2021 Volume 90 Issue 4 Pages 450-459
2021 Volume 90 Issue 4 Pages 450-459
Yellow color in dahlia flowers is conferred from chalcones, butein and isoliquiritigenin. The color intensity of yellow dahlia cultivars is diverse, but a detailed study on this has not yet been performed. In this study, we first identified structures of flavonoids by nuclear magnetic resonance imaging in ray florets of the red-white bicolor ‘Shukuhai’, which contains chalcones, flavones and anthocyanins. Four anthocyanins, four flavone derivatives, five isoliquiritigenin derivatives and five butein derivatives were identified. Among the identified compounds, butein 4'-malonylsophoroside is considered to be the final product for butein derivatives and the presence of chalcone 4'-glucosyltransferase, chalcone 4'-glucoside glucosyltransferase, and chalcone 4'-glucoside malonyltransferase for isoliquiritigenin and butein modification was predicted. Also, the biosynthetic pathway of butein and isoliquiritigenin derivatives in dahlia with butein 4'-malonylsophoroside as the final product was predicted from the identified compounds. Next, we used nine yellow cultivars and lines with different color intensities and analyzed the correlation between the b* value, an indicator of yellow color, and level of chalcones. There was no difference in the presence or absence of major peaks among the cultivars and lines. Peak area per fresh weight measured by HPLC was high in butein 4'-malonylglucoside, butein 4'-sophoroside and isoliquiritigenin 4'-malonylglucoside, suggesting these three compounds were accumulated abundantly. Among the identified chalcones, the highest correlation coefficient was detected between the b* value and butein 4'-malonylglucoside (r = 0.86), butein 4'-sophoroside (r = 0.82) or isoliquiritigenin 4'-malonylglucoside (r = 0.76). These results suggest that these three chalcones confer yellow color in dahlia ray florets. The findings in this study will contribute not only to efforts at breeding new yellow dahlia cultivars, but also to molecular breeding of yellow flowers in other species by introducing the butein biosynthetic pathway.
Dahlia (Dahlia variabilis) is one of the most popular ornamental plants as cut flowers or garden plants because of its beautiful appearance. Dahlia has huge variations in flower color, shape and size due to its high ploidy (Gatt et al., 1998). In particular, flower color is diverse, and various color intensities of red, purple, pink, ivory white, yellow and black cultivars have been bred. A determinant factor for the color intensity of cyanic cultivars was revealed by a previous study (Ohno et al., 2013b); however, a determinant factor for color intensity of yellow cultivars has not yet been elucidated.
In contrast to other Asteraceae species such as chrysanthemum or sunflower which accumulate carotenoids as yellow pigment in flowers, the yellow color in dahlia ray florets is conferred by chalcones, butein and isoliquiritigenin (Price, 1939; Nordström and Swain, 1956; Saito et al., 1970; Harborne et al., 1990). Yellow flower cultivars accumulate chalcones and flavones in their ray florets (Ohno et al., 2013a). Butein accumulation is found in dahlia leaves (Ohno et al., 2018) and in flowers of some limited species such as two Coreopsis species (C. douglasii and C. gigantea) (Geissman, 1941a, b), Cosmos sulphureus (Geissman, 1942) and Bidens (Scogin and Zakar, 1976). Alhough butein itself functions as a yellow pigment, but it is also used as a precursor of aurone in Coreopsis grandiflora, in which the step is catalyzed by polyphenol oxidase (Kaintz et al., 2014; Molitor et al., 2016). In the presumed butein biosynthesis pathway, isoliquiritigenin is first synthesized from coumaroyl-CoA and malonyl-CoA, the same substance as other flavonoids. Chalcone synthase and chalcone reductase (CHR), specific enzymes for this biosynthetic pathway, catalyze this first step in a coordinated manner, and then isoliquiritigenin is converted to butein by chalcone 3-hydroxylase (CH3H) or another isoform of flavonoid 3'-hydroxylase (Wimmer et al., 1998; Schlangen et al., 2010a, b). The final butein product in dahlia ray florets inferred from the structure is butein 4'-malonylsophoroside (Harborne et al., 1990), but the intermediate biosynthetic pathway remains unknown.
