2022 Volume 91 Issue 1 Pages 49-57
2022 Volume 91 Issue 1 Pages 49-57
Anthracnose, caused by the fungal pathogen Colletotrichum orbiculare, is one of the severest diseases in open-field cucumber (Cucumis sativus L.) cultivation in Japan. Genetically conferred host resistance is the best way to control anthracnose. In order to develop new cultivars resistant to anthracnose, identification of promising sources of resistance is vital. In this study, we screened 232 genetic resources of Asian origin preserved in the NARO Genebank and identified one accession, ‘Ban Kyuri’, with strong, although not perfect, resistance to anthracnose. Further evaluation of the resistance based on seedling and greenhouse assays confirmed resistance. We also revealed a wide variation in the virulence of three C. orbiculare strains in Japan. ‘Ban Kyuri’ exhibited stable resistance to all strains. Genotyping of CsSGR, a known gene for anthracnose resistance, indicated that ‘Ban Kyuri’ is homozygous for the wild-type allele, and its resistance level was equal to or higher than that of accessions harboring Cssgr, the mutated allele conferring resistance. This finding indicates that ‘Ban Kyuri’ harbors a novel resistance gene or a novel allele of CsSGR conferring anthracnose resistance. ‘Ban Kyuri’ is a promising new source of resistance to diverse anthracnose strains with different levels of virulence in Japan, and its use will help to further clarify the genetic mechanism of anthracnose resistance in cucumber.
Cucumber (Cucumis sativus L.) is one of the most important cucurbits worldwide, together with melon, watermelon, and squash. In Japan, cucumber is grown in greenhouses throughout the year, except for midsummer, and in open-fields from late spring to early autumn. Open-field cultivation is cost effective, but plants are more prone to disease and pests than those in greenhouses, where it is easier to control environmental conditions. Anthracnose, caused by the fungal pathogen Colletotrichum orbiculare (Berk. & Mont.) Arx [syn. C. lagenarium (Pass.) Ellis & Halst.], is a major foliage disease in open-field cultivation of cucumber, and tends to spread in warm and humid environments. The fungus can survive on plant residues in the field for 2 years, even in the absence of a suitable host. The spread of its spores and infection depends upon water, such as rain and irrigation spray. Seed transmission is also a component of its parasitic cycle. The fungus can infect aboveground organs, such as leaves, stems, and fruit. Lesions often first appear on a leaf. At the primary stage, the lesions are small, round, and pale, either chlorotic (yellow) or necrotic (brown). At the middle to late stage, lesions become larger and the leaves die. In highly susceptible hosts, symptoms appear not only on leaves but also on stems and fruit. Damage to these organs decreases the quantity and quality of fruit. Therefore, controlling this disease is very important for the stability of cucumber production in open-field cultivation.
Genetically conferred host resistance is the ideal means of controlling anthracnose. In the USA, seven races of cucurbit anthracnose have been reported, of which races 1 and 2 appear to be the most common (Goode, 1956; Jenkins et al., 1964; Sitterly, 1973; Wasilwa et al., 1993). Race 1 is highly virulent on cucumber, cantaloupe melon, and some watermelon cultivars. Race 2 is moderately virulent on most cucumber cultivars and highly virulent on most watermelon cultivars (Wasilwa et al., 1993). Cucumber accession PI 197087 has long been known to be resistant to anthracnose, and this resistance has been used widely in breeding for nearly 60 years in the USA. Recently, Pan et al. (2018) reported that STAYGREEN (CsSGR) is a candidate anthracnose resistance locus in an inbred cucumber line, ‘Gy14’, whose resistance originated from PI 197087. This recessive gene (hereafter referred to as Cssgr) carries a loss-of-susceptibility mutation and represses chlorosis under C. orbiculare infection. In addition, it confers durable broad-spectrum resistance to downy mildew and angular leaf spot (Wang et al., 2018). In watermelon, a non-synonymous SNP in Cla001017, which encodes a CC–NBS–LRR protein, results in an arginine-to-lysine substitution in the leucine-rich repeat domain and causes resistance to C. orbiculare race 1 (Jang et al., 2019). This mutation is conserved among the Cucurbitaceae and Fabaceae (Jang et al., 2019).
