2021 Volume 90 Issue 4 Pages 428-449
2021 Volume 90 Issue 4 Pages 428-449
Current carnation cultivars have a wide range of flower colors, which is one of the important traits for the flower market. Since large numbers of commercial carnation cultivars bearing various flower colors and flower color patterns have been developed over the last few decades, a comprehensive understanding of the diversity of flower color characteristics has become difficult to achieve. In this study, 56 standard carnation cultivars and 54 spray carnation cultivars were collected and evaluated in terms of flower color and flower color pattern, two visual traits, and flower pigments. Visual flower color analysis indicated that the flower color pattern of the 110 cultivars could be categorized into five typical types and two minor types, and the five typical types were further classified into 16 sub-types. Additionally, flower colors of these carnations could be categorized into 15 hues. High-performance liquid chromatography (HPLC) analysis indicated that carnation flower color is basically determined by the combination of pelargonidin and cyanidin-based anthocyanins, chalcononaringenin 2'-O-glucoside and chlorophyll, giving cyanic, yellow and green color, respectively. Microscopic observation of the petal epidermis indicated that formation of anthocyanin aggregates in the vacuoles and cell shape affect color perceptions, either metallic or velvety, and this is involved in flower color diversification. A fundamental investigation of flower color characteristics will support further developments in flower-color breeding.
Carnation (Dianthus caryophyllus L.) is one of the most economically important ornamental flowers. Originally, domesticated carnation cultivars were bred by multiple crossing wild type D. caryophyllus with several wild Dianthus species (Onozaki, 2018). Then, a typical carnation ‘Atim’, known as a first perpetual carnation, was bred from ‘Oeillet de Mahon’ in France in 1840 (Sisley, 1886). Carnation for cut flowers with various flower colors and flower color patterns were seen in old horticultural magazines of the 1800’s (Houtte, 1857; Rodigas, 1885). Recent carnation cultivars display a wider flower color range together with more varied flower color patterns. The diversification of flower appearance in terms of color and pattern can ultimately have a beneficial economic impact on the flower market.
The richness in flower colors in carnation cultivars is due to the accumulation of flavonoids in the petals. Anthocyanin is responsible for cyanic colorations of carnation flowers. Firstly, pelargonidin and cyanidin 3-O-(6-O-malyl-β-glucopyranoside) (Pg3MG and Cy3MG, respectively) were identified as Dianthus-specific anthocyanins from red and dark-red carnation flowers, respectively (Terahara and Yamaguchi, 1986; Terahara et al., 1986). Subsequently, unique chemical structures of 3,5-di-O-(β-glucopyranosyl) pelargonidin 6"-O-4,6'''-O-1-cyclic malate (Pg3,5cMdG) and 3,5-di-O-(β-glucopyranosyl) cyanidin 6"-O-4,6'''-O-1-cyclic malate (Cy3,5cMdG) were identified in deep-pink and reddish-purple carnation flowers, respectively (Bloor, 1998; Nakayama et al., 2000). To date, the biosynthetic pathway of the two cyclic-malylated anthocyanins has largely been elucidated. Non-cyclic 3,5-malyldiglucoside of anthocyanidin is produced from anthocyanidin 3-malylglucoside by glycosylation, and subsequently a macrocyclic ring in the anthocyanidin 3,5-glucoside is formed by an unknown cyclase reaction (Sasaki et al., 2013). Basically, a carnation cultivar exclusively accumulates one of the four malyl anthocyanins in the petals as the major flower pigment (Okamura et al., 2013). As exceptions, there are several carnation cultivars that accumulate both pelargonidin (Pg) and cyanidin (Cy)-based anthocyanins or accumulate mono-glucoside and di-glucoside forms of either Pg- or Cy-based anthocyanin. However, the ratio of one major anthocyanin and the other one is quite low (Ootani and Miura, 1961; Yamaguchi, 1989). Transgenic carnation cultivars bearing bluish petals, i.e., the “Moondust series”, are capable of producing delphinidin (Dp)-derivatives in the petals (Fukui et al., 2003); however, no pure blue carnation cultivars have been realized. Chalcononaringenin 2'-O-glucoside (Ch2'G) is also an important flavonoid pigment contributing to yellow coloration in carnation flowers (Yoshida et al., 2004). Basically, carnation flower color is determined by the composition of either one of the major four malyl anthocyanins (Pg3MG, Cy3MG, Pg3,5cMdG, and Cy3,5cMdG) and Ch2'G. Orange flowers arise due to the coexistence of Pg-based anthocyanin and Ch2'G (Gonnet and Hieu, 1992; Morimoto et al., 2019). There are a handful of commercial carnations that display colors distinct from general carnations, and the well-known dusky-purple-flowered carnation ‘Nazareno’ has a deacyl anthocyanin, pelargonidin 3,5-O-diglucoside (Pg3,5dG), as the major flower pigment (Okamura et al., 2012). There are other carnations with distinct flower colors that have been generated by ion-beam breeding and these varieties accumulate either pelargonidin 3-O-glucoside (Pg3G), cyanidin 3-O-glucoside (Cy3G) or cyanidin 3,5-O-diglucoside (Cy3,5dG) (Nakayama et al., 2012; Okamura et al., 2013). Petal epidermal cells with non-malylated anthocyanin often produce anthocyanic vacuolar inclusions (AVIs) in their vacuoles resulting in dusky and metallic colors depending on the pigment condensation level in the vacuolar sap (Okamura et al., 2013). The relationship between flower color and flower pigment composition in modern carnation cultivars has been examined using colorimetric, spectrophotometric and chromatographic studies in order to develop flower color breeding (Ootani and Miura, 1961; Yokoi, 1973; Maekawa and Nakamura, 1977; Yamaguchi, 1989; Gonnet and Hieu, 1992; Onozaki et al., 1999). However, to the best of our knowledge, there has been no comprehensive investigation into the flower colors of carnation cultivars since the identification of the chemical structures of Dianthus-specific true anthocyanins.
Chlorophyll and carotenoid also play an important role in the extension of petal color range in various ornamental plants (Mudalige et al., 2003; Ohmiya et al., 2017), and almost all mature petals of carnation cultivars have lower contents of chlorophyll and carotenoids (Ohmiya et al., 2013, 2014). However, a relatively large number of carnation cultivars with pale green or greenish flowers caused by chlorophyll can be seen in flower shops. Substantial chlorophyll contents were confirmed genetically in the mature petals of pale-green-flowered carnation cultivars in comparison with white-flowered cultivars due to a higher rate of chlorophyll biosynthesis (Ohmiya et al., 2014). This fact indicates that green-flowered mutants that accumulate substantial chlorophyll contents are preferentially selected for commercial production (Ohmiya et al., 2020). Production of yellow-flowered carnations that accumulate carotenoids by conventional breeding has so far been impossible (Ohmiya et al., 2020). Recent research, however, indicated that the ‘Club’ cultivar with pale yellow flowers could synthesize substantial carotenoid content in the flowers, suggesting much deeper-yellow-flowered cultivars could be bred by manipulating carotenoid biosynthesis in carnation flowers (Iijima et al., 2020).
The diversification of flower color patterns is also an important factor in increasing the appeal of carnation flowers. Several flower color patterns were listed by Imai (1936) and Yamaguchi (1992) described three typical flower color patterns (colored-bottom variegation, streak coloration pattern and picotee), as wells as a very minor pattern showing spot variegation in cut carnation flowers and an eye-spotted pattern in other Dianthus species. Furthermore, a recent report indicated 10 flower color patterns in carnation cultivars (D. caryophyllus) and a ring-like coloration pattern, a kind of eye or center of wild Dianthus flowers, in some other Dianthus species and described the relationship between formation of their patterns and genetic regulation (Nakayama, 2020). However, there are other types of flower color pattern that were not listed in these reports. Despite the fact that carnation cultivars have changed significantly over the past few decades in response to changes in consumer habits and preferences, our current understanding of the diversity of carnation flower colors is inadequate. A complete picture of the current flower color diversity of carnation cultivars is essential to create new possibilities and ideas for future flower color breeding.
The aim of this study was to assess the diversity of flower color characteristics of current cut carnation flowers. We investigated flower color patterns and the color range in 110 commercial cut carnation flowers by perceptual recognition and colorimetric analysis. The major flower pigments of each cultivar were identified using high-performance liquid chromatography (HPLC). The two data sets including flower color appearance and pigmentation were integrated to promote a comprehensive understanding of the flower color diversification of current carnation cultivars.