For molecular breeding of yellow flowers, introduction of the butein biosynthetic pathway has advantages because it only modifies the existing flavonoid biosynthetic pathway. Since introduction of a few genes may be enough to biosynthesize butein, it is much easier to introduce than the entire carotenoid biosynthetic pathway. Chalcononaringein and aurone are known as yellow flavonoids in flowers (Harborne, 1966; Miyajima et al., 1991; Itoh et al., 2002; Ono et al., 2006; Saito et al., 2011; Deguchi et al., 2016a); however, yellow flower color conferred by chalcononaringein was described as “pale-yellow” in morning glory (Saito et al., 2011), and the b* value in yellow flowers of snap dragon (62.97 ± 1.45, Tatsuzawa et al., unpublished data) was lower than yellow dahlia in this study; therefore, butein results in brighter colors compared to them, so butein is a good candidate for conferring bright yellow color in flowers. Considering artificial accumulation of butein in other species, understanding of modifications such as glycosylation and acylation is essential because different levels of glycosylation or acylation of anthocyanin affected absorption spectra, and resulted in changes in color appearance in chrysanthemum (Chrysanthemum morifolium) (Noda et al., 2017).
In this study, to develop an understanding of butein biosynthesis in dahlia ray florets, we first identified flavonoids in red-white bicolor ray florets of ‘Shukuhai’ by NMR analysis. We identified four anthocyanins, 10 chalcones (five derivatives for butein and five derivatives for isoliquiritigenin) and four flavones, and the biosynthetic pathways of isoliquiritigenin derivatives and butein derivatives were predicted. Next, we measured L*a*b* values and content of each chalcone by HPLC using 10 cultivars with different yellow color intensities, and the correlation between the b* value and pigment content was analyzed to reveal which factor, difference in modification or amount of pigment was be more important for yellow coloration in dahlia.
Dahlia cultivars and lines ‘Shukuhai’ (Fig. 1A), 1-8 (Fig. 1B), 1-10 (Fig. 1C), S1 (Fig. 1D), S2 (Fig. 1E), W4 (Fig. 1F), 16-136 (Fig. 1G), ‘Shukuhou’ (SH: Fig. 1H), ‘Ittosei’ (IT: Fig. 1I), ‘Suckle Pico’ (SP: Fig. 1J), and ‘Glenbank Honeycomb’ (GH: Fig. 1K) were used for the study. ‘Shukuhai’, 1-8, 1-10, W4, 16-136, ‘Shukuhou’, ‘Ittosei’, ‘Suckle Pico’, and ‘Glenbank Honeycomb’ were grown in an experimental field at Kyoto University (Kyoto, Japan,) and inflorescences were harvested from October to November 2018 and used for this study. For S1 and S2, cut inflorescences were purchased from the market (cultivar names are unknown). 16-136, ‘Shukuhou’, ‘Ittosei’, ‘Suckle Pico’, and ‘Glenbank Honeycomb’ were purchased from Akita International Dahlia Park (Akita, Japan). ‘Shukuhai’ and ‘Shukuhou’ were obtained from Nara Prefectural Agricultural Research and Development Center. 1-8, 1-10, and W4 are seedling lines. The fully expanded ray florets were collected to measure color components and for high-performance liquid chromatography (HPLC) analysis. The ray florets of ‘Shukuhai’ were freeze-dried and used to identify flavonoids.

Dahlia cultivars and lines used in this study. (A) ‘Shukuhai’, (B) 1-8, (C) 1-10, (D) S1, (E) S2, (F) W4, (G) 16-136, (H) ‘Shukuhou’, (I) ‘Ittosei’, (J) ‘Suckle Pico’, and (K) ‘Glenbank Honeycomb’.
