2020 Volume 89 Issue 4 Pages 343-350
2020 Volume 89 Issue 4 Pages 343-350
Haskap (Lonicera caerulea subsp. edulis) is a deciduous shrub that produces blue-black edible berries with a sour-sweet taste. By expanding fruit color variation, the value of agricultural products is enhanced. Interspecific hybrids were obtained from crossings between Haskap and red-fruit bearing Miyama-uguisukagura (Lonicera gracilipes). The fruit color of the interspecific hybrids obtained was red-purple. Fruit color in Haskap is mainly affected by the concentration of anthocyanin. However, there are no reports on the chemical determinants of fruit color in interspecific hybrids between Haskap and Miyama-uguisukagura. We evaluated anthocyanin components in these hybrids and their parents using liquid chromatography/tandem mass spectrometry, and revealed the presence of five different kinds of anthocyanins. The major anthocyanin in interspecific hybrids and Haskap was cyanidin 3-glucoside, while in Miyama-uguisukagura, it was cyanidin 3,5-diglucoside. Some genotypes among interspecific hybrids showed higher concentrations of cyanidin 3,5-diglucoside and peonidin 3,5-diglucoside, compared with their parents. The genotypes of interspecific hybrids and the parents were evaluated by principal component analysis of anthocyanin concentration. Our study contributes to the identification of anthocyanin composition in fruits of interspecific hybrids and in expanding fruit color variation when breeding new varieties.
Lonicera caerulea subsp. edulis, known as blue honeysuckle or sweet berry, belongs to the Caprifoliaceae family. Its fruits are widely harvested in Russia and North Eastern Asia, where they are eaten fresh or after processing, e.g., in the form of juices, jams, and sauces. In Japan, the plant is called “Haskap” in the Ainu language used by the indigenous Ainu people of Hokkaido. Haskap fruits have a blue-black color, and they contain saccharides, lipids, proteins, organic acids, and polyphenols as major components (Svarcova et al., 2007; Caprioli et al., 2016), with a sour to sweet taste.
Fruit color variation can enhance the value of agricultural products, such as in kiwifruit (yellow to green), grapevine (green to dark purple), and raspberry (yellow to red), among others. Haskap fruits only have a blue-black color and show no color variation. In Haskap fruits, color is known to be determined mainly by anthocyanins, with cyanidin 3-glucoside (Cy3G) being the most abundant (Terahara et al., 1993; Chaovanalikit et al., 2004; Jordheim et al., 2007; Svarcova et al., 2007; Wojdyło et al., 2013; Celli et al., 2015; Zhao et al., 2015; Wang et al., 2016). We hypothesized that changing the anthocyanin composition may expand the color variation of Haskap fruits.
Interspecific hybridization has been reported to change the chemical composition in some genera. Therefore, in Rubus spp., boysenberry and loganberry, which are interspecific hybrids between raspberry and blackberry, different anthocyanin compositions were identified compared with their parents (Torre and Barritt, 1977). Similarly, anthocyanin contents changed in interspecific hybrids of Prunus spp. as a result of hybridization (Schuster et al., 2013).
Interspecific Lonicera hybrids between Haskap and Uguisukagura (L. gracilipes) were produced recently (Miyashita and Hoshino, 2010). Further, interspecific hybridization between Haskap and Miyama-uguisukagura, a variety of Uguisukagura (L. gracilipes), and a species closely related to Haskap, was performed to improve the taste using the same procedure as that used while seeking to identify functional compounds. Ovule culture was used to produce these interspecific hybrids after artificial pollination between Miyama-uguisukagura as the female parent and Haskap as the male parent. We used a tetraploid for both parents. Therefore, amphidiploid (allotetraploid) hybrids retained fertility and successfully produced fruits. Miyama-uguisukagura and Haskap bear red and blue-black colored fruits, respectively; however, the fruits of all interspecific hybrid strains were red-purple.
In previous studies, anthocyanins have been qualified and quantitated by liquid chromatography/tandem mass spectrometry, LC/MS/MS (Mullen et al., 2002; Tian et al., 2006; Myjavcová et al., 2010; Vayupharp and Laksanalamai, 2015). By using LC/MS/MS, we could identify and compare the amount of anthocyanins in interspecific hybrids between Haskap and Miyama-uguisukagura to the amounts found in their parents. In the present study, we evaluated anthocyanin composition and concentration in fruits of Miyama-uguisukagura, Haskap, and interspecific hybrids from crosses between these two species, focusing on the variation in these compounds among the different genotypes obtained.
One Miyama-uguisukagura (Lonicera gracilipes) (2n = 4x = 36) (accession name: Lg1) genotype, three Haskap (L. caerulea subsp. edulis) (2n = 4x = 36) (accession names: Lc23, Lc27, and Lc37) genotypes, and four genotypes among interspecific hybrids from crosses between Miyama-uguisukagura and Haskap (accession names: M23-1, M27-1, M27-4, and M37-2) were used in this study. Miyama-uguisukagura was provided by Botanic Garden, Hokkaido University, Japan. Three Haskap genotypes were collected from Yufutsu mire in Hokkaido, Japan, and maintained at Experiment Farms in Hokkaido University, Japan. To produce interspecific hybrids, Lg1 (female parent) was pollinated with pollen from Haskap (Lc23, Lc27, and Lc37), and an ovule culture was used to obtain hybrid seedlings. Hybrid accessions, M23-1, M27-1, M27-4, and M37-2, were derived from male parents, Lc23, Lc27, Lc27, and Lc37, respectively.
All plants were grown at the Experimental Farms in Hokkaido University, Japan. The plants were cultivated in an open-air field with conventional management. Mature fruits of each genotype (accessions) were harvested in June and July in 2016. The fruits of each strain were harvested from one plant. Harvested fruits were immediately stored at −80°C until they were processed.