A total of 56 standard cut carnation flowers and 54 spray cut carnation flowers were used to investigate flower color characteristics. Carnations possessing cyanic petals were mainly chosen to investigate flower colors of commercial cut flowers. Randomly selected cultivars (38, 62, and 5) were provided by Inochio Fujiplants Inc. (Nishio, Japan), Miyoshi Group Co., Ltd. (Hokuto, Japan) and Koukaen (Takamatsu, Japan), respectively. Four transgenic cultivars of the “Moondust series” with bluish-colored petals were purchased at a local flower shop to compare them with the flower color of the other commercial carnations. ‘Velvet Rouge’, bred by the Kagawa Prefectural Agricultural Experiment Station (Ayagawa, Japan) with petals that are distinctively different from those of other carnations, was added to the survey. The cuttings were grown in a greenhouse at the agricultural campus of Kagawa University. All cut flowers were kept in a solution consisting of 1% glucose and 0.05% Legend MK (Cresta Japan, Chiba, Japan) followed by overnight treatment with 0.2 mM silver thiosulfate anionic complex (STS) solution. Fully opened flowers with outer petals spreading horizontally were examined in this research.
Evaluation of petal color appearanceFlower color pattern and pigmentation of both adaxial and abaxial sides of the petals were evaluated by the naked eye referring to the reports by Yamaguchi (1992) and Nakayama (2020). The flower color patterns of the adaxial side of the petals were classified. Each petal color was annotated by color name and color code using the Royal Horticultural Society Colour Chart (RHSCC) Sixth Edition (2015). When the flower color appearance differed from the petal color, the flower color was also annotated by RHSCC. The lightness (L*) and chromatic components a* and b* of the Commission Internationale de l’Eclairage (CIE) L*a*b* of each petal part were measured by a colorimeter (NR-12B; Nippon Denshoku Industries Co., Ltd., Tokyo, Japan) in one petal since there were no significant differences among flowers or among petals. The colorimetric values were measured twice, and after confirming that there was no significant deviation in the color difference values, the first measurement values were recorded. Chroma (C*) was hue angle (H°) calculated using an equation: C* = (a*2 and b*2)1/2 and arctan (b*/a*), respectively (McGuire, 1992). The carnation flower colors were grouped based on the L*a*b* components and C* from the variegation color of bicolor flowers and blade petal parts of single-colored flowers. The petal colors from the cultivars with purplish-colored flowers were grouped by the C* and H° values and the RHSCC color code. ‘Ateca’ and ‘Martina’ were classified into a color group based on the RHSCC annotation since the edge-colored areas of the petals were too narrow to be measured by the colorimeter. The colorimetric information from the base color of ‘Furby’ was used to classify the flower color group since its variegation is difficult to assess without close observation.
HPLC analysis for flavonoidsThe blade parts of the outer petals were used to investigate flower color characteristics and perform flower pigment analysis (see the single color flower in Fig. 1A). In the case of bicolor cultivars, the base part and the variegation were separated as much as possible, and then each separated part was used for the investigation. The samples were then dried at 38°C for 24 h, and stored at −25°C under dry conditions until use. The dried samples were soaked in test tubes with 1 mL of 50% acetic acid (v/v), and flavonoids were extracted from the dried samples at room temperature (ca. 25°C) for 24 h in the dark. The crude extracts were filtrated by 0.45 μm membrane filters (Advantec Toyo Kaisha, Ltd., Tokyo, Japan), and the filtrated elutes were then filled up to 1 mL with 50% acetic acid (v/v) in volumetric flasks. The filtered elutes were injected into an HPLC system. The HPLC analysis for flavonoid detection was performed according to the same protocol as Morimoto et al. (2019). Pg- and Cy-based anthocyanins were detected based on absorptions of 510 nm and 520 nm, respectively. Anthocyanins and Ch2'G of each sample were identified by HPLC comparison with the retention times of the authentic anthocyanins as follows: Pg3G, Pg3,5dG, Cy3G, and Cy3,5dG as chemical standards were purchased from Extrasynthese (Genay, France). Pg3MG, Pg3,5cMdG, Cy3MG, and Cy3,5cMdG were prepared from the petals of the carnation cultivars ‘Excerea’, ‘Beam cherry’, ‘Royal Tessino’, and ‘Trendy Tessino’, respectively (Yamaguchi et al., 2000; Matsuba et al., 2010; Nishizaki et al., 2011). Pg3,5MdG and Cy3,5MdG were obtained during the purification process of cyclic-malylated anthocyanins, and the molecular weights were confirmed as m/z 711 and m/z 727, respectively (data not shown). The molecular weights corresponded to those of non-cyclic Pg3,5MdG and Cy3,5MdG due to hydrolysis of the cyclic anthocyanins as in previous studies (Bloor, 1998; Gonnet and Fenet, 2000). Yellow flavonoid Ch2'G was extracted from a yellow-flowered carnation ‘Liberty’ (Yoshida et al., 2004).
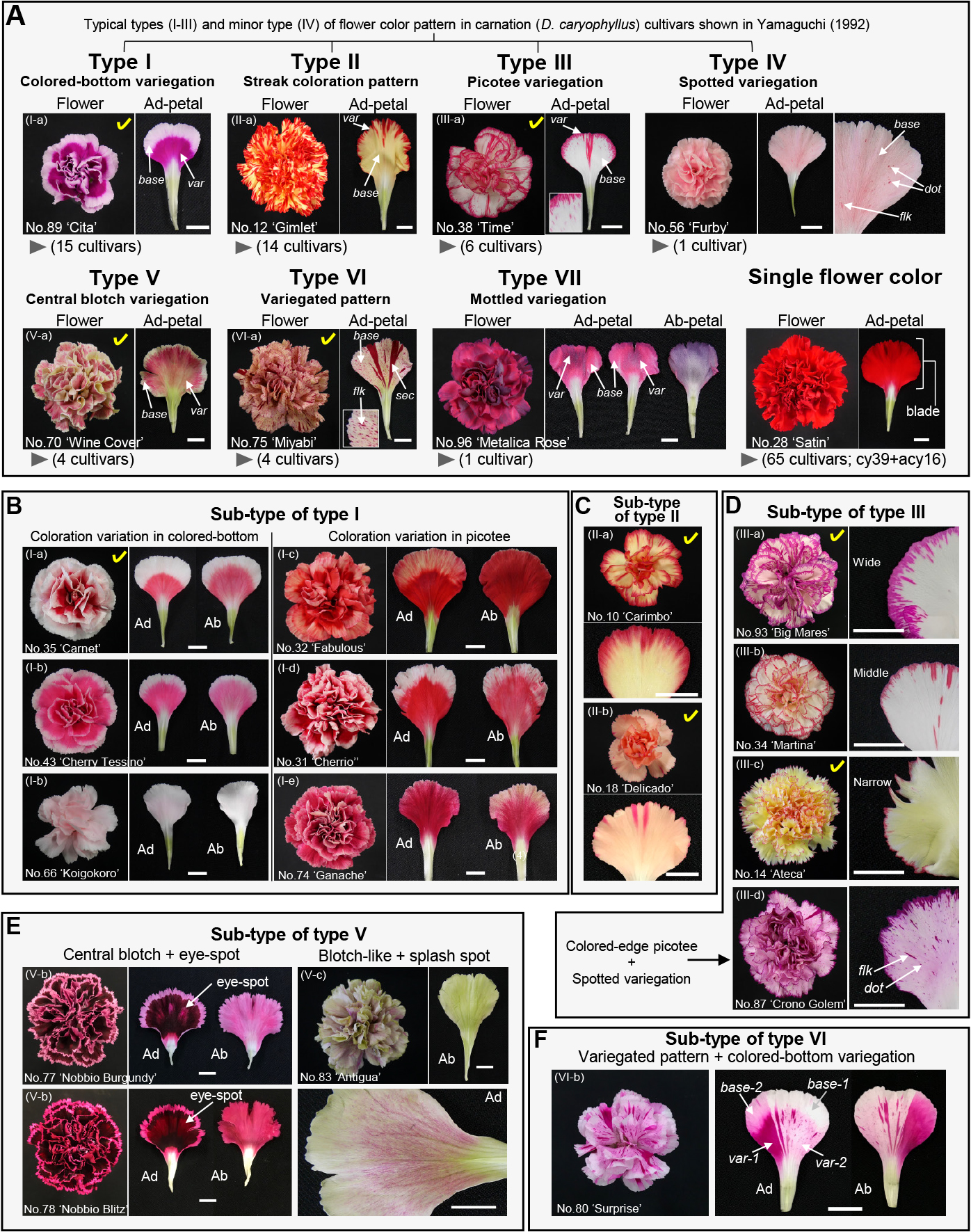
Flower color patterns of the carnation cultivars. (A) The flower photographs show flowers and petals from the representative carnation cultivars in this study. The five typical types (types I–III, V, and VI) and two minor types (types IV and VII) of flower color pattern were classified by the naked eye. The three typical types (types I–III) and a minor type (type IV) of flower color pattern were reported by Yamaguchi (1992). Check marks on all photographs in Figure 1 indicate flower color patterns introduced by Nakayama (2020). Type I, colored-bottom variegation with white or pale cyanic picotee; type II, streak coloration pattern; type III, picotee variegation with trace or several small spots; type IV, spotted variegation; type V, central blotch variegation with white or pale cyanic-colored picotee; type VI, variegated pattern (flowers variegated with flecks and sectors); VII, mottled variegation. Single color