In Japan, almost all cucurbits, except squash, have been damaged by C. orbiculare (Akai and Terasawa, 1957). In particular, the production of cucumber and watermelon in open-fields has been severely affected by this disease for a long time. Recently, watermelon cultivars highly resistant to this disease were released in Japan, but no such cucumber cultivars are yet available. Some Japanese old cultivars with anthracnose resistance have been identified in the past. For example, Akai and Terasawa (1957) reported that ‘Tsuda Sanjaku’, ‘Sekino 2’, ‘Sakata Pickle’, ‘Taiwan Kema’, and ‘Yasei Kiuri’ have moderate resistance. Meera et al. (1995) reported that ‘Shogoin Fushinari’ and ‘Sagami Hanjiro’ are resistant and ‘Ochiai Fushinari’ is moderately resistant. However, cultivars with high levels of resistance are not yet released in Japan, and such development is highly desired. For this purpose, the identification of promising sources of resistance to anthracnose is vital.
India and Nepal are considered centers of origin for cucumber, and India is also the center for cultivated cucumber (Sebastian et al., 2010). China and the Middle East are secondary centers of cucumber diversity (Meglic et al., 1996; Staub et al., 1999). Many sources of resistance to pathogens have been identified in various cucumber genetic resources from Asia. For instance, the Chinese cultivar ‘TMG-1’ is resistant to Zucchini yellow mosaic virus, Papaya ringspot virus, and Watermelon mosaic virus (Wai et al., 1997), ‘Chinese Long’ is resistant to Cucumber mosaic virus (Wasuwat and Walker, 1961), and 27028930, of Thai origin, is resistant to Melon yellow spot virus (Sugiyama et al., 2009). Among fungal and bacterial diseases, accession PI 198088, of Indian origin, is resistant to powdery mildew (Podosphaera xanthii; Morishita et al., 2002) and downy mildew (Pseudoperonospora cubensis; Palti and Cohen, 1980; Call et al., 2012a, b), and PI 200818, of Myanmar origin, is resistant to bacterial wilt (Erwinia tracheiphila; Nuttall and Jasmin, 1958). Therefore, it was worthwhile to screen genetic resources from Asia for anthracnose resistance.
Here, (1) we evaluated the anthracnose resistance of cucumber accessions that originated mainly from East and South-East Asia by seedling assay, and screened resistant accessions for practical resistance by comparing with commercial F1 hybrid cultivars in Japan. In this trial, we used C. orbiculare strain MAFF 240422 (104-T), which is known to infect cucumber and is widely used in Japan to investigate host–pathogen interactions (Kubo and Takano, 2013). (2) We re-evaluated the selected accessions by greenhouse assay and seedling assay with two other strains of C. orbiculare. (3) We checked whether the selected resistant accessions have the same resistance gene (Cssgr). (4) We examined the response of the accessions harboring Cssgr to Japanese strains of C. orbiculare. (5) We compared three assays to identify the most efficient one as an alternative to the seedling assay for genetic analysis and breeding programs that handle a lot of plants at the same time.
Plant materials used are listed in Table S1. We screened 232 accessions preserved in the Genebank of the National Agriculture and Food Research Organization, Japan (NARO Genebank), for anthracnose resistance: 136 originated in China, 30 in Cambodia, 27 in Vietnam, 9 in Malaysia, 5 in Taiwan, 4 in Thailand, 3 in the Philippines, 3 in Nepal, 3 in Turkey, 3 in Japan, 2 in South Korea, 2 in Bhutan, 2 in Bangladesh, 1 in Papua New Guinea, 1 in India, and 1 in Israel (G1–G232 in Table S1). The three Japanese accessions (G230–G232, old Japanese cultivars) were reported as resistant in a previous study (Meera et al., 1995). We also tested the resistance of 64 F1 commercial cultivars (F01–F64) released by Japanese breeding companies, six fixed lines of Japanese long type (J1–J6), a fixed line of Beit-Alpha type (B1), and a weedy-type fixed line, CS-PMR1 (W1), derived from PI 197088 and preserved at the University of Tsukuba. A Japanese old cultivar ‘Tokiwa’ (C1) was used as a control in all assays. In addition, we used accessions S1–S6, harboring the resistance mutation in CsSGR (kindly provided by Dr. M. Amano at Saitama Gensyu Ikuseikai Co., Ltd.).
Fungal materials and preparation of inoculumThe NARO Genebank preserves 23 strains of C. orbiculare collected from all over Japan. Strains MAFF 240422 (104-T), MAFF 306737 (CcM-1), and MAFF 726522, isolated from infected cucumber, were used in this study. For inoculum preparation, a mycelium plug was transferred onto potato dextrose agar (PDA; Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) and incubated at 25°C in the dark for 10 days. Spores formed on the surface of agar were then suspended in deionized water and filtered through Kimwipe tissue (Nippon Paper Crecia Co., Ltd., Tokyo, Japan). The suspension was shaken well and spores were counted with a hemocytometer. The concentration was adjusted to >5 × 104 spores/mL, and 0.5 μL/mL Tween-20 (Kanto Chemical Co., Inc., Tokyo, Japan) was added to the inoculum.