ChemicalsThe standard chemicals, cyanidin 3-glucoside (Cy3G) (Tokiwa Phytochemical Co., Ltd, Sakura, Japan), cyanidin 3,5-diglucoside (Cy35diG) (Extrasynthese S. A., Genay, France), cyanidin 3-rutinoside (Cy3R) (Extrasynthese), peonidin 3-glucoside (Pn3G) (Sigma-Aldrich Co. LLC, St. Louis, MO, USA), peonidin 3,5-diglucoside (Pn35diG) (Extrasynthese), and pelargonidin 3-glucoside (Pg3G) (Sigma-Aldrich) were used.
Characterization of interspecific hybrids in comparison with their parentsFruit color was determined using the Royal Horticultural Society (RHS) Colour Chart (2001 edition) by referring the images in Figure 1. To characterize interspecific hybrids in comparison with their parents, the individual fresh weight of 30 fruits was measured. Soluble solids content (SSC) and pH of the fruit juice were measured using a digital refractometer (Atago PAL-1; ATAGO CO., LTD., Tokyo, Japan) and a digital pH meter (LAQUAtwin; HORIBA, Ltd., Kyoto, Japan), respectively. Fruit juice was extracted from two fruits for one measurement. In all, 30 fruits were used for 15 measurements. Pollen germination frequency was evaluated by the procedures of Hirano and Hoshino (2009) and Hoshino et al. (2016), 6 h after pollen culture initiation. Pollen germination tests were conducted in triplicate.
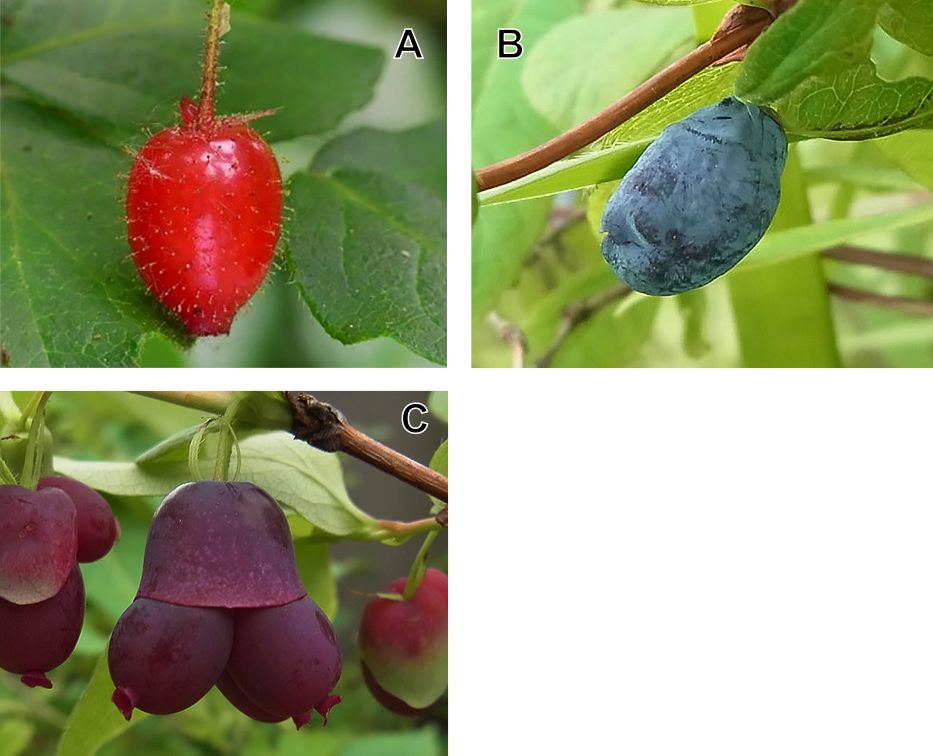
Fruits of Miyama-uguisukagura, Haskap, and an interspecific hybrid between them. (A) A Miyama-uguisukagura fruit, (B) a Haskap fruit, (C) an interspecific hybrid fruit between Miyama-uguisukagura and Haskap.
Samples were freeze-dried (FDU-2200; Tokyo Rikakikai Co., Ltd, Tokyo, Japan) and then powdered in a commercial blender (Milser IFM-700; Iwatani Corporation, Tokyo, Japan). Powdered samples (20 mg each) were sonicated with 2 mL of 5% acetone for 15 min at room temperature. One-milliliter aliquots of the extracted solutions were passed through 0.2-μm membrane filters (DISMIC-25cs Advantec; Toyo Roshi Kaisha, Ltd.). Filtered extracts were diluted five times with 5% acetone prior to analysis by LC/MS/MS.
LC/MS/MS analysisLC/MS/MS was performed with a Dionex Ultimate 3000 ultra-high-performance liquid chromatography system (Thermo Scientific, San Jose, CA, USA) coupled with a TSQ Quantum Access Max triple stage quadrupole mass spectrometer (Thermo Scientific).
LC was conducted on an Inertsil ODS-3 250 × 4.6 mm I.D. column (GL Science Inc. Tokyo, Japan). The mobile phase consisted of 0.1% formic acid aqueous solution (A) with 0.1% formic acid in 50% acetone aqueous solution (B) at a flow rate of 0.4 mL/min. Separation was performed with the column held at 35°C according to the gradient program: 0 min, 90% A; 0–20 min, 10%–50% B; 20–25 min, 50–100% B; 25–40 min, 100% B. The injection volume was 10 μL.
MS detection was performed using a total ion current chromatogram (TICC) and selected reaction monitoring (SRM) in the positive-ion mode. The parameters and conditions were as follows: spray voltage, 3.0 kV; vaporizer temperature, 375°C; sheath gas pressure, 50 psi; aux gas pressure, 15 psi; capillary temperature, 220°C; tube lens offset, 105 V; skimmer offset, −5 V; collision pressure, 1.0 mTorr. Collision energy and the lens were optimized by the compounds (Table 1).