shows the number of cyanic (cy) and acyanic (acy) cultivars. The enlarged images of the upper parts of the petals are indicated in photographs of the petals of ‘Time’ (No. 38) and ‘Miyabi’ (No. 75). The letters below each photograph show the number of cultivars categorized into each flower color pattern type. var, main variegation part; base, base parts of petals (parts excepts for variegated parts); flk, colored flecks; dot, colored dots; sec, sector as main variegation parts in type VI. Ad, adaxial side; Ab, abaxial side; blade, name of parts used for HPLC analysis. Scale bar, 10 mm (in all photographs of Fig. 1). Number and lower case letters in the upper left of all photographs of Figure 1 indicate typical types and sub-types of each typical flower color pattern, respectively. (B) Pigmentation variation in sub-type of “type I”. (I-a) and (I-b), different scale of colored bottom on both adaxial and abaxial sides of the petals. The variegation part in (I-b) occupies more than half of the adaxial side of the blade part, but not (I-a). In “sub-type I-b”, ‘Koigokoro’ (No. 66) flowers displayed much paler colored variegation than that of ‘Cherry Tessino’ (No. 43). (I-c) and (I-d), invasion of pigmentation in a marginal picotee. (I-e), ‘Ganache’ flowers (No. 74) with a reverse pigmentation pattern of typical colored-bottom variegation “sub-type I-b”. (C) Pigmentation variation in “type II”. (II-a), colored-edge picotee with pigmented streaks. (II-b), a petal with smaller number of colored streaks than ‘Gimlet’ (No. 12) presented in Figure 1A and the petal edge in “sub-type II-b” did not show picotee. (D) Different pigmentation scale of colored-edges of petals in “type III”. The largest area of the colored edge was (III-a, wide type), followed by (III-b, middle type) and (III-c, narrow type) in that order. (III-d), a ‘Crono Golem’ flower (No. 87) with a colored-edge picotee and spotted variegations. (E) Pigmentation variation in “type V”. (V-b), central blotches with eye spots in flowers and petals of two “Nobbio” cultivars (No. 77 and 78). (V-c), a central blotch with a splash spot in a flower and petals of ‘Antigua’ (No. 83). (F) Pigmentation variation in “type VI”. (VI-b), variegated pattern combined with colored-bottom variegation in a flower and petals of ‘Surprise’ (No. 80). var-1 and base-2 are shown as the main variegation and secondary base parts of the petals in “type VI”, respectively. var-2 and base-1 are shown as the secondary variegation and main base parts of the petals in “type I”, respectively.
The HPLC peak area of anthocyanin was divided by fresh weight of petals and then divided by 1 × 106 calculated as the relative anthocyanin content. The Ch2'G of each cultivar was detected at absorption of 360 nm, and the content was calculated as a relative rate when the value of Ch2'G content in the petals of ‘Liberty’ was 1.0. The total content of anthocyanins (TA) was summed in 10 types of anthocyanins. The total content of colorless or pale yellow flavonoids (TF) was detected at 360 nm, and the TF was calculated by subtracting the Ch2'G content from the total peak area divided by fresh weight and 1 × 107. The ratio of cyanidin to pelargonidin (C/P) value in cultivars accumulating pelargonidin dominantly was calculated by dividing total content of Cy-based anthocyanins by total content of Pg-based anthocyanins. The P/C value in cultivars accumulating cyanidin dominantly was calculated by dividing total content of Pg-based anthocyanins by total content of Cy-based anthocyanins.
Flavonoid contents in the variegation parts of petals in carnations with petals displaying a streak coloration pattern, colored-bottom variegation and mottled variegation were compared with those in the base parts. In the case of ‘Metalica Rose’, petal epidermises accumulating anthocyanins were separated from peculiarly colored and non-peculiar colored parts using cellophane tape and used for anthocyanin analysis.
Chlorophyll analysisThe third and fourth petals were sampled from any standard or spray carnations of each flower color group, respectively. Blade parts of these petals from each carnation sample were used for chlorophyll analysis. The blade petal parts were frozen in liquid nitrogen and stored at −25°C until use. The frozen samples were ground into powder in liquid nitrogen. Then, 3 mL of acetone was added to the powered petals. The solutions were centrifuged at 12,000 g for 10 min. Then, 800 μL of supernatants were dried under reduced pressure, and the crude pigments were dissolved completely with 900 μL of methanol. The solutions were filtrated by 0.45 μm membrane filters (GL Sciences Inc., Tokyo, Japan). Subsequently, the filtrated solutions were filled up to 1 mL with methanol in volumetric flasks. Ten μL of the filtrated solutions was injected into an HPLC system for chlorophyll detection, according to the method of Takamura and Morino (2019). Chlorophyll a and b were confirmed by comparing with chromatograms and the maximal absorption wavelength of the pale-green-flowered carnation ‘Prado Mint’ based on absorption at 650 nm (Ohmiya et al., 2013). Presence or absence of chlorophyll in the petals was assessed by the detection, or below the detection limit, of chlorophyll peaks.
Microscopic observation of petal epidermal cellsThe peculiar-colored carnations ‘Metalica Rose’, ‘Velvet Blue’, and ‘Velvet Rouge’ were used to observe the structure of petal epidermal cells. The petals were embedded in 8% agar. Transverse sections (ca. 75 μm thick) of the petals were prepared using a micro slicer (DIK-1000; Olympus Corporation, Tokyo, Japan) and photographed using an optical microscope (BX51; Olympus Corporation) and a digital microscopy camera (DP72; Olympus Corporation). Subsequently, the height and width of 30 epidermal cells from three petals (10 cells/a petal) from two velvety cultivars (‘Velvet Blue’ and ‘Velvet Rouge’) and non-velvety cultivar ‘Princes Blue’ were measured and the average of the aspect ratio was calculated. Significant differences in the aspect ratios were evaluated using the Mann-Whitney U test.