Thirty grams of freeze-dried ‘Shukuhai’ ray florets were immersed in 20 L of MAW (methanol:acetic acid:water = 4:1:5 v/v/v) for 24 h at room temperature, and flavonoid pigments were extracted. After concentration, the eluates were fractionated by paper chromatography using BAW (n-butanol:acetic acid:water = 4:1:2 v/v/v). The crude extracts were divided into three fractions and further purified by paper chromatography (BAW or 5% acetic acid 50% methanol) and SephadexTM LH-20 (GE healthcare Japan Corp., Tokyo, Japan) column chromatography. The amounts of purified 1-13 obtained from the ‘Shukuhai’ ray florets were as follows: 1 (23 mg), 2 (4 mg), 3 (5 mg), 4 (58 mg), 5 (3 mg), 6 (45 mg), 7 (7 mg), 8 (3.5 mg), 9 (23 mg), 10 (3.5 mg), 11 (3 mg), 12 (12 mg), 13-1 (12 mg), and 13-2 (136 mg).
Identification of chalcones and flavones by nuclear magnetic resonance (NMR)Ten chalcones and four flavones were identified by high-resolution fast atom bombardment mass spectroscopy (HR-FABMS) and nuclear magnetic resonance (NMR). NMR spectra were recorded on JNM AL-400 (JEOL Ltd., Tokyo, Japan) at 400 MHz for 1H spectra and 100 MHz for 13C spectra using CD3OD-d4 as a solvent. Chemical shifts are indicated on the δ-scale from tetramethylsilane as the internal standard, and coupling constants (J) are in Hz. 3 calc. for C24H25O12: 505.1346. Found: 505.1336; 7 calc. for C21H23O9: 419.1342. Found: 419.1345; 6 calc. for C24H25O13: 521.1295. Found: 521.1287; 8 calc. for C27H33O13: 565.1921. Found: 565.1920; 9 calc. for C30H35O18: 683.1823. Found: 683.1820; 10 calc. for C27H33O14: 581.1870. Found: 581.1868; 11 calc. for C27H31O14: 579.1714. Found: 579.1730; 12 calc. for C21H23O10: 435.1291. Found: 435.1296; 13-2 calc. for C27H33O15: 597.1819. Found: 597.1821; 13-1 calc. for C27H31O15: 595.1663. Found: 595.1685. Isoliquiritigenin (1), butein (4), apigenin (2), and luteolin (5) were identified by comparing them with a commercial standard.
Measurement of ray floret color components in yellow cultivarsColor components of the CIE L*a*b* co-ordinate were measured with a colorimeter (CR-200; KONICA MINOLTA Inc., Tokyo, Japan). The middle part on the adaxial surface of each fully expanded ray floret was measured and D65 was used for the analysis spectrum. A mean score of three different ray florets from three different inflorescences was calculated. The C* value was calculated as C* = (a*2 + b*2)1/2, and the h value was calculated as h = tan−1 (b*/a*).
HPLC analysisRay florets used for the color components were weighed and freeze dried with liquid nitrogen until analysis. Frozen ray florets were homogenized with 1 mL of extraction buffer (acetic acid:methanol:water = 1:4:5 v/v) and centrifuged for 10 min at 4°C at 15,000 rpm and the supernatant was collected. The supernatant was diluted 10-fold with the extraction buffer and absorption at 400 nm was measured by HPLC. HPLC analysis was conducted using an HPLC system (SCL-10AVP, SPD-M10AVP, CTO-10AVP. SIL-10ADVP, LC-10ADVP, DGU-12A, LC solutions software; Shimadzu Corp., Kyoto, Japan) with a C18 column (Nihon Waters K.K., Tokyo, Japan) that was maintained at 40°C. The injection volume was 20 μL. The HPLC analysis process was performed according to Ohno et al. (2011). The peak area score was divided by the fresh weight and peak area per fresh weight (mg) was calculated.