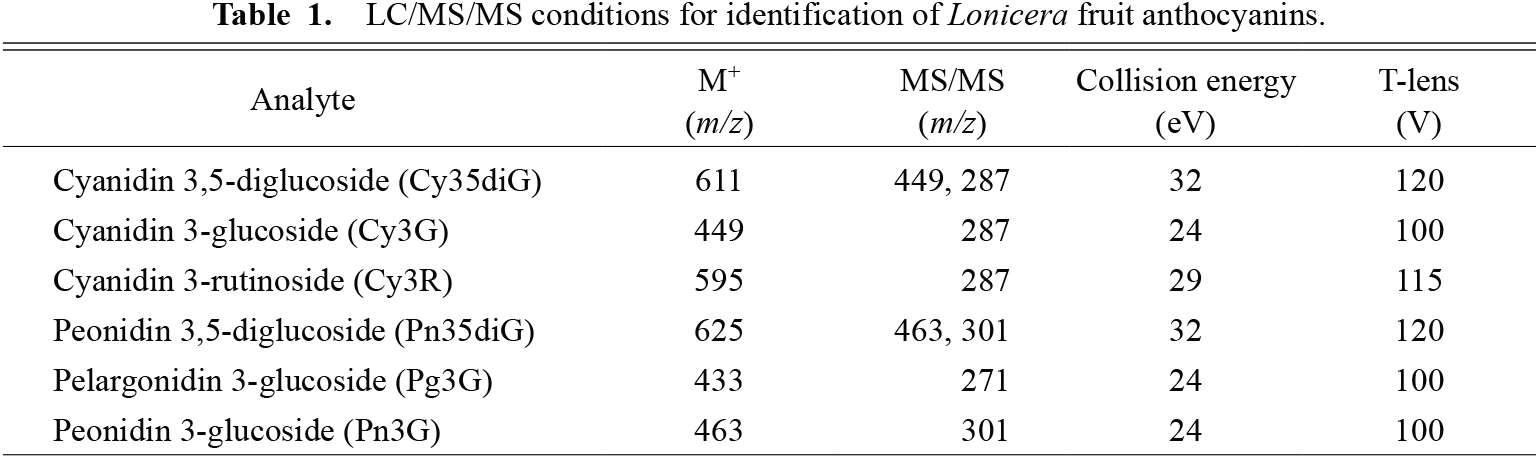
LC/MS/MS conditions for identification of Lonicera fruit anthocyanins.
LC/MS/MS analysis was conducted in triplicate for each strain. Compound identity was confirmed by retention times and mass spectra. Anthocyanin concentration was calculated by comparison with commercial standards. Spectral data were processed with Xcalibur software (Thermo Scientific).
Statistical analysisTo visualize the group of genotypes under study, the six compounds identified were subjected to principal component analysis (PCA) using R version 3.4.3 software. Data shown are means ± S.D. The results were compared using Tukey’s HSD test. Pollen fertility results were processed by angular transformation.
Representative fruits of Miyama-uguisukagura, Haskap, and an interspecific hybrid are shown in Figure 1. Fruit colors of interspecific hybrids and parents were characterized using the Royal Horticultural Society (RHS) Colour Chart (2001 edition); Miyama-uguisukagura (Fig. 1A) was included in the Red group (44B in the chart), Haskap (Fig. 1B) was included in the Violet-Blue group (95A in the chart), and the hybrid (Fig. 1C) was included in the Red-Purple group (64A in the chart).
Table 2 summarizes the fruit parameters measured for the interspecific hybrids obtained and for their parents. Fruit fresh weights of interspecific hybrids were lower than or similar to those of their pollen parents. The pH of the fruit juice extracted from Haskap, the interspecific hybrids, and Miyama-uguisukagura were 2.8 to 2.9, 3.2 to 3.4, and 4.6, respectively. The pH values of the fruit juice extracted from all Haskap (male) strains were lower than those of Miyama-uguisukagura and all interspecific hybrids analyzed. SSC in Haskap fruits and the interspecific hybrids ranged from 13.2% to 15.5%, and 14.3% to 16.1%, respectively, but averaged only 8.8% in Miyama-uguisukagura fruits. SSC values of the fruit juice of all male strains and interspecific hybrid strains were higher than those of the female parent, Miyama-uguisukagura. There was a significant difference in pollen germination frequency between Lc23 and M37-2, and Lc23 and Lg1, but not among any other strains. The pollen germination frequency of all interspecific hybrids was higher than for Miyama-uguisukagura (Lg1). Compared with the male parent, in terms of pollen fertility, interspecific hybrids did not lose the ability to germinate. All plants set fruit by open pollination.
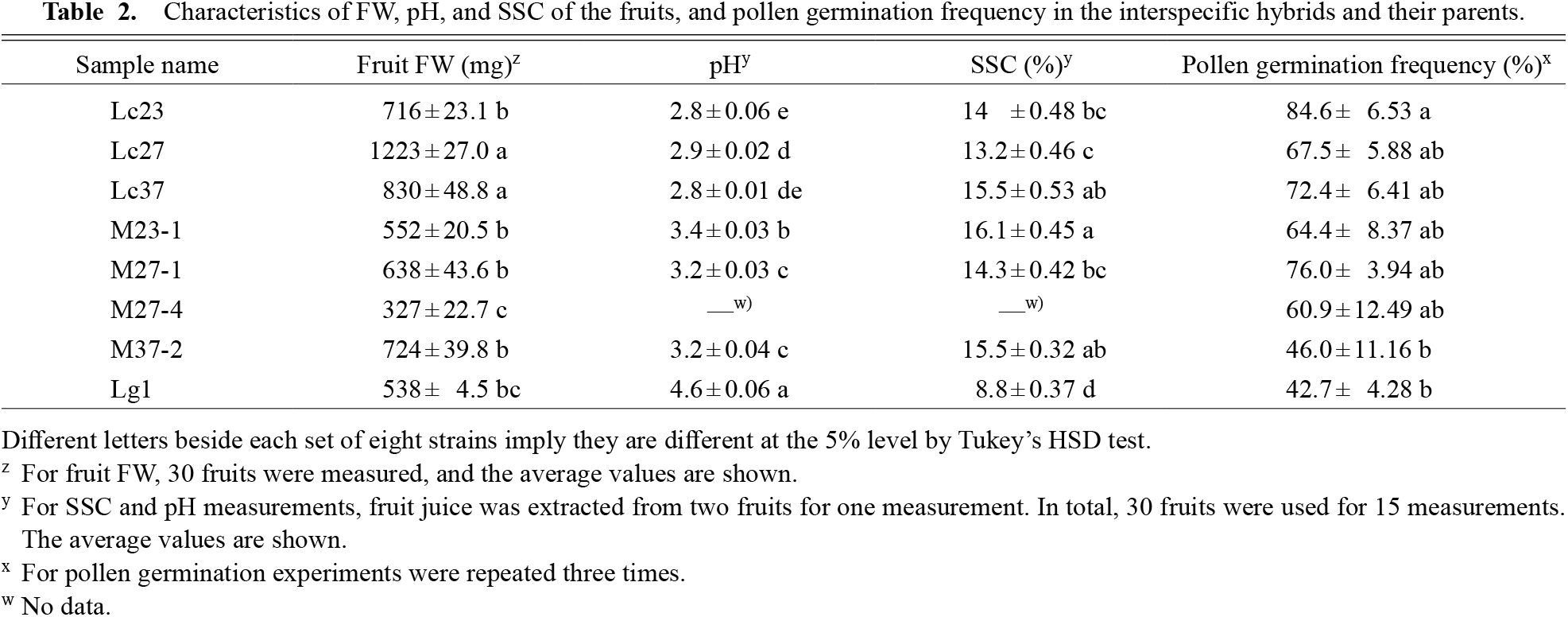
Characteristics of FW, pH, and SSC of the fruits, and pollen germination frequency in the interspecific hybrids and their parents.
