2020 Volume 89 Issue 4 Pages 481-487
2020 Volume 89 Issue 4 Pages 481-487
We investigated the molecular mechanisms underlying the pigmentation patterns of the dorsal petals in torenia (Torenia fournieri Lind. ex Fourn.) cultivars. ‘Piccolo Mix’ consists of lines exhibiting different pigmentation patterns in the limb of the dorsal petal, i.e., entirely pigmented (all-pigmented line), picotee with a pigmented margin (half-pigmented line), and entirely pale (pale line). In the all- and half-pigmented lines, expression of T. fournieri CYCLOIDEA2 (TfCYC2), which is involved in dorsal–ventral floral asymmetry, was inhibited by integration of Ty1/Copia-like LTR retrotransposon TORE2 into the exon of TfCYC2 (TfCYC2TORE2). The all-pigmented line was homozygotic for TfCYC2TORE2, while the pale line was homozygotic normal-type TfCYC2 (TfCYC2+). The half-pigmented line was heterozygotic TfCYC2TORE2/TfCYC2+. Therefore, the extent of pigmentation of the dorsal petal is negatively correlated with the gene dosage of TfCYC2+. Further, ‘Crown Violet’, another torenia cultivar exhibiting the same pigmentation pattern as the all-pigmented line of ‘Piccolo Mix’, was also homozygotic for TfCYC2TORE2. These results indicate that TfCYC2TORE2 is responsible for the marked enrichment of flower pigmentation patterns seen in torenia cultivars.
Flower symmetry is an important characteristic affecting the value of ornamental floricultural plants. Variations in floral symmetry diversify the appearance of the flowers, offering more choice to consumers. For instance, although the wild type snapdragon (Antirrhinum majus) bears typical bilaterally-symmetric personate corolla with a nectar guide, bell-shaped or butterfly-type cultivars bearing relatively radially-symmetric flowers without a nectar guide are also popular. The flower pigmentation patterns are also often varied within a species and diversify the observed flower symmetry. For example, flower pigmentation patterns of pansy (Viola × wittrockiana) cultivars are distributed gradually from a bilaterally symmetric pattern, i.e., dorsal, lateral, and ventral petals pigmented with different colors, to a radially symmetric pattern, i.e., all petals are pigmented with the same color (Huxley et al., 1997).
It has been suggested that evolution of elaborate bilaterally symmetric flowers from simple radially-symmetric flowers occurred independently in various species through coevolution with their pollinators (Citterne et al., 2010). The CYCLOIDEA (CYC) gene, a TEOSINTE BRANCHED 1/CYCLOIDEA and PROLIFERATING CELL FACTOR (TCP) transcription factor, is involved in this evolution (Luo et al., 1996, 1999; Feng et al., 2006; Busch and Zachgo, 2007; Broholm et al., 2008; Wang et al., 2008; Zhang et al., 2010). In flowers of A. majus, dorsal–ventral (DV) asymmetry is controlled by TCP transcription factors CYC and DICHOTOMA (DICH), and by MYB transcription factors RADIALIS (RAD), DIVARICATA (DIV), and DIV-and-RAD interacting factor (DRIF). CYC and its homolog DICH express specifically in dorsal petals, and directly induce RAD (Corley et al., 2005; Costa et al., 2005). A complex of DIV and DRIF proteins promotes ventralization of petals (Raimundo et al., 2013). Although DIV and DRIF express in all petals, RAD competes with DRIF for formation of a DIV–DRIF complex in dorsal petals, inhibiting ventralization, and in turn promoting dorsalization of dorsal petals.
Torenia (Torenia fournieri Lind. ex Fourn.) is a popular summer bedding plant in Japan bearing a bilaterally symmetric flower consisting of five petals, although the two dorsal petals are fused (Fig. 1). In the conventional cultivar ‘Common Violet’ resembling the wild ancestor of T. fournieri (Aida et al., 2000; Nishijima et al., 2013), lateral and ventral petals are pigmented a dense violet (described as “pigmented” in the following text), while dorsal petals are faintly pigmented with pale violet (described as “pale” in the following text). The ventral petal has an oval nectar guide pigmented yellow. The flower colors of torenia cultivars have been diversified since the 1980s. White, pink, and pale-yellow flowers have been bred in addition to the original dark violet flowers (Aida, 2008). The corolla pigmentation pattern has also been diversified. There are also concolor-type cultivars in which all petals are pigmented uniformly, as well as cultivars in which only the lateral petals are pigmented.