Statistical analysisCorrelations between the b* value of each ray floret and average score of peak area per fresh weight (mg) of each chalcone were analyzed by Pearson’s correlation coefficient.
The pigments accumulated in ‘Shukuhai’ ray florets were identified by HPLC and NMR analysis. We identified four anthocyanins (cyanidin 3,5-di-O-glucoside, cyanidin 3-O-[6-O-(malonyl)-glucoside]-5-O-diglucoside, pelargonidin 3,5-di-O-glucoside, and pelargonidin 3-O-[6-O-(malonyl)-glucoside]-5-O-diglucoside), the same anthocyanins detected in other cultivars previously (Deguchi et al., 2016b). As for flavones and chalcones, four flavones (apigenin, apigenin 7-O-[6-O-(rhamnosyl)-glucoside], luteolin and luteolin 7-O-[6-O-(rhamnosyl)-glucoside]) and 10 chalcones (butein, butein 4'-O-glucoside (butein 4'-glucoside: Bt4'G), butein 4'-O-[6-O-(malonyl)-glucoside] (butein 4'-malonylglucoside: Bt4'MG), butein 4'-O-[2-O-(glucosyl)-glucoside] (butein 4'-sophoroside: Bt4'S), butein 4'-O-[2-O-(glucosyl)-6-O-(malonyl)-glucoside] (butein 4'-malonylsophoroside: Bt4'MS), isoliquiritigenin, isoliquiritigenin 4'-O-glucoside (isoliquiritigenin 4'-glucoside: Iso4'G), isoliquiritigenin 4'-O-[6-O-(malonyl)-glucoside] (isoliquiritigenin 4'-malonylglucoside: Iso4'MG), isoliquiritigenin 4'-O-[2-O-(glucosyl)-glucoside] (isoliquiritigenin 4'-sophoroside: Iso4'S) and isoliquiritigenin 