Two anthocyanins were detected by LC/MS/MS in Miyama-uguisukagura, and six were detected in all genotypes of Haskap and their interspecific hybrids (Fig. 2). Peak assignment was based on matching product ions from standard anthocyanins in MS/MS analyses or previous reports. Retention times and molecular ions (m/z) of representative anthocyanins are listed in Table 3.
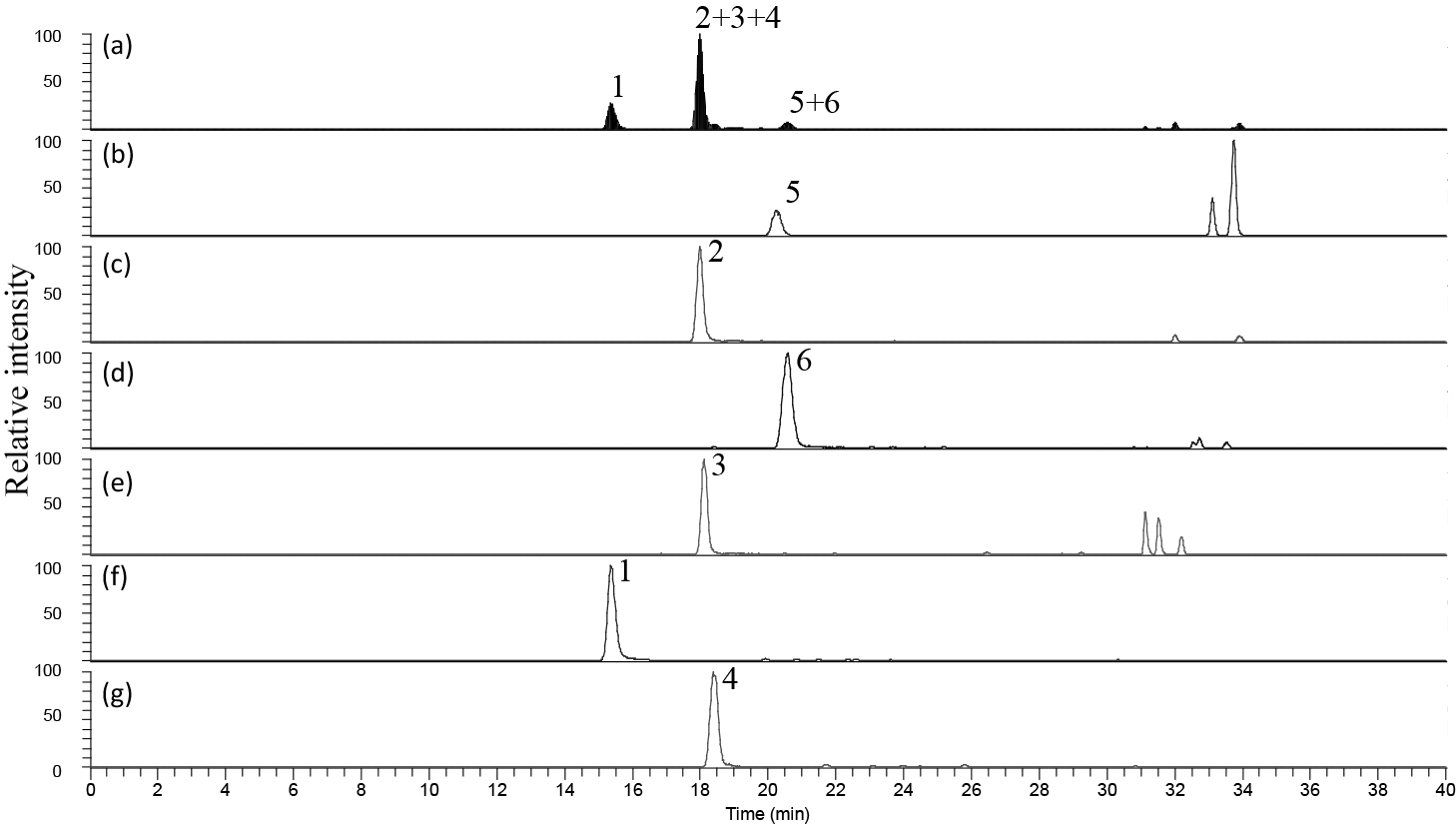
LC/MS/MS chromatogram of anthocyanins from the fruit extracts of the interspecific hybrids between Miyama-uguisukagura and Haskap. (a) TICC (total ion current chromatogram) of the fruit extract of an interspecific hybrid (M27-1). (b–g) Representative SRM (single reaction monitoring) chromatogram of the fruit extract of an interspecific hybrid, detected ion at m/z (b) 271 from 433, (c) 287 from 449, (d) 301 from 463, (e) 287 from 595, (f) 287 from 611, (g) 301 from 625. Peak numbers correspond to 1) cyanidin 3,5-diglucoside; 2) cyanidin 3-glucoside; 3) cyanidin 3-rutinoside; 4) peonidin 3,5-diglucoside; 5) pelargonidin 3-glucoside; and 6) peonidin 3-glucoside.
