storage. Additionally, longkong fruit coated with silver particle-alginate containing 0.45 and 0.90 mg·L−1 LPE showed a higher total phenolic content than the control fruits. The fruits coated with silver particle-alginate alone, and those containing 0.45 mg·L−1 LPE, showed no differences in titratable acidity on the 3rd and 6th days of storage, while longkong fruit treated with 0.90 mg·L−1 LPE had a lower titratable acidity than any other treatments after three days of storage. The total soluble solid content of longkong fruit for all of the treatments changed slightly during the nine days of storage. Further research is warranted to determine the functional significance of key sugar accumulation-related genes using specific primers based on the genome sequences of longkong.