Seedling assayAnthracnose resistance of all accessions was first tested by seedling assay using MAFF 240422 from 2015 to 2017. Eight individuals per accession were tested in two experimental replications. Seeds were placed in Petri dishes lined with wet filter paper (Toyo Roshi Kaisha, Ltd., Tokyo, Japan) and incubated at 28°C in the dark. Germinated seeds were transplanted into 7.5-cm-diameter plastic pots filled with wet culture soil (Nihon Hiryo Co., Ltd., Fujioka, Japan) and incubated at 28°C for a 12-h photoperiod in a climate chamber (Nippon Medical and Chemical Instruments Co., Ltd., Osaka, Japan). After cotyledon unfolding, the temperature in the chamber was set to 25°C for 7 or eight days. After the first true leaves unfolded, the temperature was set to 25°C during the day (12 h) and 18°C at night for 10 days. Plants were put in plastic boxes (16 plants per box) (Akasaka Co., Ltd., Atsugi, Japan), and inoculum was sprayed on the upper surface of each true leaf (ca. 0.4 mL) and cotyledon (ca. 0.2 mL) with a spray bottle. The boxes were then closed to maintain 100% relative humidity, and kept in the dark for 24 to 36 h. The lids were then opened, and the plants were grown at 25°C for a 12-h photoperiod in the climate chamber. The resistance of each inoculated plant was scored two weeks after inoculation. Scoring was based on infection severity (IS) levels: 0, no lesions; 1, a few small lesions (< 2 mm in diameter); 2, several small lesions or a few medium lesions (2–5 mm); 3, many small lesions and a few to several medium lesions, or a few large lesions (> 5 mm); 4, less than half of the cotyledon or leaf is withered; 5, half or more of the cotyledon or leaf is withered. As a standardized index for comparison among experiments, we calculated relative infection severity (RIS) as the ratio to the average IS of C1.
The resistance level of three accessions (G100, G209, G213) with low RIS scores in the first trial were re-checked by the seedling assay as described above with 10 Japanese commercial F1 cultivars with a wide range of RIS scores, eight fixed lines (J1–J6, B1, and W1), and C1 as a control. In this trial, 20 individuals per accession, cultivar, or line were tested in two experimental replications. Additional seedling assays with three strains (MAFF 240422, MAFF 306737, and MAFF 726522) were performed to compare the resistance level of G100 and three other cultivars or lines (C1, J4, and F36). In these assays, 10 individuals per accession, cultivar, or line were tested in two experimental replications.
Greenhouse assayGreenhouse assays were performed twice using 6 accessions (C1, G100, W1, F36, B1, and F1 progeny of a cross between G100 and B1) between September and December 2019; the first trial started in September and the second in October. In each trial, 20 individuals per accession were tested in two experimental replications. Seeds were sown in 7.5-cm-diameter plastic pots filled with wet culture soil (Nihon Hiryo), and seedlings were grown for 14–20 days in the climate chamber. The seedlings were then transplanted into rectangular planter boxes (30 L) filled with Peat Mix (Hokkaido Peatmoss Inc., Konosu, Japan) for the first trial or a 1:1 mixture of culture soil and peat mix soil (Sakata Seed Corp., Yokohama, Japan) for the second trial, and placed into a polyhouse. From the late autumn, the polyhouse was warmed with a heater to keep the temperature above 18°C. The plants were grown vertically; the main stem was cut between the 11th and 12th nodes, and all lateral branches and true leaves at the 1st to 5th nodes were removed. The plants were given nutrient solution (N:15, P:8, K:17, OAT Agrio Co., Ltd., Tokyo, Japan) to prevent leaf yellowing caused by fertilizer deficiency. When the leaves at the 11th node were fully expanded, inoculation was performed by spraying on the upper surface of leaves at the 9th to 11th nodes of the main stem. After inoculation, the inner curtain of the polyhouse was closed to maintain darkness for 1 day. On the day after inoculation, a sprinkler attached to the top of the polyhouse was activated for 3–6 h to produce water droplets on the leaves and polyhouse surfaces to maintain high air humidity. About two weeks after inoculation, the symptoms of leaves at the 9th to 11th node were evaluated, and the SI of each plant was calculated.