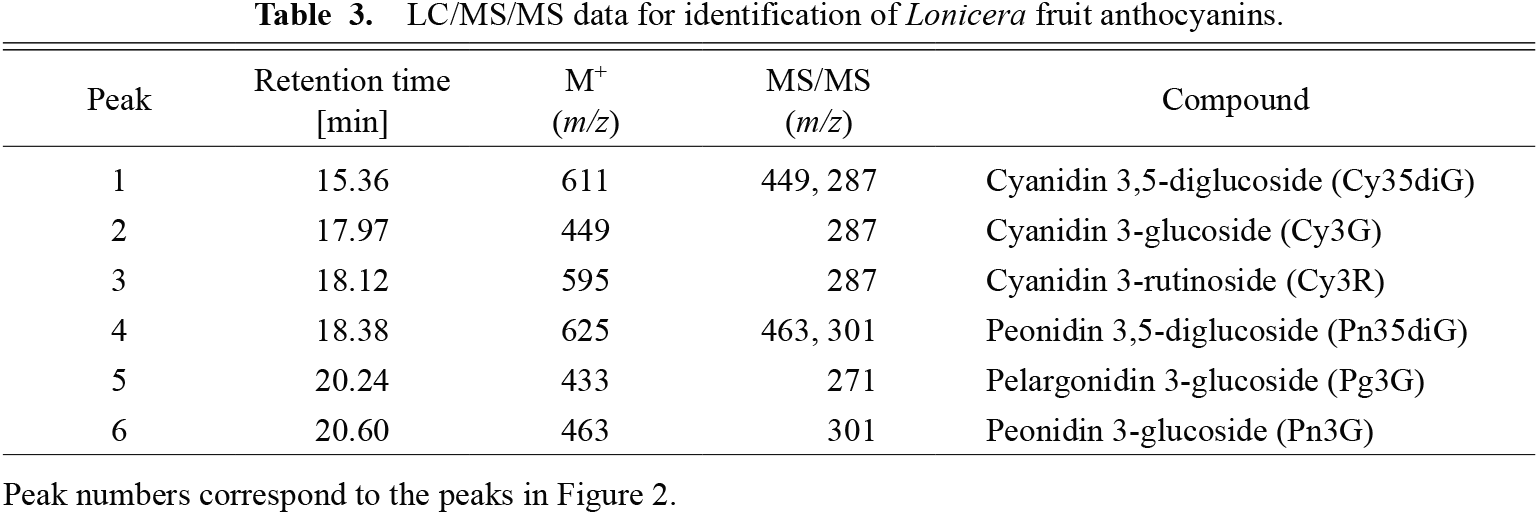
LC/MS/MS data for identification of Lonicera fruit anthocyanins.
Peak 1 had a main ion peak at M+ m/z 611 in the mass spectrum, which yielded MS/MS product ions at m/z 449 ([M-162]+, loss of hexosyl unit) and at m/z 287 (cyanidin, [M-324]+, loss of dihexosyl unit). The Cy35diG standard corresponded with this peak; therefore, this compound was assumed to be Cy35diG.
Peak 2 had an ion peak at M+ m/z 449, which yielded an MS/MS product ion at m/z 287 (cyanidin, [M-162]+, loss of hexosyl unit). The Cy3G standard corresponded with this peak; therefore, this compound was assumed to be Cy3G.
Peak 3 had an ion peak at M+ m/z 595, which yielded an MS/MS product ion at m/z 287 (cyanidin, [M-308]+, loss of rutinosyl unit). The Cy3R standard corresponded with this peak; therefore, this compound was assumed to be Cy3R.
Peak 4 had an ion peak at M+ m/z 625, which yielded MS/MS product ion peaks at m/z 463 ([M-162]+, loss of the hexosyl unit) and at m/z 301 (peonidin, [M-324]+, loss of the dihexosyl unit). This corresponded to the fragmentation pattern of peonidin-dihexoside. The Pn35diG standard corresponded with this peak; therefore, this compound was assumed to be Pn35diG.
Peak 5 had an ion peak at M+ m/z 433, which yielded an MS/MS product ion peak at m/z 271 (pelargonidin, [M-162]+, loss of the hexosyl unit). This corresponded to the fragmentation pattern of pelargonidin-hexoside. The Pg3G standard corresponded with this peak; therefore, this compound was assumed to be Pg3G.
Peak 6 had an ion peak at M+ m/z 463, which yielded an MS/MS product ion peak at m/z 301 (peonidin, [M-162]+, loss of the hexosyl unit). This corresponded to +the fragmentation pattern of peonidin-hexoside. The Pn3G standard corresponded with this peak; therefore, this compound was assumed to be Pn3G.
Quantitative analysisAs mentioned above, the six anthocyanins detected in this study were quantified by LC/MS/MS (Table 4). All six anthocyanins were detected in Haskap parents and their interspecific hybrids, whereas only two, namely Cy35diG and Pn35diG, were detected in the female parent Miyama-uguisukagura. The total anthocyanin concentration in the fruits of interspecific hybrid strains was higher than that of the Miyama-uguisukagura strain and less than that of the Haskap strains, respectively.