Lines selected for experiments from ‘Piccolo Mix’ exhibiting different pigmentation patterns of the dorsal petal. P, pale line; H, half-pigmented line; A, all-pigmented line. D, dorsal petal; L, lateral petal; V, ventral petal. White bars indicate 1 cm.
The pigmentation pattern of the corolla is known to be induced by post-transcriptional silencing of the genes encoding an anthocyanin biosynthesis enzyme. In petunia cultivars, post transcriptional silencing of the chalcone synthase (CHS) A gene induces star and picotee flowers consisting of a colored center with a white margin (picotee) and alternating colored and white parts in a radial pattern (star) (Koseki et al., 2005; Morita et al., 2012). Reduction of CHS expression by antisense gene induces wavy-patterned corolla in addition to uniformly pale corolla in torenia (Aida et al., 2001).
The pigmentation pattern of the torenia corolla has also been shown to be controlled by temporal and positional expression of genes involved in regulation of DV asymmetry. We previously isolated a “Begonia” mutant from the “flecked” line which had the active DNA transposon Ttf1 in the genetic background of ‘Common Violet’ (Niki et al., 2016). Both dorsal and ventral petals are pale in the “Begonia” mutant through dorsalization of the ventral petal. This dorsalization occurred because TfCYC1–3 and TfRAD1, which are orthologs of CYC and RAD, respectively, express both in dorsal and ventral petals (Niki et al., 2016). Su et al. (2017) showed that overexpression of TfCYC1, TfCYC2, and TfRAD1 induces dorsalization of petals and represses pigmentation, while knockdown and knockout of these genes by RNAi and CAS9, respectively, induced ventralization of petals, promoting pigmentation.
In this study, we investigated the effect of genes regulating anthocyanin biosynthesis and floral DV asymmetry on the pigmentation of the dorsal petal in torenia cultivars. The horticultural significance of this molecular mechanism is discussed.
Seeds of ‘Piccolo Mix’ (Takii & Co., Ltd., Kyoto, Japan) and ‘Crown Violet’ (Sakata Seed Corporation, Yokohama, Japan) were sown on 280-cell plug trays filled with horticultural soil (Prime mix TKS-1; Sakata seed), and grown in a glasshouse (18°C minimum and 23°C ventilation temperatures) in Tsukuba city (140°05'E, 36°02'N). Seedlings were transplanted to another horticultural soil (Yokabaido; Hokkaido peatmoss Inc., Kounosu, Japan) in plastic pots (9 cm in diameter and 10 cm deep). As ‘Piccolo Mix’ is a mixture of several lines with various petal colors and patterns, we selected three lines bearing violet flowers and exhibiting different pigmentation patterns in the limb of the dorsal petal. One line had faint pigmentation in the entire limb and was designated “pale”, while another line with completely dense pigmentation was designated “all-pigmented” (Fig. 1). In the third line, only the margin of the limb was densely pigmented, and this line was designated “half-pigmented”. We propagated these lines using cuttings and grew them in an incubator kept at 23°C under 16 h/day illumination from fluorescent lamps at 85 μmol·m−2·s−1. Plants that flowered were used for the experiments. For ‘Crown Violet’, exhibiting the same flower pigmentation pattern as the all-pigmented line of ‘Piccolo Mix’, plants raised from seeds were used directly for the experiments.
Quantitative analysis of anthocyaninAnthocyanin analysis was performed using methods described previously (Nishijima et al., 2013). In brief, we collected 20 mg fresh weight of the dorsal and lateral limbs of opened flowers. The samples were frozen in liquid nitrogen and stored at −80°C until analysis. Anthocyanins were extracted with 5% aqueous acetic acid. We analyzed 10 μL of the extract using a 1100 high-performance liquid chromatography system with a photodiode array detector (Agilent Technologies, Inc., CA, USA) and an Inertsil ODS-2 column (4.6 × 250 mm; GL Sciences Inc., Tokyo, Japan) at 40°C with a flow rate of 0.8 mL·min−1. Absorption spectra were monitored at 220–600 nm. A linear gradient of 20% to 100% of solvent B (1.5% H3PO4, 25% acetonitrile, 20% acetic acid) in solvent A (1.5% H3PO4) was run over 40 min.