Detection of polymorphism at CsSGR using a CAPS marker and comparison of resistance of G100 with that of accessions harboring CssgrGenomic DNA was extracted from cotyledons of C1, G100, B1, and S1–S6 using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol with minor modifications. To confirm the CsSGR genotype of each accession—wild type (susceptible) or carrying the mutation (resistant)—we used the cleaved amplified polymorphic sequence (CAPS) marker constructed by Dr. M. Amano (personal communication) (forward primer: 5'-TCTTCTCGGTATTTTGAATCTGAA-3'; reverse primer: 5'-CCCTTAACTTTCTTCCATTCTCC-3'). PCR mixtures (5 μL) contained template DNA, Blend Taq-Plus-, 10 × buffer, 2 mM dNTPs (Toyobo Co., Ltd., Osaka, Japan), autoclaved Milli-Q water, and primer mix. The PCR conditions were as follows: 94°C for 2 min; 35 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 30 s; and a final extension at 72°C for 3 min. Amplified DNA was digested with Bsp119I (AsuII) (Thermo Fisher Scientific, Waltham, MA, USA) mixed with 10 × Buffer Tango and autoclaved Milli-Q water at 37°C for 2 h. The digested PCR products were electrophoresed in 8% polyacrylamide gel. The gel was stained with GelRed (0.03 μL·mL−1, Cosmo Bio Co., Ltd., Tokyo, Japan) and visualized under UV light. Using the seedling assay as described above, we compared the resistance of the accessions to MAFF 240422 and MAFF 306737. In each assay, 10 individuals per accession were tested in two experimental replications.
Comparison of three different assaysUnlike in the seedling assays described above, here we used detached cotyledons, detached true leaves, or rooted cuttings, and compared these three assays using accessions C1, G100, J4, and B1. J4 is one of the fixed lines derived from Japanese F1 cultivar, and included in this test to identify the most efficient one as an alternative to the seedling assay for genetic analysis and breeding programs of Japanese type cucumber. In each assay, 20 individuals per accession were tested.
In the detached cotyledon assay, germinated seeds were transferred to a 38-mm-diameter plug tray filled with wet culture soil (Nihon Hiryo), and incubated at 28°C for 12 h (photoperiod time) in the climate chamber. After cotyledon unfolding, the temperature was set to 25°C for three days, and then to 25°C during the day and 18°C at night for four days. Then, the hypocotyl was cut and set in a plastic box with water. Inoculation was performed as in the seedling assay, and the infection severity of each cotyledon was evaluated a week after inoculation.
In the detached true leaf assay, germinated seeds were transferred to 7.5-cm-diameter plastic pots filled with wet culture soil and incubated at 28°C for a 12-h photoperiod in an environmental room. After cotyledon unfolding, the temperature was set to 25°C for 7–8 days. After the first true leaves fully expanded, the temperature was set to 25°C during the day and 18°C at night for at least 10 days. During this period, the plants were given liquid fertilizer to prevent leaf yellowing. The 3rd and 4th true leaves were cut and planted in a 5-mL tube filled with 0.5% agar. They were kept in a plastic box to maintain high humidity until evaluation was complete. Inoculation was performed as in the seedling assay, and the infection severity of each leaf was evaluated a week after inoculation.
In the rooted cutting assay, branches from a well-grown plant were stuck upright in wet vermiculite (Nittai Co., Ltd., Osaka, Japan). The rooted cuttings were kept in a plastic case for approximately 10 days to maintain high humidity and promote rooting. After rooting, the plants were grown at 25°C during the day (12 h) and 18°C at night for approximately five days. Inoculation was then performed as in the seedling assay, and the infection severity of each plant was evaluated two weeks after inoculation.
The resistance of 232 accessions, mainly of Asian origin (Table S1), was tested with the C. orbiculare strain MAFF 240422 in 2015–2017. We observed a wide variation in IS among accessions; RIS variation in cotyledons is shown in Figure 1A and that in true leaves is shown in Figure 1B. In these trials, significantly positive correlations between cotyledon and true leaf IS scores were observed in 2016 (Pearson’s correlation coefficient r = 0.97), 2017 (r = 0.96), and 2018 (r = 0.95). No accession was completely resistant with no symptom in both cotyledon and true leaf (RIS = 0), but we selected 13 accessions as moderate resistance based on both cotyledon and true leaf RIS scores (< 0.5): 6 from Vietnam, 4 from China, 2 from Cambodia, and 1 from Papua New Guinea. The resistance of these 13 accessions was re-evaluated several times in 2016–2018, and accessions G100 from China and G209 and G213 from Cambodia were finally selected as moderately resistant. Among 64 Japanese commercial F1 cultivars, variation in RIS was narrow (Fig. 1A, B), and no cultivars had the same level of resistance as the three selected accessions. In addition, the RIS scores of previously reported resistant (G230 and G232) and moderately resistant (G231) accessions were 1.0 to 1.18 in cotyledon, and 1.0 to 1.26 in true leaf, respectively. Thus, these three accessions were considered to be as susceptible at the same level as C1 rather than resistant.