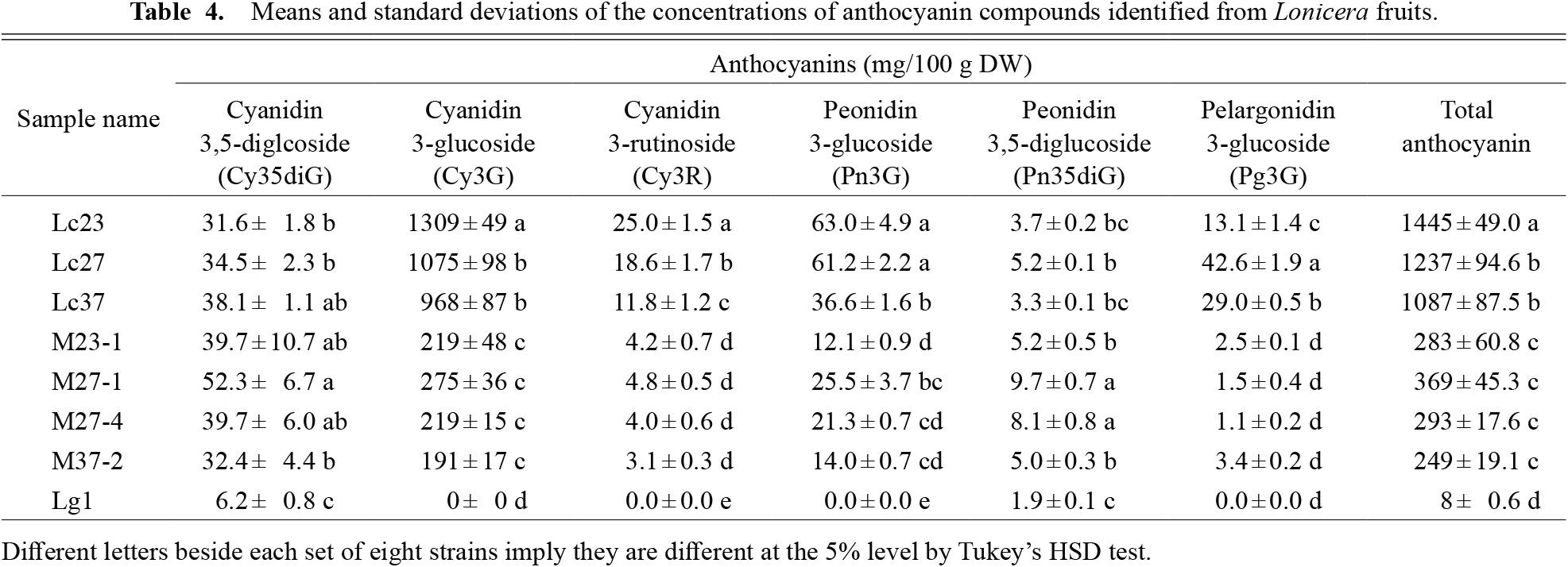
Means and standard deviations of the concentrations of anthocyanin compounds identified from Lonicera fruits.
Cyanidin 3-glucoside was the most abundant anthocyanin in Haskap (968–1309 mg/100 g DW). Similarly, the proportion of Cy3G in interspecific hybrids was the highest among all anthocyanins. However, the amount of Cy3G found in interspecific hybrids was about one-fourth the amount found in Haskap. The proportion of minor anthocyanins varied with genotype. In Miyama-uguisukagura, the major anthocyanin was Cy35diG (6.2 mg/100 g DW), but its concentration was the lowest among all genotypes.
Figure 3 schematically summarizes the common biosynthetic pathways and relative concentrations of anthocyanins detected in this study in Miyama-uguisukagura (Lg1), in the interspecific hybrid (M27-1), and in Haskap (Lc27). In the M27-1 strain, concentrations of Cy3G, Pn3G, and Pg3G were between those of their parents. On the contrary, the concentrations of Cy35diG and Pn35diG were higher than those of both the parents. Cy35diG and Pn35diG are produced from Cy3G and from Pn3G, respectively, by 5GT (anthocyanin 5-glucosyltranferase).
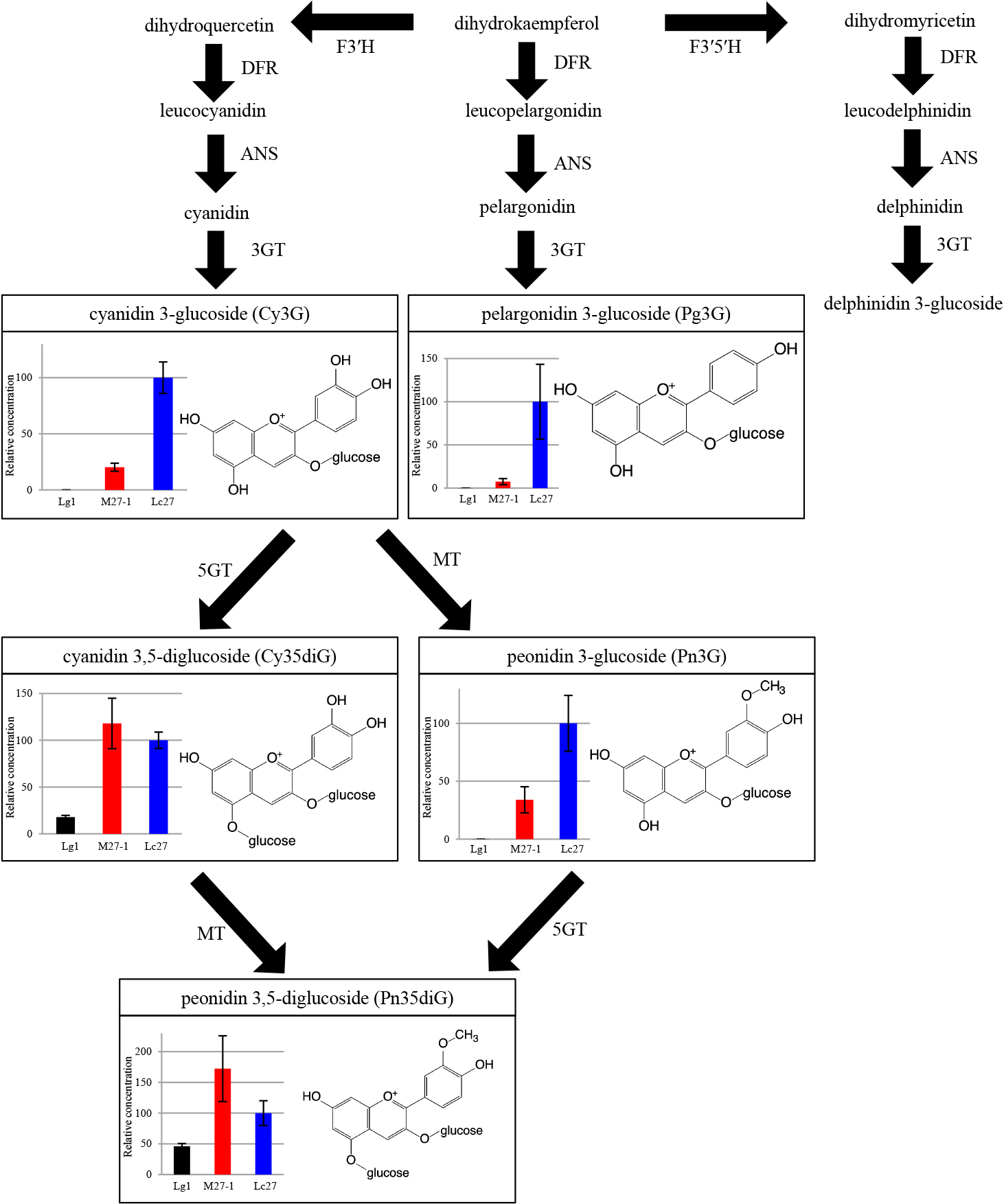
Anthocyanin biosynthesis and anthocyanin relative concentration in the present study. Black, red, and blue bars represent means of the relative concentration of anthocyanins in Miyama-uguisukagura (Lg1), interspecific hybrid (M27-1), and Haskap (Lc27) with error bars indicating SD. Each anthocyanin