Expression analysis of genes responsible for anthocyanin biosynthesis and DV asymmetryThe expression levels of genes involved in anthocyanin biosynthesis and floral DV asymmetry were quantified by real-time polymerase chain reaction (RT-PCR) analysis as described previously (Nishijima et al., 2013; Niki et al., 2016). Total RNA was extracted from young corolla at two stages, 3–5 mm and 8–10 mm corolla length. Total RNAs were then reverse transcribed to generate cDNA as described previously (Nishijima et al., 2013). cDNA was synthesized on total RNA (150 ng) using PrimeScript RT Master Mix (Takara Bio Inc., Shiga, Japan). A Thermal Cycler DICE Real Time System 800 (Takara Bio) and SYBR Premix Ex Taq (Takara Bio) were used for RT-PCR analysis. The primer sequences and thermal programs used were described previously (Nishijima et al., 2013; Niki et al., 2016). Standard curves were obtained using full-length cDNA in a cloning vector, pGEM T-Easy (Promega Corp., WI, USA). TfACT3 was used as an internal control. Three independent replicates of the experiment were conducted.
Structural analysis of TfCYC2Genomic DNA of TfCYC2 was amplified by PCR using high fidelity DNA polymerase (KOD Plus Neo; TOYOBO Co., Ltd., Osaka, Japan) with the primer sets shown in Table S1. The thermal program was set following the manufacturer’s instructions. The amplified DNA was cloned and the nucleotide sequence was analyzed as previously described (Nishijima et al., 2013).
Correlation between the TfCYC2 genotype and mutant phenotypeGenomic DNA was extracted from S1 plants derived by self-pollination of the half-pigmented line as described above. Short sequences specific to the target genotypes were amplified by PCR using combinations of primers P1, P2, and P3 (Table S1; Fig. 5). The combination of P1 and P2 amplifies the 247-bp sequence specific to TfCYC2 harboring retrotransposon TORE2 (designated TfCYC2TORE2 as described below), while the combination of P1 and P3 amplifies the 333-bp sequence specific to TfCYC2 without TORE2 insertion (designated TfCYC2+ as described below). GoTaq (Promega) was used for PCR reaction according to the manufacturer’s instructions. The thermal program was set following the manufacturer’s instructions, while the extension reaction was set for 30 seconds to avoid amplification of long non-specific products.
The dorsal petal of the pale line of ‘Piccolo Mix’ was not pigmented (Fig. 1). The all-pigmented line was fully pigmented in the dorsal limb of the petal, while only the margin of the dorsal limb was pigmented in the half-pigmented line. In the developing flower buds, petal pigmentation was not observed in any of the lines at a corolla length of 4 mm (Fig. S1). When the corolla length reached 8 mm, the limbs of the lateral and ventral petals were entirely pigmented in all lines, but pigmentation of the dorsal petal was seen only in the all-pigmented line. At a corolla length of 12 mm, the limb of the dorsal petal was entirely pigmented in the all-pigmented line, while in the half-pigmented line pigmentation was restricted to the limb margin.
Analysis of anthocyanins and related compoundsThe anthocyanins and flavones detected in flowers of ‘Piccolo Mix’ were the same as those detected previously in ‘Common Violet’. They were: malvidin 3,5-diglucoside, petunidin 3,5-diglucoside, delphinidin 3,5-diglucoside, peonidin 3,5-diglucoside and cyanidin 3,5-diglucoside (anthocyanins); and apigenin 7-glucronide, luteolin 7-glucronide, and luteolin 7-glucoside (flavones) (Fig. S2). The lateral petals showed high anthocyanin and flavone concentrations in all lines tested. In contrast, anthocyanin concentrations in the dorsal petal were very low in the pale line and only slightly higher in the half-pigmented line; in the all-pigmented line anthocyanins were much higher, almost at the same level as for the lateral petals. These results clearly show that the anthocyanin concentration in the dorsal petal positively correlates with the extent of observed pigmentation. The flavone concentration in the dorsal petal positively correlated with the anthocyanin concentration, although even the pale line had a substantial concentration of flavones. It was interesting that the flavone concentration in the lateral petals also seemed positively correlated with the anthocyanin concentration in the dorsal petal.
Expression profiles of the genes encoding anthocyanin biosynthesis enzymesIn the lateral and ventral petals, expression levels of the genes involved in anthocyanin biosynthesis were not greatly different among lines (Fig. 2). In the dorsal petal, expression levels of TfCHS and TfCHI did not differ greatly among lines. In contrast, expression levels of TfMYB1, TfF3H, TfDFR, TfANS, and TfUFGT in the dorsal petal were very low in the pale line and only slightly higher in the half-pigmented line; levels in the all-pigmented line were much higher and comparable to levels in the lateral petals. Since TfMYB1 encodes the R2R3-MYB transcription factor and is a main promoter of flower pigmentation in torenia (Nishijima et al., 2013), we initially predicted mutation of TfMYB1 in the half- and all-pigmented lines. However, no mutation was detected in the base sequence of the TfMYB1 gene.