Results Categorization of flower color patternThe flower color pattern of adaxial side of carnation petals was roughly categorized into seven types, typical types (types I–III, V, and VI) and minor types (types IV and VII), together with a single color type as shown in Figure 1A. Furthermore, the typical flower color patterns could be divided into 16 sub-types depending on the pigmentation scale and combination of flower color patterns. The single flower color type accounted for the majority of the investigated carnations, and the proportion was 59.1%. Colored-bottom variegation “type I” displayed a deep color in the proximal petal part, whereas the distal part displayed either paler coloration like the base color in comparison with the variegated part or white coloration. In several “type I” cultivars, the variegation scale and pigmentation pattern in the petals differed depending on the cultivar; consequently, a further five sub-types of flower color pattern were generated (Fig. 1B). The areas of the colored part of the adaxial and abaxial sides of the “sub-type I-a” petals were smaller than those of the “sub-type I-b” petals. The flower color pattern of ‘Koigokoro’ (sample number 66, refer to Table 1) could not be classified without careful observation since the petals displayed very pale coloration. The flower color patten could be classed as “sub-type I-b”. The variegations extended to the marginal parts of the adaxial and abaxial petal sides of ‘Fabulous’ (No. 32) and ‘Cheerio’ (No. 31), respectively. The color patterns of the adaxial and abaxial sides of ‘Ganache’ (No. 74) petals were similar to the patterns of the abaxial and adaxial sides of ‘Cherry Tessino’ petals (No. 43), respectively. A notch in the marginal parts of petals were pigmented in a streak coloration pattern in “type II”, which includes the “chintz” pattern called cited by Imai (1936). Additionally, the size and number of streak variegations varied depending on the cultivar and “type II” was divided into two sub-types (Fig. 1C). The “sub-type II-a” flowers showed colored-edge picotee, but “sub-type II-b” did not. The proportions of “type I” and “type II” were prominent as compared with the other types of flower color pattern. The number of cultivars with colored-edge picotee with trace or several small marks were largely “type III”, followed by “type I” and “type II”. “Type III” is familiar as a common “picotee variegation”. The edge parts of the petals displaying picotee variegation “type III” were finely, jaggedly colored and “type III” differed from “sub-type II-a”. Furthermore, the flowers in the “type III” group displayed three pigmentation scales depending on the colored-edge size (Fig. 1D). Only the “type III” ‘Crono Golem’ (No. 87) displayed dotted variegation in the proximal parts of the petals. Thus, “type III” was classified into four sub-types according to the pigmentation scale of the petal edge and presence of dotted variegation in the proximal parts. Of the 110 cultivars investigated here, only ‘Furby’ (No. 56) had flowers with spotted variegation “type IV”, showing scattered pigmented dots in the petals. The spotted pattern of ‘Furby’ petals was similar to that of ‘Crono Golem’ with picotee variegation. The color of the edge of the petals in the central blotch variegation pattern “type V” was paler than the colored-circle part and was similar to the abaxial color of the petals. ‘Nobbio Burgundy’ (No. 77) and ‘Nobbio Blitz’ (No. 78) displayed eye spots or butterfly wing-like spots, as reported by Umiel et al. (1987), in addition to the typical central blotch that ‘Wine Cover’ (No. 70) petals displayed (Fig. 1E). Additionally, ‘Antigua’ (No. 83) exhibited a splashed-spotted pattern as a central blotch. Thus, “type V” could be classified into three sub-types. Pale-colored petals variegated with deep-colored streaks and flecks are familiar as conventional flower color pattern “type VI” (variegated pattern). One carnation cultivar, ‘Surprise’ (No. 80), had flowers combining two flower color patterns of “type VI’ and “type I” (Fig. 1F). ‘Surprise’ had petals variegated with deep reddish purple flecks and sectors, as in “type VI”. Moreover, their flecks and sectors displayed “type I”. The paler-colored petal parts except for the flecks and sectors displayed “type I”. In this study, we classified ‘Surprise’ as “type VI” as the major flower color pattern based on the petal color intensity. Thus, “type VI” was classified into two sub-types. Mottled carnation flowers of “type VII” displayed uneven petal coloration as described by Markham et al. (2000), and the abaxial side of the petals fully displayed the same variegation color as the adaxial petals (Fig. 1A).

Colorimetric characteristics of carnation petal colors.

Continued

Continued
According to the color name annotation of the RHSCC and the colorimetric components’ L*a*b* values in the deeper-colored parts of the adaxial side of petals evaluated by the colorimeter, the flower color in the 110 carnation cultivars was categorized into 15 color groups roughly as follows; yellow, pale brown (sometimes known as tea color), orange, red, deep pink, pink, pale pink, dark red, purple, pale purple, lilac, pale violet, bluish purple, pale green, and white (Fig. 2; Table 1). The RHSCC color codes of ‘Cassata’ (No. 6) corresponded to the orange group; however, the flower color was classified as pale brown by perceptual recognition (Table 1).

Distribution of carnation flower color based on chromatic components a* and b*. The a* and b* values from variegations of bicolor flowers and blade parts of single-colored flowers are plotted in this graph. For ‘Maracuya’ (No. 16), ‘Lion King’ (No. 20), and ‘Leon Salmone’ (No. 21), their base colors were evaluated. The chromatic components of the adaxial side (Ad), abaxial side (Ab) and variegation parts (var) of the petals of the pale violet group are plotted in this graph. Photographs of the representative flowers from each flower color group are shown in this graph.
In the yellow group, the lightness component L* values of the petals ranged around 90, and the chromatic components a* and b* values were in the range of −14.8 to −6.6 and 22.8 to 49.7, respectively (Table 1). In this study, we classified bicolor cultivars with petals comprising cyanic distal parts and yellowish proximal parts into the orange group. Approximately 13.6% cultivars of the investigated flowers belonged to the orange group, and all bicolor cultivars except for ‘Ateca’ (No. 14) in the orange group were the “type II” flower color pattern. The flower color pattern of ‘Ateca’ was “type III”. The flower color from cultivars in the orange group distributed widely between yellow and red groups based on the colorimetric data. The variegation colors of the orange group displayed deep pink, pink, and red colors in addition to orange. On the other hand, the base color of the petals was limited to the same color as the yellow and orange groups. Only ‘Cameron’ (No. 17) had petals with pale-pink-colored variegation and a yellow base. The L* values of the petals in the red group ranged from 36.6 to 48.7, and the a* and b* values ranged from 48.2 to 64.0 and 23.5 to 38.4, respectively. Pink-flowered cultivars accounted for the majority of the investigated cultivars, and they could be divided into three groups by the a* and b* components (Fig. 2). The highest number of cultivars belonged to the deep pink group in the investigated carnation samples, and the percentage was ca. 18.2%. The L* value of the deep pink flowers was in a range from 46.2 to 74.9, and the a* and b* values ranged from 31.9 to 66.9 and from −5.6 to 19.4. The L* values of the pink and pale pink groups tended to be larger in comparison with those of the deep pink group, whereas their a* and C* values tended to be smaller. The b* values did not differ markedly among the deep pink, pink and pale pink groups. The a* and C* values from the pale pink group were lower than those in the pink group, resulting in separation of the pale pink group from the pink group. The L* and C* values of the petals of the dark red group were relatively smaller in comparison with those of the other groups and dark-red-flowered cultivars were separated from cultivars in the red and deep pink groups (Fig. S1). The a* and b* values in the dark-red-grouped petals ranged from 23.8 to 60.0 and from 4.0 to 20.6, respectively. The base parts of ‘Royal Tessino’ (No. 76) and the two Nobbio cultivars, ‘Nobbio Burgundy’ (No. 77) and ‘Nobbio Blitz’ (No. 78) visually had a more bluish petal color in comparison with the other cultivars in the dark red group. The petal color of the purple group tended to be darker than that of the other groups in the dark red group. The a* and b* values of petals of the purple group except for ‘Purple Night’ (No. 79) and ‘Antigua’ (No. 83) ranged from 56.7 to 69.5 and −21.5 to −10.2, respectively. The colorimetric values of purplish-red-flowered ‘Purple Night’ and ‘Antigua’ with petals colored like brash marks were distributed as outliers (Figs. 2 and 3). It was not easy to visually distinguish the petal color of the pale purple group from that of the pale pink group, although the b* values tended to be smaller than those of the pale pink group (Fig. 2). The a*-b* and C*-H° values of cultivars with lilac-colored flowers were different from the cultivars in the purple group (Figs. 2 and 3). The L* values of the petals in the lilac group ranged from 51.5 to 66.7, and the a* and b* values ranged from 44.4 to 46.8 and −20.1 to −12.3, respectively. There were two cultivars, ‘Metalica’ (No. 95) and ‘Metalica Rose’ (No. 96), that displayed peculiar colors as described by Markham et al. (2000) and Okamura et al. (2013). The adaxial and abaxial sides of the petals of ‘Metalica’ displayed bluish and grayish coloration and their flower colors appeared pale violet to the naked eye (Fig. 2). The variegation and abaxial side of the petals of ‘Metalica Rose’ also displayed bluish and grayish petal coloration the same as ‘Metalica’, whereas the base color of the adaxial side displayed a deep pink color. In the colorimetric analysis using a* and b* values, the two cultivars with peculiar-colored petals were separated from the other cultivars in the purple and lilac groups. Additionally, the peculiar-colored petals had lower C* values than those of the purple group (Fig. 3), suggesting greyish coloration. Here, ‘Metalica’ and ‘Metalica Rose’ were categorized into the pale violet group to distinguish them from the purple and lilac groups. The four transgenic cultivars in the “Moondust series” (No. 97–100), known as blue carnations, were also categorized into the bluish purple group to distinguish them from the other cultivars in the purple, pale purple, lilac, and pale violet groups (Table 1). The cultivars in the bluish purple group had lower H° values in comparison with the purple, lilac and pale violet groups. The petals of the pale green group displayed narrow ranges of lightness and chromatic values. The petal color of the pale green group could be distinguished from that of the yellow group by RHSCC color code and L* values, but not by a* and b*. The L* values ranged 80.6 to 86.1, and the L* values tended to be lower than those of the yellow group. The a* and b* values of almost all the petals of the pale green group ranged from −17.2 to −10.5 and 27.9 to 50.3, respectively. On the basis of the RHSCC color code, ‘Jig’ (No. 106) had a more yellowish color in comparison with the other cultivars in the pale green group (Fig. 2; Table 1). The petals of the white group ranged around 90 in terms of L* value, and the a* and b* values were close to zero.