concentration was indexed relative to the anthocyanin concentration in Haskap (Lc27) fruits as 100. Enzyme abbreviations: F3'H, flavonoid 3'-hydroxylase; F3'5'H, flavonoid 3'5'-hydroxylase; DFR, dihydroflavonol 4-reductase; ANS, anthocyanidin synthase; 3GT, anthocyanin 3-glucosyltransferase; 5GT, anthocyanin 5-glucosyltransferase; MT, methyltransferase.
Principal component analysis was used to examine the similarity among genotypes (Fig. 4). The results showed that the genotypes studied were distinguished by the concentration of Pg3G, Cy3G, Pn3G, Cy3R, Cy35diG, and Pn35diG. PC1 and PC2 accounted for 60.5% and 30.6% of total variance, respectively. Lg1 was clearly distinct from other genotypes (Fig. 4). The genotypes of interspecific hybrids and Haskap clustered close together. Haskap genotypes showed variance to the PC1 vector, while interspecific hybrids showed variance to the PC2 vector. The vectors of Cy3G, Pn3G, Pg3G, and Cy3R were directed to the x-axis (PC1), while the vectors of Cy35diG and Pn35diG were directed to the y-axis (PC2).
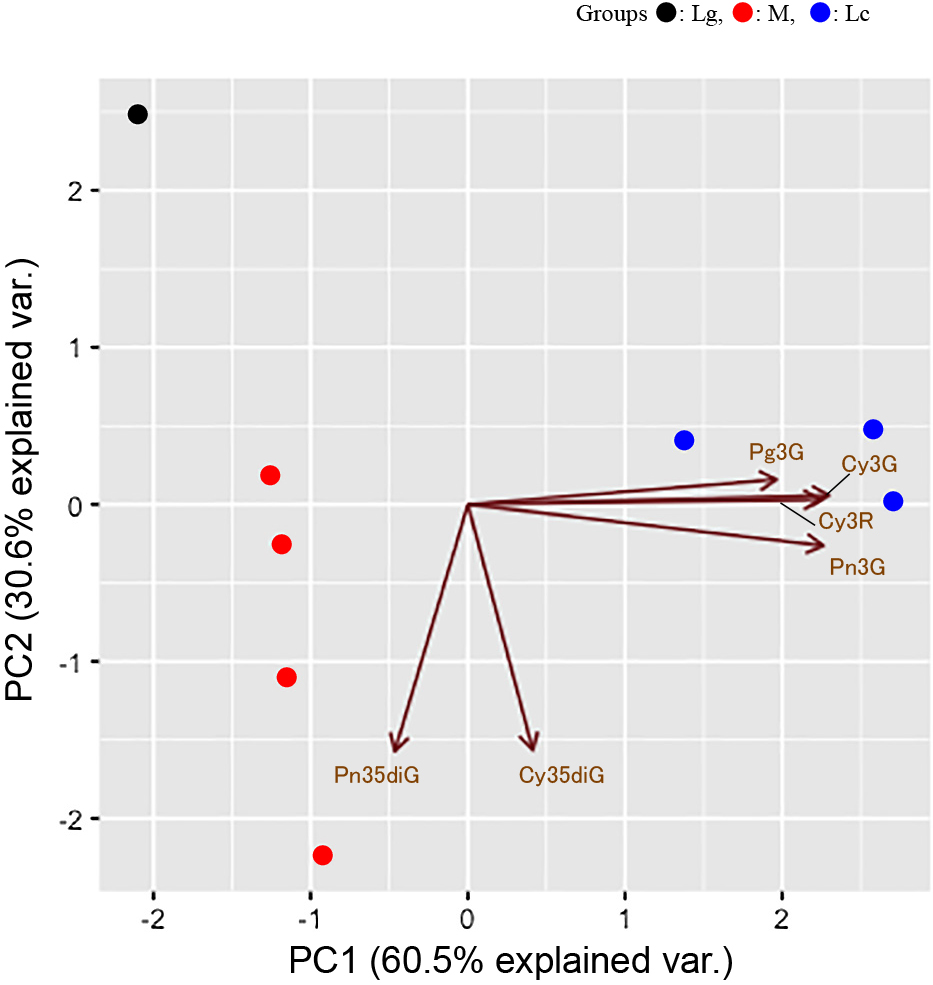
PC scores for positions of the anthocyanin profiles of Lonicera fruits (PC1 vs. PC2). The plots represent strains, while black, blue, and red dots correspond to Miyama-uguisukagura (Lg), Haskap (Lc), and interspecific hybrids (M), respectively. Each vector represents the compounds, while the direction and length of the vector indicates how each ratio readout contributed to the two principal components in the plots.