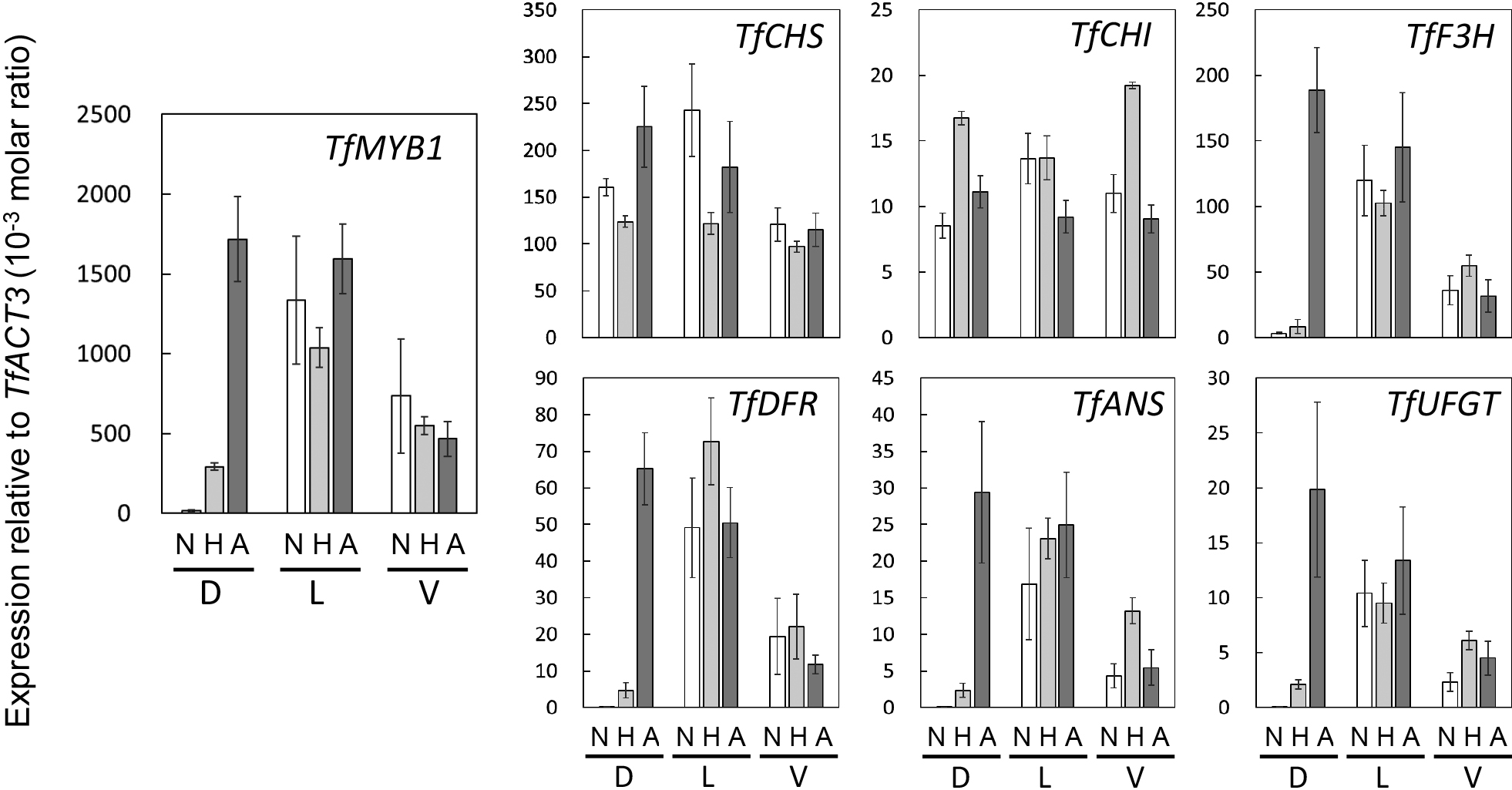
Expression levels of the genes involved in anthocyanin biosynthesis in young petals (11–13 mm) of ‘Piccolo Mix’. P, pale line; H, half-pigmented line; A, all-pigmented line; D, dorsal petal; L, lateral petal; V, ventral petal. Values are means ± S.E. (n = 3).