Flower color distribution of flowers in the bluish purple and the purple groups. Bluishness of single color and variegation color of petals from cultivars in the two flower color groups were compared by hue angle value. Hue angles from 270 to 360 show as a blue to red color. Flower colors of the “Moondust series” (No. 97–100) are distributed in a hue angle around 320, appearing as purple. Flower colors of the purple, lilac and pale violet groups (No. 79–96) are distributed between hue angles 330 to 350 appearing reddish purple. Hue angle around 360 shows a reddish color. ba, base part of a petal; var, variegation of a petal; ad, adaxial side of a petal; ab, abaxial side of a petal.
The composition of anthocyanins, Ch2'G, other colorless or pale yellow flavonoids and chlorophyll in each individual cultivar was determined by HPLC analysis. The cultivars in the yellow group had Ch2'G as a major flower pigment and no or trace amount of anthocyanins in the petals (Fig. 4; Table 2). The cultivars except for ‘Chappy’ (No. 2) and ‘Golden Chappy’ (No. 3) tended to accumulate less TA as compared with the other flower color groups. Chlorophyll was detected in the petals of ‘Golden Chappy’, although the cultivar belonged to the yellow group. The petals of ‘Cassata’ (No. 6) in the pale brown group accumulated a small amount of Pg-based anthocyanins and trace Ch2'G content in addition to chlorophyll. The variegations of the petals of the orange-grouped cultivars had either Pg3MG or Pg3,5cMdG as the major pigments. Otherwise, the petals of the orange group accumulated an equal or larger amount of Ch2'G than the yellow-flowered carnation ‘Liberty’ (No. 1). However, no cultivars accumulating Cy-based anthocyanins and Ch2'G as the major flower pigments were observed.

HPLC chromatograms of color flavonoids and chlorophyll in representative carnation cultivars.

Composition of flower pigments in petals of carnation cultivars.

Continued

Continued
The petals of cultivars in the red group accumulated Pg3MG and Pg3G as the major and secondary anthocyanins, respectively. The cultivars in the red group had no or trace amount of Ch2'G. Chlorophyll was detected in the petals of ‘Fabulous’ (No. 32) and ‘Napoleon’ (No. 33) in addition to anthocyanins. The petal colors of deep pink, pink, and pale pink groups were correlated with the concentration of anthocyanin contents in the petals. In the petals of many cultivars in the deep pink and pink groups, Pg3,5cMdG was detected as a major anthocyanin and Pg3,5MdG and Pg3,5dG were detected as secondary anthocyanins. However, the ‘Mini Tiara Pink’ (No. 45) petals in the deep pink group accumulated Pg3,5MdG and Pg3,5dG as the major flower pigments and a small amount of Ch2'G in the absence of Pg3,5cMdG accumulation, suggesting that the Pg3,5MdG was a precursor of its macrocyclic form. The major flower pigment of ‘Carnet’ (No. 35) and ‘Mini Tiara Spinel’ (No. 36) in the deep pink group and ‘Furby’ (No. 56) in the pink group was Pg3MG, and was responsible for red petal coloration. The petals of ‘Montezuma’ (No. 58) in the pink group mainly accumulated Cy3MG and Cy3G. The variegated flowers of ‘Pink Montezuma’ (No. 60) in the pink group, accumulated both Cy- and Pg-based anthocyanins as major flower pigments. The variegation of the petals of ‘Pink Montezuma’ had Cy3MG as the major flower pigment, whereas the base parts showed predominant accumulation of Pg3MG. In the deep pink and pink groups, chlorophyll was detected in the petals of only ‘Ipanema’ (No. 37). The petals of almost all cultivars in the pale pink group had Pg3,5cMG as the major pigment, whereas the petals of ‘Panther’ (No. 62) and ‘Subaru’ (No. 67) showed dominant accumulation of Pg3MG and Cy3,5cMdG, respectively. The petals of cultivars in the dark red group had Cy3MG and Cy3G as the major and secondary pigments, respectively. The cultivars except for ‘Wine Cover’ (No. 70) in the dark red group had trace amounts, or the absence of, Ch2'G in the petals. Chlorophyll was also detected in the petals of ‘Wine Cover’, ‘Ganache’ (No. 74) and ‘Miyabi’ (No. 75). The petals of the purple, pale purple and lilac groups accumulated Cy3,5cMdG and Cy3,5MdG as major and secondary pigments, respectively, with trace amounts or the absence of, Ch2'G. In the petals of ‘Antigua’ (No. 83) in the purple group, chlorophyll was detected. Otherwise, the peculiar-colored petals of ‘Metalica’ (No. 95) and ‘Metalica Rose’ (No. 96) in the pale violet group had deacyl anthocyanins, whereas no malyl anthocyanins were detected. In the petals of these cultivars, Pg3,5dG was detected as the major anthocyanin, and Pg3G and Cy3,5dG were also detected as the minor anthocyanins. The four “Moondust series” cultivars belonging to the bluish purple group can synthesize Dp-derivatives in the petals (Fukui et al., 2003). In addition to Dp-based anthocyanins, Cy-based anthocyanins were also detected in the petals of ‘Aqua Blue’ (No. 97) and ‘Lilac Blue’ (No. 100), whereas ‘Velvet Blue’ (No. 98) and ‘Princes Blue’ (No. 99) accumulated Pg3,5dG together with Cy-based anthocyanins. Additionally, Ch2'G was not detected in the petals of the “Moondust series” cultivars. The cultivars in the pale green group accumulated chlorophyll in the petals, but the cultivars contained no anthocyanins and only a trace amount of Ch2'G. Only ‘Jig’ (No. 106) had Ch2'G as a major flower pigment in addition to chlorophyll, resulting in a more yellowish flower color in comparison with other cultivars in the pale green group. No anthocyanins, or only a very small amount, were detected in the petals of the white group, and Ch2'G was not detected in this group except for ‘Star Snow Tessino’ (No. 108). The cultivars in the white group accumulated colorless or pale yellow flavonoids mainly in the petals, and especially ‘Star Snow Tessino’ had a larger TF as compared with the other cultivars. On the other hand, ‘Mantua’ (No. 109) bearing pure white petals only had a markedly small level of TF.
The ratio of the accumulation of Pg- and Cy-based anthocyanins (C/P or P/C) in an individual carnation petal was calculated. Almost all the cultivars had a ratio value less than 0.05 in the petals without the basal part, suggesting that carnation flowers that originated from conventional breeding exclusively synthesize either Pg- or Cy-based anthocyanins. However, the variegation of the orange-grouped flower ‘Cameron’ (No. 17) and the red-grouped flower ‘Cicas’ (No. 27) had C/P values of 0.103 and 0.118, respectively (Table 2). Moreover, the ratio value of the transgenic blue carnation ‘Princes Blue’ (No. 99), with Dp-based anthocyanins, was 0.373.