Distributions of (A, B) RIS (the ratio to the average IS of C1) of genetic resources (white) and commercial F1 cultivars (grey) in (A) cotyledons and (B) true leaves in the 1st screening, and (C, D) RIS in (C) cotyledons and (D) true leaves in the 2nd screening. F1s, 10 selected commercial F1 cultivars.
Accessions G100, G209, and G213 were re-tested with 10 Japanese commercial F1 cultivars, with a wide range of RIS scores in the first test, 8 fixed lines (J1–J6, B1, and W1), and C1 as a control. The three selected accessions had lower averages of both cotyledon and true leaf RIS than the others except for two F1 cultivars (F36 and F42). G100 had a few medium lesions or many small lesions, whereas in B1, which had the highest RIS score in true leaves, lesions consistently occupied the whole leaf area and some leaves died. In the seedling assay, cotyledon was under more aging stress than true leaves and more compatible to anthracnose infection. In addition, the proportion of true leaves is far larger than that of cotyledon in a whole plant, and thus the phenotype of the true leaf is more important than that of the cotyledon to evaluate resistance. Considering these points, G100 had the lowest RIS true leaf score (Fig. 1D), indicating that it had the highest resistance to anthracnose.
Re-confirmation of the resistance of G100 by additional evaluationsAdditional seedling assays with three strains were performed to compare the resistance level of G100 and three other cultivars or lines (C1, J4, and F36). Although a variation of IS scores within accession (among plants) was observed, G100 showed broad resistance to all three C. orbiculare strains (Fig. 2). The symptoms of G100 were most severe with MAFF 306737. With this strain, G100 had more and larger lesions than with the other strains, whereas plants of the three other cultivars/lines tested (C1, J4, F36) almost died. In contrast, all plants of C1, J4, and F36 did not die when inoculated with MAFF 726522 and MAFF 240422. The symptoms with MAFF 726522 and MAFF 240422 were similar in all three cultivars/lines (C1, F36, J4): they had many large lesions in true leaves. In contrast, G100 had only a few small lesions. These results suggested variation in the virulence levels and compatible levels with host cucumber among the three strains tested. Because plant growth of G100 was not affected, even by the most virulent strain, MAFF 306737, we consider G100 to have promising resistance to a wide range of C. orbiculare strains in Japan.
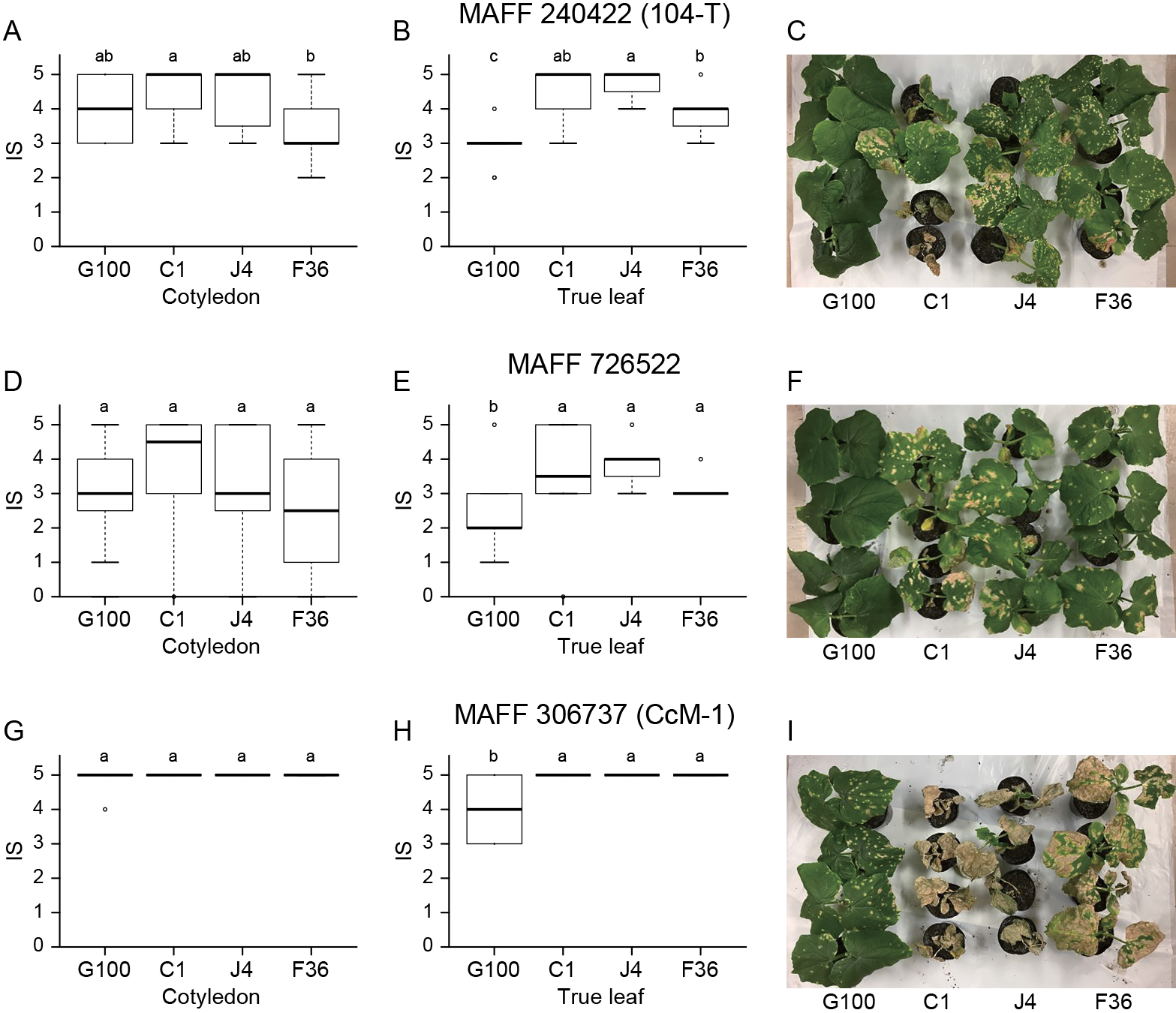
Distribution of IS in cotyledon and true leaf and examples of seedlings inoculated with (A–C) MAFF 240422, (D–F) MAFF 726522, and (G–I) MAFF 306737. C1 as the control. Different uppercase letters in the boxplots denote a significant difference at P < 0.05 by Steel–Dwass test; vertical bars indicate SD.