4'-O-[6-O-(rhamnosyl)-glucoside]) (Tables 1–3; Figs. 2 and S1) were identified. Apigenin 7-O-[6-O-(rhamnosyl)-glucoside] was reported previously (Nordström and Swain, 1953). From these results, we constructed a putative biosynthetic pathway of butein and isoliquiritigenin derivatives in dahlia ray florets (Fig. 3).

NMR spectroscopic data for isoliquiritigenin derivatives in dahlia ‘Shukuhai’ ray florets in CD3OD-d4.

NMR spectroscopic data for butein derivatives in dahlia ‘Shukuhai’ ray florets in CD3OD-d4.
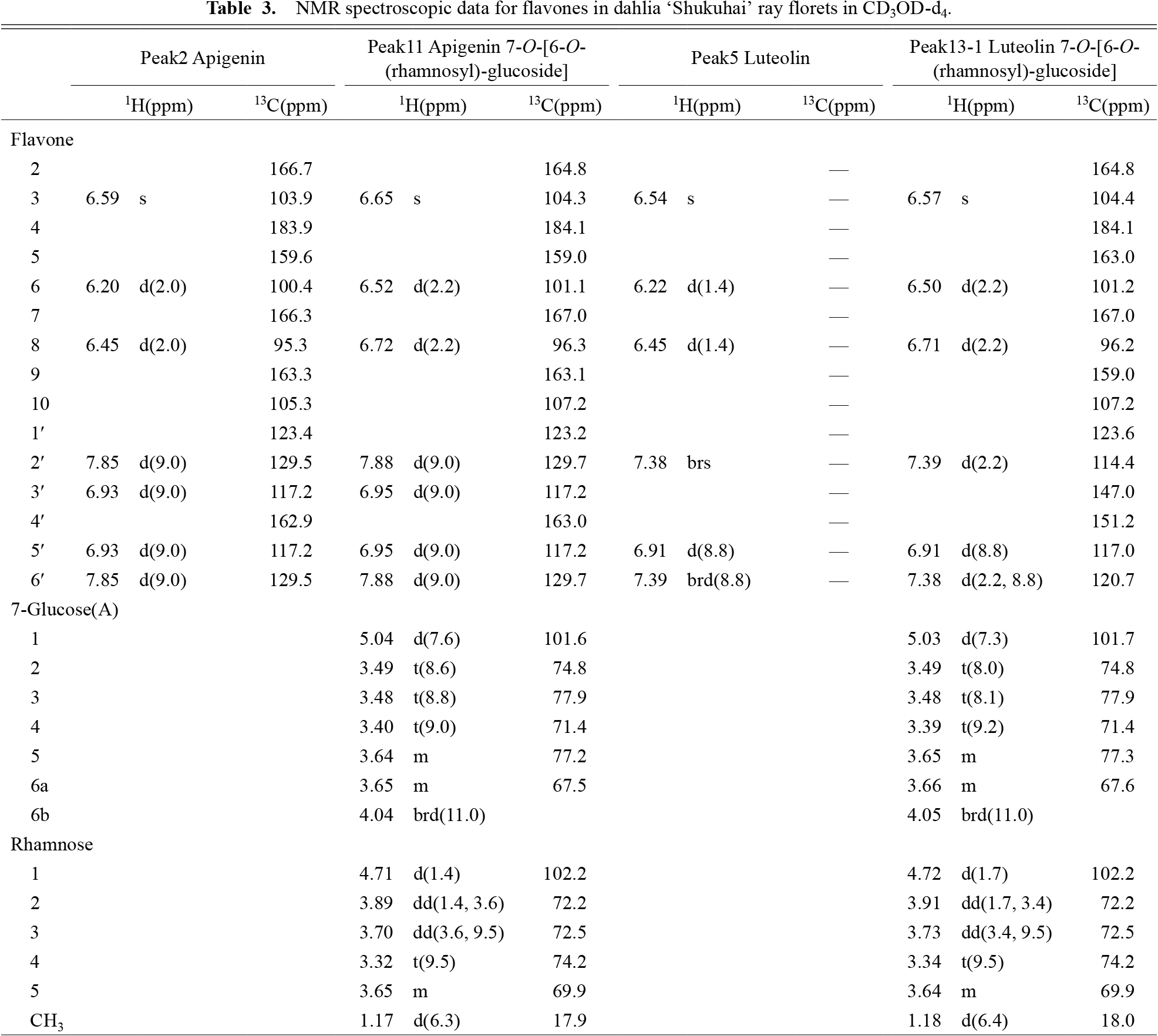
NMR spectroscopic data for flavones in dahlia ‘Shukuhai’ ray florets in CD3OD-d4.

HPLC chromatograph of flavonoids detected in extracts from ‘Shukuhai’ ray florets (A) at 350 nm and (B) at 400 nm. The peaks indicated represent the following compounds: 1, isoliquiritigenin; 2, apigenin; 3, isoliquiritigenin 4'-O-[6-O-(malonyl)-glucoside]; 4, butein; 5, luteolin; 6, butein 4'-O-[6-O-(malonyl)-glucoside]; 7, isoliquiritigenin 4'-O-glucoside; 8, isoliquiritigenin 4'-O-[6-O-(rhamnosyl)-glucoside]; 9, butein 4'-O-[2-O-(glucosyl)-6-O-(malonyl)-glucoside]; 10, isoliquiritigenin 4'-O-[2-O-(glucosyl)-glucoside]; 11, apigenin 7-O-[6-O-(rhamnosyl)-glucoside]; 12, butein 4'-O-glucoside; 13, butein 4'-O-[2-O-(glucosyl)-glucoside] and luteolin 7-O-[6-O-(rhamnosyl)-glucoside] (peak 13 contains two different compounds).