Flavonoid metabolism and flower color patternsWe investigated the metabolism of flavonoids in the variegation and base parts of petals in bicolor carnation flowers displaying the streak coloration pattern “type II”, colored-bottom variegation “type I” and mottled variegation “type VII”. The variegation part of the petals of the cultivars displaying “type II” tended to have larger values of both TA and TF (Fig. 5A; Table 2). Additionally, the Ch2'G contents did not differ between variegation and base parts in the petals. The cultivars with flowers exhibiting “type I” showed a tendency towards larger TA in the variegation parts than the base part, whereas no difference in TF was observed (Fig. 5B). To understand why differences in flower color intensity occur among the metallic and non-metallic petal parts from ‘Metalica Rose’ (No. 96), petal epidermises with different flower colors were used for the HPLC analysis (Fig. 1A). The adaxial surface of the ‘Metalica Rose’ petals had mottle variegation displaying a peculiar color, and the whole surface of abaxial petals displayed the same color as the mottle variegation color (Fig. 1A). The petal epidermises had a bluish and greyish color with accumulated anthocyanic vacuolar inclusions (AVIs), that is, anthocyanin aggregations, and their vacuolar sap was clear (Fig. 5C), and the petal epidermises displaying the base color had dissolved anthocyanins in the vacuoles. Furthermore, HPLC analysis using the separated petal epidermises of the adaxial and abaxial sides showed that the amount of Pg3,5dG in the variegation parts was significantly larger than the non-peculiar colored parts (Fig. 5D).

Flavonoid metabolism in different petal parts of bicolor cultivars. A. The level of flavonoid contents in flowers exhibiting streak coloration pattern “type II”, except for ‘Liberty’ (No. 1). Boxes in the diagram indicate the 25 to 75 percentiles containing horizontal lines showing the median. Whiskers show the range of the flavonoid contents. Box plots indicate outliers of flavonoid contents in variegation (var) and base parts (base) of petals of all cultivars. Asterisks indicate significant differences according to the Wilcoxon signed-rank test (P < 0.01). NS, not significant; TA, total contents of 10 types of carnation anthocyanins; TF, total flavonoids contents except for Ch2'G contents detected at 360 nm. B. The level of flavonoid contents in flowers exhibiting colored-bottom variegation “type I”. C. Transverse section of a petal from ‘Metalica Rose’ (No. 96). Scale var, 50 μm. AVI, anthocyanic vacuolar inclusion; Ad, adaxial side; Ab, abaxial side. D. The relative level of deacyl anthocyanin contents in the petal epidermises of different petal parts of ‘Metalica Rose’. Different alphabetical letters show significant differences in anthocyanin levels according to Tukey’s multiple comparison test (P < 0.05, n = 3).
The epidermal cells of velvety and non-velvety flowers were observed by microscopy. The adaxial side of the velvety flower ‘Velvet Blue’ (No. 98) had conical-papillate-like cells, but dome-shaped cells were observed in the abaxial side (Fig. 6A). On the other hand, the velvety flower ‘Velvet Rouge’ (No. 72) and non-velvety flower ‘Princes Blue’ (No. 99) had dome petal epidermal cells in both the adaxial and abaxial sides. All observed epidermal cells from ‘Velvet Blue’ and ‘Princes Blue’ had completely dissolved anthocyanins in the vacuole sap. However, ‘Velvet Rouge’ had many cells with bluish anthocyanic aggregations in the adaxial and abaxial petal sides. The aspect ratio of adaxial petal epidermal cells from the velvety flower ‘Velvet Blue’ was higher than that of the non-velvety flower ‘Princes Blue’, whereas the aspect ratio did not differ between abaxial petal epidermal cells (Fig. 6B). The aspect ratio of the adaxial petal epidermal cells from ‘Velvet Rouge’ was not different from that of ‘Princes Blue’, but a difference in the aspect ratio was noted in the abaxial petal sides.

Shape and aspect ratio of petal epidermal cells in two velvety flowers ‘Velvet Blue’ (No. 98) and ‘Velvet Rouge’ (No. 72) and a non-velvety flower ‘Princes Blue’ (No. 99). A. Photographs of adaxial petal epidermal cells (upper) and height and width averages of petal epidermal cells (n = 30). Anthocyanic condensation (AC) is shown by an arrow. Ad, adaxial side; Ab, abaxial side; Scale bar, 50 μm. B. Comparison of the aspect ratio of petal epidermal cells of velvety flowers with non-velvety flowers. Asterisks indicate significant differences in the aspect ratio according to Wilcoxon’s signed-rank test (P < 0.01, n = 30). NS, not significant.
Many cultivars with various flower color patterns have been bred from many ornamental plants including azalea, petunia and morning glory because the combination of different colors and shades of flowers makes a strong impression on people. Flowers of current carnation cultivars also have various color patterns. In the investigated carnations, the flower color pattern of the adaxial sides of the petals was visually categorized into five typical types (types I–III, V, and VI) and two minor types (types IV and VII) (Fig. 1A). Moreover, differences in the size and number of variegations contributed to an increase in the color pattern variation (Fig. 1B–F). Actually, the flower color pattern of the carnation cultivars used in this study could be classified into 16 sub-types. Here, we found seven common types from the 110 investigated cultivars in the 11 flower color patterns reported by Nakayama (2020), but the 110 samples did not include cultivars displaying the four flower color patterns he observed, that is, (1), cultivars with a few spotted variegations with a clear border to base color in a petal; (2), cultivars with all splash-spotted petals; (3), cultivars with colored-edge picotee petals with spotted stripes and clear border coloration; (4), cultivars with a ring-like coloration pattern. On the other hand, we newly found clearly different flower color patterns from those reported by Yamaguchi (1992) and Nakayama (2020), and especially the two “Nobbio” cultivars (No. 77 and 78), ‘Antigua’ (No. 83), ‘Surprise’ (No. 80), and ‘Metalica Rose’ (No. 96) displayed different patterns; a central blotch with an eye-spot, splash spots, a variegated pattern combined with colored-bottom variegation and mottled variegation, respectively (Fig. 1A, E, F). Almost all the carnation cultivars displayed the same pigmentation scale in both the abaxial and adaxial sides of the petals, whereas a difference in the pigmentation between the adaxial and abaxial sides of the petals was observed in flowers displaying central blotch variegation “type V” (Fig. 1E). This characteristic flower color appearance will surely give rise to new flower colors with two hues.