Expression levels of TfCYC1, TfCYC2, TfCYC3, and TfRAD1 in the pale line were high in dorsal, and low in lateral and ventral, petals in both stages tested, i.e., 3–5 and 8–10 mm corolla length (Figs. 3 and S3). Expression levels of these genes in lateral and ventral petals did not differ significantly among lines. However, expression levels of TfCYC2 and TfRAD1 in dorsal petals decreased slightly in the half-pigmented line and significantly in the all-pigmented line compared to the pale line (Figs. 3 and S3). Since expression of TfCYCs and TfRAD1 promote dorsalization (Niki et al., 2016; Su et al., 2017), the dorsal petal was very probably ventralized by the low expression of TfCYC2 and TfRAD1, resulting in complete pigmentation of the limb, the same as for the lateral petals. The dorsal petal in the half-pigmented line may have been partially ventralized because of the slight decrease in TfCYC2 and TfRAD1 expressions, although it is not clear why partial ventralization of the dorsal petal restricted pigmentation to the margin.

Expression levels of genes regulating dorsal–ventral floral asymmetry in young corolla (3–5 mm in length) of ‘Piccolo Mix’. P, pale line; H, half-pigmented line; A, all-pigmented line; D, dorsal petal; L, lateral petal; V, ventral petal. Values are means ± S.E. (n = 3).
Since CYC directly promotes the expression of RAD1 (Costa et al., 2005) and knockdown of TfCYC2 induces pigmentation of the dorsal petal of torenia (Su et al., 2017), we assumed that TfCYC2 was mutated in the half- and all-pigmented lines in which the expression levels of both TfCYC2 and TfRAD1 were low.
Sequence analysis of TfCYC2Insertion of a 2498-bp fragment was detected in the 1st exon of TfCYC2 in the all-pigmented line (Fig. 4; LC511041). This fragment may be the Ty1/Copia retrotransposon, because a 270-bp long terminal repeat (LTR)-like tandem repeat at both ends flanked a Gag-Pol-like coding sequence. We named this sequence “TORE2”, to follow in sequence from TORE1, the first retrotransposon-like sequence reported in torenia (Nishihara et al., 2014). For notation of TfCYC2 genotypes, we designated TfCYC2 with TORE2 insertion as TfCYC2TORE2 and TfCYC2 without TORE2 as TfCYC2+ in the following text.

Genomic structure of TfCYC2 detected from the pale line of ‘Piccolo Mix’. Ty1/Copia-like retrotransposon TORE2 is integrated in the complementary direction. P1, P2, and P3 were primers used to evaluate the genotype. The gray box indicates an exon. LTR, long terminal repeat; ATG, start codon; TGA and TAG, stop codon.
A low-level expression of TfCYC2 was observed in the homozygote of TfCYC2TORE2 in parallel with low-level TfRAD1 expression (Figs. 3 and S3). This contradicted the prediction that function and expression of TfCYC2TORE2 would be strongly inhibited by TORE2 insertion in the 1st exon. Although the cause of this phenomenon is not clear, we cannot rule out the possible presence of an unknown homolog of TfCYC2 that acts to induce TfRAD1; expression of this putative homolog could be detected by RT-PCR.
Correspondence between genotype and phenotypeOnly TfCYC2+ was detected in the pale line, while the reverse was the case in the all-pigmented line in which only TfCYC2TORE2 was detected (data not shown). Both TfCYC2+ and TfCYC2TORE2 were detected in the half-pigmented line. Therefore, we predicted that TfCYC2+ and TfCYC2TORE2 are allelic and inherit semi-dominantly. Of the 36 F1 plants derived from crossing between the pale and all-pigmented lines, all were half-pigmented. The segregation among 48 S1 plants derived from self-pollination of the half-pigmented line was as follows: 13, 24, and 11 plants were pale, half-pigmented, and all-pigmented, respectively. The theoretical segregation ratio of the S1 plants is 1:2:1 of pale, half-pigmented, and all-pigmented plants, respectively, if single-factor semi-dominant inheritance is assumed. The actual segregation of S1 plants was not significantly different from the theoretical ratio according to the χ2 test (P = 0.92). Analysis of the TfCYC2 genotype of the S1 plants showed that only TfCYC2+ was detected in the pale segregants, while only TfCYC2TORE2 was detected in the all-pigmented segregants (Fig. 5). Both TfCYC2+ and TfCYC2TORE2 were detected in the half-pigmented segregants. These results indicate that TfCYC2+ and TfCYC2TORE2 are allelic and inherit semi-dominantly, and that the genotypes of the pale, half-pigmented, and all-pigmented lines are TfCYC2+/TfCYC2+, TfCYC2+/TfCYC2TORE2, and TfCYC2TORE2/TfCYC2TORE2, respectively.