Presumed biosynthetic pathway for isoliquiritigenin and butein derivatives in dahlia. Bt, butein; Bt4'G, butein 4'-O-glucoside; Bt4'MG, butein 4'-O-[6-O-(malonyl)-glucoside]; Bt4'MS, butein 4'-O-[2-O-(glucosyl)-6-O-(malonyl)-glucoside]; Bt4'S, butein 4'-O-[2-O-(glucosyl)-glucoside]; CH3H, chalcone 3-hydroxylase; CHR, chalcone reductase; CHS, chalcone synthase; C4'GT, chalcone 4'-glucosyltransferase; C4'GGT, chalcone 4'-glucoside glucosyltransferase; C4'GMaT, chalcone 4'-glucoside malonyltransferase; C4'GRhT, chalcone 4'-glucoside rhamnosyltransferase; Iso, isoliquiritigenin; Iso4'G, isoliquiritigenin 4'-O-glucoside; Iso4'MG, isoliquiritigenin 4'-O-[6-O-(malonyl)-glucoside]; Iso4'RG, isoliquiritigenin 4'-O-[6-O-(rhamnosyl)-glucoside]; Iso4'S, isoliquiritigenin 4'-O-[2-O-(glucosyl)-glucoside].
L*a*b* values were measured in 10 yellow cultivars (Table 4) and the chroma (C*) was calculated. The L* value indicates lightness (black: 0 to white: 100). Positive a* values indicate redness and negative a* values indicate greenness, while positive b* values indicate yellowness and negative b* values blueness. C* indicates purity or saturation of the color. Among 10 yellow cultivars, L* and a* values were similar, but b* and C* values were different. Deep yellow cultivars had higher b* and C* values, while pale yellow cultivars had lower values (Table 4). We calculated the coefficient of determination between a* and C* or b* and C*, and R2 was 0.4834 between a* and C*, while R2 was 0.9991 between b* and C*, indicating the b* value is a good indicator of yellow color intensity in these cultivars. Based on the b* value, 1-8 (B), 1-10 (C), and ‘SH’ (H) were classified as deep yellow cultivars, ‘GH’ (K), W4 (F), S1 (D) and ‘SP’ (J) were classified as yellow cultivars, and ‘IT’ (I), S2 (E), and 16-136 (G) were classified as pale yellow cultivars (Fig. 1).

L*a*b*C*h values on the middle part of fully expanded ray florets.
Absorbance at 400 nm of extracted pigments was analyzed by HPLC. Among 10 cultivars, although some small peaks were different, there was no difference in the presence or absence of major peaks. Among 10 identified chalcones, although two compounds could not be specified due to small peaks, eight compounds were predicted from the retention time and the order of detected peaks. In addition, a small luteolin peak was detected from all samples. Three detected major peaks were presumed to be Bt4'S, Bt4'MG, and Iso4'MG; deep yellow cultivars had a higher peak area and pale yellow cultivars had lower peak area than other cultivars (Fig. 4). We analyzed the correlation between the b* value and each chalcone amount (Fig. 5). The type of aglycone (Bt: r = 0.41–0.86, Iso: r = 0.54–0.76), and the type of glycoside (no glycosylation: r = 0.54–0.58, glucoside: r = 0.41–0.86, sophoroside: r = 0.60–0.82) did not correlate with the b* value, while the presence of a malonyl group bond (without malonyl bond: r = 0.42–0.82, with malonyl bond: r = 0.75–0.86) tended to have a higher b* value. The highest correlation coefficient was detected between the b* value and Bt4'MG (r = 0.86), followed by Bt4'S (r = 0.82) and Iso4'MG (r = 0.76) (Fig. 5). Therefore, yellow color intensity is regulated by the amount of major chalcones, Bt4'S, Bt4'MG, and Iso4'MG, rather than the type of compound.

Peak area of chalcones in yellow cultivars and lines. Bars indicate standard error (n = 3). Compounds: Bt4'S, butein 4'-O-[2-O-(glucosyl)-glucoside]; Bt4'G, butein 4'-O-glucoside; Iso4'S, isoliquiritigenin 4'-O-[2-O-(glucosyl)-glucoside]; Bt4'MS, butein 4'-O-[2-O-(glucosyl)-6-O-(malonyl)-glucoside]; Bt4'MG, butein 4'-O-[6-O-(malonyl)-glucoside]; Bt, butein; Iso4'MG, isoliquiritigenin 4'-O-[6-O-(malonyl)-glucoside]; Iso, isoliquiritigenin. Cultivars: SH, ‘Shukuhou’; GH, ‘Glenbank Honeycomb’; SP, ‘Suckle Pico’; IT, ‘Ittosei’.