In the first trial of the greenhouse assay, G100 was moderately resistant to MAFF 306737. In this trial, the inoculum concentration was quite high (ca. 4.2 × 106 spores·mL−1). About a week after inoculation, the first symptom was observed in B1 (the most susceptible cultivar in the seedling assay). Then the symptoms spread to the whole plant, and many B1 plants died. Cultivars C1 and F36 also had severe symptoms on all inoculated leaves, and growth of many plants was strongly suppressed. In G100, W1, and F1 progeny of B1 × G100, lesions were observed on all inoculated leaves, but the effect of the disease on plant growth was lower than in the other three cultivars (Fig. S1). The IS values of B1, F36, and C1 were higher than those of G100, F1 progeny of B1 × G100, and W1 (Fig. 3). In the second trial, the concentration of inoculum was decreased (ca. 3.0 × 105 spores·mL−1). Unfortunately, downy mildew occurred on all plants during this trial in addition to anthracnose. As in the first trial, C1 and B1 were highly susceptible and G100 and W1 were moderately resistant. Unlike in the first trial, the IS of F36 was low, and that of W1 was lower than in first trial (Fig. 3). The IS scores of F1 were intermediate between those of parents B1 and G100 in the first trial, but were almost the same as B1 in the second trial. Although the phenotype was affected by environmental conditions, the result of the greenhouse assay suggested that the resistance of G100 to MAFF 306737 may be inherited recessively.

IS in the 1st and 2nd greenhouse assays. Different uppercase letters in the boxplots denote a significant difference at P < 0.05 by Steel–Dwass test; vertical bars indicate SD.
G100 was found to be homozygous for the wild-type allele of CsSGR (Fig. 4A). The susceptible cultivars B1 and C1 were also homozygous for this allele. Using the seedling assay, we compared the resistance of G100 and S1–S6 (homozygous or heterozygous for the mutant allele, Cssgr), to MAFF 240422 and MAFF 306737. With MAFF 240422, all six accessions (S1–S6) had low true leaf IS values, although their cotyledon IS tended to be high (Fig. 4B, C). Among those, S2 (homozygous for Cssgr) was most resistant in true leaf, and its resistance level was the same as that of G100, although its cotyledon IS was higher than that of G100. With MAFF 306737, all six accessions (S1–S6) had high cotyledon and true leaf IS values. Although S2 was also the most resistant in all six accessions (S1–S6), its symptoms, including chlorosis, were more severe than those of G100 (Fig. S2). Interestingly, although S1 was homozygous for Cssgr, it showed higher IS cotyledon and true leaf scores to both MAFF 240422 and MAFF 306737.