Genotype of TfCYC2 in S1 segregants derived from the half-pigmented line of ‘Piccolo Mix’. Pigmentation of the dorsal petal of the segregants was identical either to the pale (P), half-pigmented (H) or all-pigmented (A) lines shown in Figure 1. The primer sets specific to normal- and mutant-type TfCYC2, indicated respectively as TfCYC2+ and TfCYC2TORE2, were used for PCR (Fig. 5; Table S1). M: molecular weight marker.
Since overexpression, knockdown and knockout of TfCYC2 result in a greater change in the petal pigmentation pattern than for TfCYC1, it may be that TfCYC2 is the main factor regulating floral DV asymmetry (Su et al., 2017). In this study, the TfCYC2 mutation in ‘Piccolo Mix’ resulted in a significant change in dorsal petal pigmentation, from no pigmentation to complete pigmentation, supporting the hypothesis that TfCYC2 is the main factor regulating floral DV asymmetry in torenia.
Effects of genes regulating floral DV asymmetry on pigmentation patterns in torenia cultivarsAs described above, Su et al. (2017) showed that overexpression of TfCYC1, TfCYC2, and TfRAD1 induce dorsalization of the lateral and ventral petals, repressing pigmentation. Conversely, knockdown and knockout of these genes by RNAi and CAS9 induces ventralization of the dorsal petal, promoting pigmentation. These results clearly show that positional regulation of gene expression involved in floral DV asymmetry is crucial for the pigmentation pattern. This is true not only in genetic recombinants, but also in natural mutants and commercial cultivars. In the natural torenia mutant “Begonia”, expression levels of TfCYC1–3 and TfRAD1 increase in the ventral petal to a level comparable to that in the dorsal petal (Niki et al., 2016). This positional change of expression dorsalizes the ventral petal and represses pigmentation, forming a disymmetric corolla similar to begonia. In this study, retrotransposon-induced mutation of TfCYC2, i.e., TfCYC2TORE2, ventralized the dorsal petal and promoted pigmentation in a commercial cultivar, ‘Piccolo Mix’. Further, TfCYC2TORE2 was also detected in another commercial cultivar, ‘Crown Violet’, in which the flower pigmentation pattern was the same as the all-pigmented line of ‘Piccolo Mix’ (Fig. S4). The base sequence of TfCYC2TORE2 was identical between ‘Piccolo Mix’ and ‘Crown Violet’. TfCYC2TORE2 may be homozygous in ‘Crown Violet’ because TfCYC2+ was not detected in this cultivar (Fig. S4); further, expression levels of TfCYC2 and TfRAD1 were low, as in the all-pigmented line of ‘Piccolo Mix’ (Fig. S5).
‘Crown Violet’ is one of the ‘Crown’ series cultivars released by the PanAmerican Seed Co. in 1988 (Aida, 2008). The ‘Crown’ series included the first torenia F1 cultivars that exhibited marked improvements as ornamental plants, i.e., a wider variation in flower color and pattern together with a compact plant volume suitable for bedding and pot plants. As described above, TfCYC2TORE2 can lead to three pigmentation patterns of the dorsal petal (pale, half-pigmented, and all-pigmented) through allelic combination with TfCYC2+. Therefore, TfCYC2TORE2 contributed to improvement of torenia cultivars through an epoch-making enrichment in variation of flower pigmentation patterns.
As mentioned above, the gene dosage of TfCYC2 regulated the extent of margin-preferential pigmentation in the dorsal petal. The molecular mechanism of this phenomenon could be elucidated by detailed analysis of temporal and spatial expression of the related genes. Meanwhile, this phenomenon enables us to predict that the width of the pigmented margin is regulated by the severity of TfCYC2 mutation. Integration of various TfCYC2 mutations of varying severities and other color pattern mutations such as “Begonia” described above may greatly expand variations of pigmentation patterns in torenia corolla.
We thank Mrs. Toshie Iida for her technical assistance. Luteolin 7-glucuronide and apigenin 7-glucronide for HPLC standards were a kind gift from Tsukasa Iwashina of the Tsukuba Botanical Garden, National Museum of Nature and Science.