In this study, the produced interspecific hybrids were amphidiploid because the parental species used were tetraploid. Therefore, the interspecific hybrids showed pollen fertility and proved to be capable of setting fruit.
Cy3G, Cy35diG, Cy3R, Pn3G, Pn35diG, and Pg3G were detected in Haskap fruits. These anthocyanins are known as major anthocyanins in Haskap strains (Terahara et al., 1993; Chaovanalikit et al., 2004; Jordheim et al., 2007; Svarcova et al., 2007; Wojdyło et al., 2013; Celli et al., 2015; Zhao et al., 2015; Wang et al., 2016).
Compared to its parents, the interspecific hybrid M27-1 showed significantly higher concentrations of Cy35diG and Pn35diG; moreover, interspecific hybrid M27-4 showed a significantly higher concentration of Pn35diG. Previous studies reported that some volatile sulfur compounds (e.g. propanethiol, dipropyl disulfide, and allyl ethyl sulfide) increased following interspecific hybridization In Allium spp. (Storsberg et al., 2004). In Prunus spp., interspecific hybrids contained higher levels of several kinds of anthocyanins (e.g. cyanidindiglucoside and cyanidinrutinoside) (Schuster et al., 2013). In the present study, the concentration of Cy35diG and Pn35diG in the fruits of the interspecific hybrids were higher compared to their parents. This phenomenon seemed to be mediated by changes in the biosynthesis of precursors, as well as in biosynthesis-related gene expression. The mechanism of anthocyanin biosynthesis in Haskap remains unknown; however, common anthocyanin biosynthesis mechanisms in various plant species have been well documented (Holton and Cornish, 1995; Bogs et al., 2006; Castellarin et al., 2007). As shown in Figure 3, 5GT seems to be expressed strongly compared in the fruits of the M27-1 strain relative to the parent strains. Knowledge of the quality and the quantity of specific compounds in the fruit may be improved by investigating the amounts of precursors, as well as gene expression and cloning of biosynthesis-related genes.
Here, no novel synthesized compounds were detected in any of the interspecific hybrids studied. Nakaji et al. (2015) analyzed phylogenetic relations of chloroplast DNA among 69 Lonicera species. They reported that L. gracilipes and Haskap were the most closely related of those in the subgenus Lonicera clade in a maximum-likelihood phylogenetic tree based on chloroplast DNA sequences. Novel compounds are created by changes in biosynthesis pathways due to altered gene expression derived from two different genome sets. However, the parents of interspecific hybrids in the present study were relatively closely related. The biosynthetic pathways in the hybrids are likely to be similar to the pathways in the parents; therefore, it is not surprising that we did not detect any novel compounds. It is expected that novel compounds will result from induction of new characteristics gained from other species by distant hybridization. On the contrary, the data on PCA for anthocyanins (Fig. 4) indicated that the interspecific hybrids were distinct from the parents in terms of the concentrations of different anthocyanins they contained. Although the biosynthetic pathways are probably common, the pattern of gene expression may be specific to the interspecific hybrids.
Interspecific hybrids from crosses between Haskap and Miyama-uguisukagura—with low anthocyanin concentrations—had lower concentrations of anthocyanin than those observed in Haskap. Therefore, we believe we can obtain anthocyanin-rich and anthocyanin genotypes with various composition by hybridizing Haskap to related species that have a high anthocyanin content.
Anthocyanins are an important factor determining the color of various plant organs. The color of fruits is slightly different among Haskap and interspecific hybrids. The difference may be caused by total anthocyanin levels, as well as those of Cy3G, a major component of Haskap anthocyanin. Anthocyanins may change fruit color by altering pH, aglycone, glycone, and co-pigmentation (Harborne, 1958; Yabuya et al., 1997; Heredia et al., 1998; Takeda et al., 2010). Cy3G, Cy35diG, Pn3G, Pn35diG, and Pg3G detected in this study showed maximum absorbance at 525, 522, 523, 523, and 506 nm, respectively (Harborne, 1958). All anthocyanins, except for Pg3G, showed almost the same maximum absorbance. It is unlikely that the different fruit colors resulted solely from anthocyanin composition because the main anthocyanins in this study were Cy3G and Cy35diG. Therefore, one of the causes for the differences in fruit color is likely to be the difference in total anthocyanin concentration. Furthermore, carotenoids are major components among red pigments in plants. Therefore, we propose that in addition to total anthocyanins content, the red color of the fruits produced by Miyama-uguisukagura and interspecific hybrids in this study may be due to carotenoid pigments. We plan to elucidate how color is inherited from parents by interspecific hybrids by analyzing carotenoids, in addition to anthocyanins, in a future study.
A major finding of this study is that some anthocyanins that were not contained in Miyama-uguisukagura were found in the fruits of interspecific hybrids between Haskap and Miyama-uguisukagura. Interestingly, anthocyanins found in Haskap fruits were more diverse than those in Miyama-uguisukagura. Furthermore, Haskap fruits contained all the anthocyanins detected in Miyama-uguisukagura and anthocyanins detected in interspecific hybrids were the same as those found in Haskap fruits. Therefore, anthocyanin composition was influenced by interspecific hybridization. In some of the interspecific hybrids studied, the concentrations of Cy35diG and Pn35diG increased relative to the parents. These research findings will have a strong positive impact on breeding methods for novel Haskap varieties that show fruit color variation.
We gratefully acknowledge the Botanic Garden, Hokkaido University, for providing the plant material, Miyama-uguisukagura. The authors thank M. Tsujita and N. Sakai for their technical assistance to produce the interspecific hybrids. We are grateful to Professor H. Nakashima and H. Nakano for their support with plant management. We also wish to thank Dr. H. Hyodoh for providing the LC-MS/MS equipment.