(A) Fragment patterns of the CAPS marker of CsSGR, (B) seedlings inoculated with MAFF 240422 and MAFF 306737, and (C, D) variations of IS of cotyledon and true leaf inoculated with (C) MAFF 240422 or (D) MAFF 306737. In A, the lower band (ca. 150 bp) is CsSGR with the mutation, and the upper band (ca. 200 bp) is the wild-type CsSGR. Different uppercase letters in the boxplots denote a significant difference at P < 0.05 by Steel–Dwass test; vertical bars indicate SD.
We tested three assays using detached cotyledons, detached true leaves, and rooted cuttings with MAFF 240422 and four accessions (including C1 as control) with different resistance levels. The RIS scores obtained by each assay were compared with cotyledon and true leaf RIS scores in the seedling assay (Fig. 5A, B). In the detached cotyledon assay, G100 had the lowest RIS, followed by J4 and B1 (Fig. 5C); this order was identical to that of cotyledon RIS in the seedling assay. In the detached true leaf assay, the RIS difference between G100 and the other two accessions was significant (Fig. 5D), as in the seedling assay, although significant RIS differences were not detected between G100 and J4 in both the detached cotyledon assay (Fig. 5C) and rooted cuttings assay. In the rooted cuttings assay, B1 had high RIS, and J4 and G100 had low RIS (Fig. 5E). Although the differences among the three accessions differed among the three assays, G100 had the lowest RIS, indicating that all three assays can be used as alternatives to the seedling assay.

Differences in RIS among three accessions in (A) cotyledon and (B) true leaf seedling assays, (C) detached cotyledon and (D) detached true leaf assays, and (E) rooted cuttings assay. Different lowercase letters in the boxplots denote a significant difference at P < 0.05 by Steel–Dwass test; vertical bars indicate SD.
We identified one accession, G100 (‘Ban Kyuri’), with strong, although not perfect, resistance to anthracnose. For a long time, it has been considered that no cultivars in Japan have strong resistance to anthracnose. Indeed, our results confirm that all major Japanese commercial cultivars have no strong resistance, although some of them exhibited moderate resistance. The resistance level of G100 was much higher than those of the current Japanese F1 cultivars, and G100 exhibited different degrees of resistance to three strains of C. orbiculare derived from different regions in Japan. Therefore, this accession can be used to develop new cultivars with anthracnose resistance.
Pan et al. (2018) and Wang et al. (2018) reported STAYGREEN (CsSGR) as a candidate gene for anthracnose resistance in cucumber. The recessive gene Cssgr, carrying a loss-of-susceptibility mutation, is effective against C. orbiculare race 1 in the USA. Here, S2 and S3 harboring homozygous for Cssgr showed moderate to high resistance in true leaf to both the lower virulence strain MAFF 240422 and the highly virulent strain MAFF 306737 (Fig. 4), indicating that it can provide efficient field resistance to Japanese C. orbiculare strains with a broad range of virulence. However, accession S1, homozygous for Cssgr, was susceptible to two C. orbiculare strains (Fig. 4C, D). This result suggests that S1 harbors a gene or genes suppressing the function of Cssgr or an additional mutation in CsSGR that cancels the loss of susceptibility. In contrast, G100 was homozygous for the wild-type allele of CsSGR, although its resistance levels were equal to or higher than those of accessions harboring Cssgr. Observation of symptoms also indicated the difference in resistance between G100 and accessions harboring Cssgr (Fig. S2). These results suggest that G100 harbors a novel resistance gene or a novel allele of CsSGR that confers anthracnose resistance. In the former case, this would be the second anthracnose resistance gene in cucumber (after Cssgr reported by Pan et al., 2018) and the third one in the Cucurbitaceae (after Cssgr and Cla001017 reported by Jang et al., 2019). In addition, the IS value of the F1 between G1 and B1 was close to the susceptible parent B1 in the second trial of the greenhouse assay, suggesting that the resistance of G100 to MAFF 306737 may be inherited recessively. Further progeny tests under stable environmental conditions will be necessary to confirm the inheritance of the resistance, since the result of greenhouse assay is affected by environmental condition.