Correlation between the b* value and peak area per fresh weight (mg) measured by HPLC in ray florets. ‘Shukuhou’ was not included in this figure because it is a yellow-white bicolor cultivar and contains abundant white parts in its ray florets. Each plot indicates a different ray floret. Compounds: Bt4'S, butein 4'-O-[2-O-(glucosyl)-glucoside]; Bt4'G, butein 4'-O-glucoside; Iso4'S, isoliquiritigenin 4'-O-[2-O-(glucosyl)-glucoside]; Bt4'MS, butein 4'-O-[2-O-(glucosyl)-6-O-(malonyl)-glucoside]; Bt4'MG, butein 4'-O-[6-O-(malonyl)-glucoside]; Bt, butein; Iso4'MG, isoliquiritigenin 4'-O-[6-O-(malonyl)-glucoside]; Iso, isoliquiritigenin. Cultivars: GH, ‘Glenbank Honeycomb’; SP, ‘Suckle Pico’; IT, ‘Ittosei’.
Butein is a yellow pigment found in limited species such as dahlia, Coreopsis species, Cosmos sulphureus, and Bidens species (Price, 1939; Geissman, 1941a, b, 1942; Scogin and Zakar, 1976). Previous studies characterized the structure in dahlia as butein 4'-malonylsophoroside and butein 4'-malonylglucoside (Harborne et al., 1990), however, the detailed biosynthetic pathway including intermediate compounds has not been elucidated. In this study, we identified 4'-glucoside, 4'-malonylglucoside and 4'-sophoroside for both isoliquiritigenin and butein in ray florets of a red-white bicolor ‘Shukuhai’ dahlia (Fig. 2). One difference in modification between isoliquiritigenin and butein was that 4'-O-[6-O-(rhamnosyl)-glucoside] was detected for isoliquiritigenin, while 4'-malonylsophoroside was detected for butein. We could not identify the compounds that caused small peaks; however, these results indicate that in addition to CHR and CH3H, the presence of chalcone 4'-glucosyltransferase (C4'GT), chalcone 4'-glucoside glucosyltransferase (C4'GGT), and chalcone 4'-glucoside malonyl transferase (C4'GMaT) for isoliquiritigenin and butein modification. In addition, rhamnosyltransferase for isoliquiritigenin was indicated (Fig. 3).
The final compound was inferred by structure as butein 4'-malonylsophoroside (Fig. 3; Harborne et al., 1990). Abundant compounds were butein 4'-malonylglucoside, butein 4'-sophoroside and isoliquiritigenin 4'-malonylglucoside (Fig. 4). These three compounds showed the highest correlation coefficient with the b* value (Fig. 5), suggesting bright yellow color in dahlia is likely to be conferred by these three chalcones.
Butein is a good candidate for bioengineering bright yellow color flowers because most plant species can accumulate flavonoids in their flowers. Most flavonoids are accumulated with some modifications such as glycosylation. The basic compound for butein could be butein 4'-glucoside, or coreopsin, found in Cosmos sulphureus, Coreopsis lanceolata and C. saxícola (Shimokoriyama and Hattori, 1953). For biosynthesis of butein 4'-glucoside, CHR, CH3H, and C4'GT are required (Fig. 3). CH3H was isolated in Cosmos sulphureus (Schlangen et al., 2010b), but CHR and C4'GT for butein and isoliquiritigenin biosynthesis and modification have not yet been isolated. Thus, future isolation of these two genes will be useful to introduce the butein biosynthesis pathway to other species, as well as to clarify their expression of functions.