Although molecular biological studies concerning the formation of sectorial variegations and streak coloration pattern have been reported in several cultivars (Itoh et al., 2002; Umemoto et al., 2009; Morimoto et al., 2020), the mechanisms for the formation of other types of flower color pattern have remained unknown. The results from flower color investigation and metabolic analysis in this study give clues to clarify the formation of several flower color patterns genetically (types I, II, and VI) in carnations. TA and TF of the variegation parts of the petals displaying a streak coloration pattern were larger than those of the base parts, and the Ch2'G amount between the variegations and the base part showed no difference (Morimoto et al., 2020). Furthermore, it was suggested that the amount of flavonoids accumulated in the variegation and base parts was regulated by spatial expression of genes coding for cinnamoyl-CoA reductase-like, which is involved in lignin biosynthesis branched from the flavonoid biosynthetic pathway. In this study, the flowers with streak variegations also had higher TA and TF contents in the variegation parts in comparison with those of the base parts, while the biosynthetic activity for Ch2'G did not differ (Fig. 5A). Therefore, the molecular regulation to form streak variegation may be common to all carnation cultivars with flowers displaying a streak coloration pattern. As for colored-bottom variegation, only a difference in anthocyanin contents was noted between the base part and variegation, whereas there was no difference in TF between the two petal parts (Fig. 5B). Some petunia cultivars have picotee flowers with a white-colored margin and a cyanic colored base part. The flower color pattern of petunia is similar to the colored-bottom variegation pattern of carnations. The white-colored parts of petunia flowers are caused by larger accumulation of cinnamic acids and smaller accumulation of flavonols through post-transcriptional regulation, namely silencing, of the chalcone synthase gene which plays a role in anthocyanin biosynthesis (Saito et al., 2007; Morita et al., 2012). Our preliminary analysis for detection of flavonoids containing cinnamic acid and caffeic acid derivatives suggested that larger amount of the products could be detected at 330 nm absorption in the base parts of the petals than the variegation (Fig. S2). Therefore, the colored-bottom variegation in carnations may be involved in silencing, but more detailed analysis using flower development according to analysis of picotee in petunia is required. In many ornamental flowers with variegated patterns, excision of a transposable element from anthocyanin biosynthetic genes is related with variegation in the flower color (Itoh et al., 2002). The investigated carnation cultivars possessing the variegated pattern “type VI” displayed deeper cyanic-colored variegation on the pale-colored base of the petals. Therefore, the flower color pattern of these cultivars is very likely caused by excision of a transposable element from flavonoid biosynthetic genes. Interestingly, only ‘Surprise’ (No. 80) of the investigated carnations had colored-bottom variegation in addition to the variegated pattern (Fig. 1F). The variegated pattern also displayed colored-bottom variegation and the pigmentation scale of the distal part of the variegation corresponded to that of the base part. Therefore, the flower color pattern of ‘Surprise’ is related to both silencing and transposition. The flowers of the four cultivars, containing ‘Nobbio Burgundy’ (No. 77) and ‘Nobbio Blitz’ (No. 78), displayed central blotch variegation “type V” and mainly accumulated Cy-based anthocyanin. Dr. Giacomo Nobbio (Sanremo city, Italy) produced the “Nobbio series”, including these cultivars, by using wild type Dianthus species, especially D. chinensis, to develop carnation flowers with novel flower color patterns (Sparnaaij and Demmink, 1983). Umiel et al. (1987) crossed spray carnations with several wild Dianthus species and produced carnations with various flower color patterns, including eye and ring variegation patterns. Many wild flowering plants have flowers with spots for pollinators and their spot patterns are formed via a spatiotemporal expression pattern of duplicated gene copies coding for dihydroflavonol 4-reductase (DFR) and temporal expression of flavonoid 3'-hydroxylase (F3'H) to produce cyanidin in Clarkia gracilis (Glover et al., 2013). Therefore, the central blotch variegation in carnations may also be formed by expression patterns of flavonoid biosynthetic genes regulated spatiotemporally in the petals in the same manner as C. graculis since almost of the wild Dianthus species and their hybrids accumulate Cy-based anthocyanin dominantly and possess multiple DFR genes in their genomes (Yagishita et al., 2003). The ‘Antigua’ (No. 83) flowers exhibited splashed spotted coloration like blush marks in addition to central blotch variegation. The colored patterns were similar to the flower color pattern, namely a flake-variegated pattern, generated by operation of two key genes in modern sweet peas (Yagishita et al., 2018). This implies the mechanism of blush mark pigmentation in carnations may have something in common with that of sweet pea. In ‘Metalica Rose’ (No. 96) with a mottled variegation pattern (Fig. 1A), the metallic color parts of the petals accumulated significantly larger amounts of deacyl anthocyanins as compared with the non-peculiar-colored parts (Fig. 5D). The preliminary experiment with completely metallic-flowered carnation ‘Metalica’ (No. 95) and a light control, which is a well-known regulator for anthocyanin biosynthesis, showed that when the bud harvested at a young flower bud stage and bloomed under interior light, the flower color was deep pink, but not metallic blue grey (Fig. S3). These results suggested that the formation of AVIs, a cause of peculiar color, can be attributed to the high level of anthocyanin accumulation; however, the cause of the mottled variegation was unclear. Although the putative mechanisms for the formation of picotee and spotted variegations “types III and IV” remain unknown, the genetically putative mechanisms of the other flower color patterns will contribute to the development of studies on genetic regulation to form flower color patterns in carnations.
According to the visual investigation of flower color using the RHSCC and colorimetric analysis, the flower color range in the current carnations was more extensively distributed in comparison with those of the cultivars investigated by Yokoi (1973), Maekawa and Nakamura (1977), and Yamaguchi (1989). In the commercial cultivars used here, the red and pink groups were prominent the same as the former investigations of flower color in the 20th century, while the proportions of cultivars bearing orange, dark red and purple flowers increased. The investigated materials included ‘Nobbio Blitz’ (No. 78) displaying black coloration in the variegation that accumulated the largest TA than the other cyanic cultivars (Tables 1 and 2). The black-colored carnation flowers are likely due to the high concentration of Cy-based anthocyanin the same as in black-flowered dahlias (Deguchi et al., 2016). Interestingly, there were no cultivars composed of Cy-based anthocyanins with Ch2'G as the major flower pigment, which yields a maroon flower color (Yamaguchi, 1989). This fact suggests that commercialization of carnation flowers displaying a chestnut flower color is limited in the current flower market. Conversely, the 110 carnation cultivars included a relatively large number of pale-green commercial plants accumulating chlorophyll as the major flower pigment. Additionally, the pale-green-grouped carnation ‘Jig’ (No. 106) displayed a more yellowish flower color, which was likely caused by the accumulation of a large amount of Ch2'G in comparison with the other pale-green-grouped cultivars (Table 2); however, we did not compare the concentration of carotenoid contributing to yellow coloration among the cultivars. Cultivars with greenish flowers were found in this investigation, and they accumulated chlorophyll in addition to anthocyanins. The correlation between phenotypic color and the combination of anthocyanin and chlorophyll has been studied in vegetables and fruits, but not adequately in flowers. High concentrations of chlorophyll and anthocyanin lead to an increase in darker colors and a reduction in the a* value in the fruit skin color of eggplants and apples (Nothmann et al., 1976; Lancaster et al., 1994). However, the effect of chlorophyll accumulation on the petal color of carnation cultivars that accumulate a large amount of anthocyanin was small. Additionally, the chlorophyll concentration in pale green carnation flowers is thought to be low (Ohmiya et al., 2014). Therefore, the masking of the color influence due to chlorophyll accumulation may be attributable to an overly high ratio of anthocyanin concentration to chlorophyll concentration. In dark-purple-colored spot tissues of the inner perianthes from Alstroemeria cultivars, anthocyanin accumulation, along with the presence of chlorophyll and carotenoid, is thought to add a darkish brown color to the original color (Tatsuzawa et al., 2003). The colored petals of ‘Wine Cover’ (No. 70) and ‘Antigua’ (No. 83) and the base parts of the petals of ‘Fabulous’ (No. 32) and ‘Ganache’ (No. 74) accumulated both anthocyanin and chlorophyll and visually displayed a dull color which may be produced in the same manner as the spot tissue colorations of Alstroemeria flowers. In ‘Cassata’ (No. 6), the base part of ‘Miyabi’ petals (No. 75) and the distal part of the abaxial side of ‘Ganache’ petals were a pale brown color (Fig. 1A, B). The apple fruit ‘Granny Smith’ has a bronze skin color caused by a very low ratio of anthocyanin concentration to chlorophyll (Lancaster et al., 1994). The pale brown color of carnation petals may be also attributable to a low ratio of anthocyanin to chlorophyll content in the petals. Further quantitative analysis of pigments is needed to clarify the relationship between flower color and the concentration ratio of anthocyanin to chlorophyll in the petals.
The eight major anthocyanin types contributing to flower coloration have been isolated from non-transgenic carnation flowers (Sasaki et al., 2013), and unidentified anthocyanin (An1) and Pg3,5dG were detected as the major flower pigments of the spray carnation ‘Mini Tiara Pink’ (No. 45) and its sport ‘Mini Tiara Coral Pink’ (Sugihara et al., 2019). A putative non-cyclic malylated diglucoside of cyanidin or pelargonidin was detected as a minor anthocyanin in the petals of almost all the cultivars dominantly accumulating Cy3,5cMdG or Pg3,5cMdG. These results suggest that the two ring cleavage anthocyanins are counterparts of macrocyclic malyl anthocyanins. In the flavonoid biosynthetic pathway of carnation flowers, the enzymatic pathway concerning “cyclase”, which plays an important role in the cyclic malyl diglucoside synthesis of anthocyanin, remains unknown (Sasaki et al., 2013). Therefore, genetic analysis using ‘Mini Tiara Pink’ and its sport ‘Mini Tiara Coral’ will elucidate the final reactive step from non-cyclic to macrocyclic anthocyanins.