So far, the divergence among C. orbiculare strains in their virulence has not been examined in Japan, although several strains were collected from all over the country and preserved in the NARO Genebank. Race differentiation in C. orbiculare has also not been reported in Japan. This is because the first cucurbit cultivar resistant to anthracnose (watermelon; Nanto Seed Co., Ltd., Kashihara, Japan) was released in 2015, and no breakdown has been observed so far. In this study, symptoms differed greatly among three C. orbiculare strains. For example, G100 plants had no or a few small lesions (low IS) when inoculated with MAFF 240422 and MAFF 726522, but several large lesions (high IS) when inoculated with MAFF 306737 (Fig. 2). Plants of accession J4 had many large lesions (low IS) with MAFF 240422, but a greater number of large lesions or almost dead leaves (high IS) with MAFF 306737. These results suggest that the virulence of C. orbiculare in Japan has diversified, and it may be not an overstatement to say that race differentiation of C. orbiculare has occurred in Japan. Therefore, it is important to carefully select strains in research on and breeding for anthracnose resistance.
As mentioned above, G100 is a promising material for breeding of cultivars with resistance to diverse anthracnose strains in Japan. However, fruit morphology and other traits are quite different between G100 and Japanese commercial F1 cultivars. This indicates that much effort will be needed to develop new cultivars with high anthracnose resistance and fruit traits demanded by consumers in Japan. In a future breeding program using G100, it will be important to efficiently evaluate resistance levels of numerous individual plants or lines. Needless to say, the use of a DNA marker is an ideal method for efficient selection in breeding, but the development of a DNA marker linked to anthracnose resistance of G100 needs genetic analyses using numerous plants of segregated generations. The seedling and greenhouse assays used in this study seemed to work well. However, IS score sometimes varied a lot even among plants within the same accession, suggesting that these assays are vulnerable to environment. Therefore, it is important to use a more efficient and accurate method to phenotype anthracnose resistance, which can handle many materials at the same time.
To find such a method, we compared three assays using detached cotyledons, detached true leaves, and rooted cuttings. Generally, assays using detached cotyledons or true leaves of young plants are superior in terms of minimizing environmental effects and cost because they do not require much space or time, and plants to be tested can be grown in a completely artificial environment, such as a growth chamber. The rooted cuttings assay is inferior to the other two assays in terms of efficiency, but is more accurate because the effect of inoculation on plant growth can be evaluated in addition to the symptoms on two or more expanded true leaves. All three methods do not require transplanting plants with C. orbiculare spores to the breeding field. G100 was the most resistant in all three methods, as in the seedling assay. However, in the detached true leaf assay, the RIS ranges of all accessions tested were similar to each other (Fig. 5D), possibly because of a relatively large effect of the stress of detaching on the growth of the specimen before and after inoculation. Therefore, this assay may be useful only to select plants with the strongest resistance, but not when it is necessary to detect and evaluate a continuous difference in genetic analysis, although improvement of the method may enable more accurate evaluation. The remaining two assays could detect the difference between moderately resistant and susceptible accessions. Cotyledons and true leaves differ in time of emergence, organ structure, and so on. However, as mentioned earlier, the results of the seedling assay suggested that the IS scores of cotyledons and true leaves are positively correlated, indicating that disease symptoms on cotyledons are predictors of those in true leaves, and thus can be used as an effective indicator to determine the resistance degree of plants. Therefore, the detached cotyledon assay was the most efficient and would be of practical value in breeding and genetic analysis in which many individual plants must be evaluated. However, even in the detached cotyledon assay, the environmental effect cannot be completely eliminated, and so it will be important to assess the resistance of lines or plants by using several kinds of assays or repeated assays.
In conclusion, G100 is a promising new source of resistance to diverse anthracnose strains with different levels of virulence in Japan. Since resistance can be accurately evaluated by the detached cotyledon assay, breeding and genetic analysis using G100 can be performed efficiently. The challenge that lies ahead is to develop a DNA marker linked to the resistance gene(s) of G100. We anticipate that the results reported here will contribute not only to the development of new cucumber cultivars with high resistance to anthracnose, but also to further analyses aimed at understanding the genetic mechanisms of resistance to anthracnose, and thereby the development of a marker-assisted selection system for this trait.
We thank Y. Ishiga, N. Nishi, H. Matsumura, M. Kikuchi, K. Kawakita, H. Tsuji, T. Iwaya, T. M. Ho, F. Fitriyah, and Y. Yamamoto at the University of Tsukuba for technical advice and assistance. We also thank N. Tomooka, Y. Kawazu, and other members of the Plant Genetic Resources (PGRAsia) project for collecting and providing plant materials. We are grateful to M. Amano at Saitama Gensyu Ikuseikai Co., Ltd., for providing materials and CAPS marker information. Most of the plant materials and fungal strains were kindly provided by the NARO Genebank. This work was supported by the Ministry of Agriculture, Forestry and Fisheries of the Government of Japan (Grant number: JPJ007117).