According to the chromatographic analysis, cyanic-colored carnations basically had one major anthocyanin type in the petals and accumulated either Pg- or Cy-based anthocyanins dominantly as reported in previous studies (Ootani and Miura, 1961; Okamura et al., 2012, 2013). This study also indicated that no carnation cultivars except for a variegated carnation ‘Pink Montezuma’ (No. 60) had both Pg- and Cy-based anthocyanins as the major flower pigment in a petal. Previously, Yamaguchi (1992) introduced an interspecific hybrid of D. caryophyllus and other Dianthus species with flowers possessing center spots and base parts accumulating Cy-based and Pg-based anthocyanins, respectively. There are some ornamental flowers that accumulate both Pg- and Cy-based anthocyanins as major flower pigments and the varying ratios of Pg and Cy extend the flower color range (Iwata et al., 1985; Torskangerpoll et al., 2005; Deguchi et al., 2016). However, carnation cultivars accumulate either Pg- or Cy-based anthocyanin dominantly. In the case of Dianthus plants, the coexistence of pelargonidin and cyanidin is limited by a single gene, F3'H, and its heterozygous form causes dominant biosynthesis of Cy-based anthocyanins since carnation DFR prefers dihydroquercetin (precursor of cyanidin) as a substrate rather than dihydrokaempferol (precursor of pelargonidin) (Stich et al., 1992; Momose et al., 2013). These facts may lead to a lower than 0.05 C/P ratio value, and vice versa. ‘Chicas’ (No. 27) with red flowers had a relatively high C/P ratio, whereas the petal color did not differ from the other typical red cultivars. In rose (Rosa × hybrida) flowers, coaccumulation of pelargonidin and cyanidin did not affect the petal colors (Biolley and Jay, 1993). Therefore, exclusive accumulation of cyanidin and pelargonidin in variegations and base parts of petals as in ‘Pink Montezuma’ may be a breakthrough that enables novel flower color breeding in carnations.
Transgenic blue carnations with the flavonoid 3',5'-hydroxylase gene that is absent in native carnation cultivars, have been marketed since 1997 (Tanaka et al., 2005). The molecular biological approach succeeded in producing carnations with more bluish colored flowers. In this study, the colorimetric analysis showed that the flower colors of transgenic carnation flowers accumulating dominantly blue pigment Dp-based anthocyanins were more bluish as compared with the other native cultivars used in this study due to the additional hydroxylation of the anthocyanin B-ring (Fukui et al., 2003; Noda et al., 2017). Meanwhile, the non-transgenic carnations ‘Metalica’ (No. 95) and ‘Metalica Rose’ (No. 96) with a metallic flower appearance also displayed a relatively bluish hue (Fig. 3). AVIs are present in the vacuoles of petal epidermal cells of cultivars with non-malylated anthocyanins, resulting in a bluish petal coloration known as “grey-blue” (Markham et al., 2000; Okamura et al., 2013). Microscopic observation also found the presence of AVIs in the petal epidermal cells of the two peculiar-colored carnations (Fig. 5C), and a high condensation level of anthocyanin was observed in metallic-colored petals the same as reported by Okamura et al. (2013). Additionally, chromatographic analysis revealed that the peculiar and bluish flower color is caused by AVIs formation since ‘Metalica’ and ‘Metalica Rose’ lacked chlorophyll and had only trace amounts or lacked Ch2'G. Breeding for the cultivars with petals forming Dp-derived AVIs will enable production of more bluish flowers and the coaccumulation of AVI-formed anthocyanins and other color pigments (e.g., chlorophyll and Ch2'G) may produce novel flower colors.
Flower color is known to be affected by the presence of colorless or pale yellow flavonoids. Onozaki et al. (1999) investigated flower color and the flavonoid compositions in white-flowered cultivars, and the white flowers were classified into three types based on their pigment composition. However, we did not identify flavonols and flavones, which were identified by Fukui et al. (2003), Galeotti et al. (2008), and Iwashina et al. (2010), in this investigation. The white-grouped ‘Mantua’ (No. 109) had a much smaller TF as compared with the other white cultivars, resulting in an almost pure white petal color, the same results as those reported by Onozaki et al. (1999). Colorless or pale yellow flavonoids play an important role as co-pigments to change flower color (Fukui et al., 2003).
The cultivars in the purple group accumulated Cy3,5cMdG as a major flower pigment and this resulted in bluish flower coloration, whereas ‘Purple Night’ (No. 79) flowers and the variegated parts (var-1) of ‘Surprise’ (No. 80) visually looked more reddish than the other purple-grouped cultivars (Table 1). However, an investigation of the ratio of the TF to TA (copigmentation index, CI) did not clarify any flower color difference between the two cultivars and those of the other purple cultivars (data not shown). The base parts of the petals of ‘Royal Tessino’ (No. 76), ‘Nobbio Burgundy’ (No. 77), and ‘Nobbio Blitz’ (No. 78) displayed a stronger blue color than the other cultivars in the dark red group, even though they accumulated Cy3MG as the major flower pigment the same as the other cultivars in the dark red group (Tables 1 and 2). In addition, a higher CI ratio was found in the base parts of the two “Nobbio” cultivars, except for ‘Royal Tessino’, in comparison with the variegation parts. The flower color of non-transgenic cultivars may also be influenced by co-pigmentation, but the marginal coloration system of ‘Royal Tessino’ is unknown. We examined colorimetric components in other Dianthus species including hybrids with D. chinensis to understand the genetic background of “Nobbio” cultivars and the tendency was for the b* value to be negative even though their flowers accumulated Cy3MG dominantly (Fig. S4). Therefore, the regulation of flower coloration in the “Nobbio” cultivars may be strongly affected by the coloration system from wild Dianthus flowers. Additionally, these flower color investigations indicated that the breeding reproduction of cultivars such as the “Nobbio” series with the flower color pattern “sub-type V-b” was possible by interspecific crossing using D. chinensis with carnations.
Optical properties are also one of the factors that influence the perceived color. “Velvety lustre texture” is an important petal appearance characteristic in the flower market. The ornamental flowers exhibiting velvety texture generally have the petal conical papillary cells that accumulate a high level of anthocyanins (Zhang et al., 2015). ‘Velvet Blue’ (No. 98) with Dp-based anthocyanin as the major flower pigment also had velvety petals, whereas the petals of other cultivars accumulating Dp-based anthocyanin did not have a velvety texture. To investigate what is involved in the velvety texture, microscopic observation of petal epidermis was performed. The result indicated that the aspect ratio of length to width in ‘Velvet Blue’ with oblong-shaped petal epidermal cells was significantly higher as compared with that of ‘Princes Blue’ (No. 99) with dome-shaped cells (Fig. 6A). As a result, the velvety texture of ‘Velvet Blue’ may be caused by the shape of petal epidermal cells and a high concentration of Dp-based anthocyanins. A velvety cultivar ‘Velvet Rouge’ (No. 72) also had petals with a lustrous velvety texture, but the aspect ratio of ‘Velvet Rouge’ did not differ markedly from the non-velvety ‘Princes Blue’ (Fig. 6B). Furthermore, the petals of ‘Velvet Rouge’ contained epidermal cells with blue AVI-like anthocyanic condensations as observed in the abaxial sides of petal epidermal cells of metallic crimson carnations (Okamura et al., 2013). Even though the uneven presence of many AVI-like aggregations was found, the petals had a velvety appearance, but not metallic or dusky colors. Furthermore, the petal color was similar to those of ‘Bagnacavallo’ (No. 71) and ‘Arabesque’ (No. 73) in the dark red group (Table 1). Interior reflected light (IRL) from inside the petal cells is an important element that determines velvety appearance (Zhang et al., 2015). Therefore, we hypothesize that the scatter of anthocyanic aggregations affects our texture perception through changes in IRL reflection strength.
In this study, we categorized flower color patterns and flower colors in commercially available carnation cultivars. Visual and colorimetric analyses indicated that flower color appearance had more variation in comparison with previous studies of flower color characteristics prior to the 21th century. Through chromatographic analysis, the relationship between flower color and flower pigment composition was clarified. The participation of green pigment chlorophyll together with multiple cyanic pigment anthocyanins and yellow pigment Ch2'G contributed to the extension of the flower color range. In addition to the conventional Pg- and Cy-based anthocyanins, the introduction of Dp-based anthocyanins produced by a transgenic process expanded the flower color distributions and is expected to be a turning-point to increase the number of flower pigment combinations. Flower color is affected by pigment composition, but also petal surficial texture, anthocyanin condensation, and copigmentation. Furthermore, in carnations with various flower color patterns composed of different flower colors, the combination of variegation and base color is also important to increases the number of new cultivars. The fundamental information concerning flower color appearance and floral pigmentation clarified in this study will contribute to the further development of carnation breeding and production of commercial products with novel flower colors.
We gratefully thank Inochio Fujiplants Inc., Miyoshi Group Co., Ltd., Koukaen and Kagawa Prefectural Agricultural Experiment Station for providing cut carnation flowers and information concerning carnation cultivation. We would like to thank Dr. Masayoshi Nakayama, NARO Institute of Vegetable and Floriculture Science, for fruitful discussions.