2022 Volume 91 Issue 2 Pages 209-220
2022 Volume 91 Issue 2 Pages 209-220
Dissatisfaction with the flavor quality of young aromatic Thai coconut ‘Nam-Hom’ usually grows when stored at 2–4°C after harvest, represented by off-flavor development and aroma loss. To understand the mechanism of postharvest flavor deterioration, young coconut fruit were stored at 4°C and 25°C for 15 days. Sensory evaluation was performed, volatile compositions were analyzed, enzyme activities and transcript levels of genes related to the synthesis of volatiles were investigated. Off-flavor was observed after six days of storage at 4°C and tended to be more serious after that. A total of 45 volatiles were identified in the young coconut fruit. Partial least square discriminant analysis (PLS-DA) revealed two volatiles, 1-heptanol, 1-octanol, and nonanal, which were associated with off-flavor development. Transcript levels of CnLOX1, CnHPL1, and CnADH2, relating to the volatile production catalyzed by the LOX pathway, increased during storage at low temperatures. Their respective enzyme activities were also found to increase. The loss of the aromatic nature of young ‘Nam-Hom’ coconut at 4°C was found to be associated with an increase in CnBadh2 gene expression, resulting in a lower 2-acetyl-1-pyrroline (2-AP) content as compared to that at 25°C. Wrapping the fruit with polyethylene (PE) instead of polyvinyl chloride film reduced the off-flavor by reducing the oxygen concentration; consequently, this prevented enzyme activities and expression of genes from the LOX pathway. However, the loss of the aromatic nature of young ‘Nam-Hom’ coconut fruit at low temperature storage could not be mitigated with PE packaging.
Young coconut has become a popular fruit for its refreshing and high electrolyte water, in addition to the tender, soft jelly-like endosperm (kernel). It has abundant sugars, vitamins, minerals, amino acids, and phytohormones (Yong et al., 2009). In general, young coconut fruit is harvested at 6 to 7 months after full bloom, when it contains 6–7% soluble solids in the coconut water, and approximately 20% of lipids in the kernel (Yong et al., 2009). After harvest, the husk (mesocarp) is trimmed or almost totally removed. They are then dipped in 3–5% sodium metabisulfite (SMS) for 3–5 min (Mohpraman and Siriphanich, 2012), the trimmed fruit is then wrapped with PVC stretch film, followed by storage or shipping at 2–4°C (Siriphanich et al., 2011).
Cold storage is an effective means to prolong the postharvest shelf-life of crops; however, this postharvest procedure spoils the flavor of young coconut fruit (Meethaworn and Siriphanich, 2015; Meethaworn et al., 2019). Similar observations were reported in other lipid-rich fruits such as olives (Kalua et al., 2008) and avocados (Prabath-Pathirana et al., 2013). For trimmed young coconut fruit, the development of off-flavor caused by postharvest cold storage at 4°C was positively correlated with volatiles derived from the lipid oxidation catalyzed by the lipoxygenase (LOX) pathway, including heptanal, octanal, nonanal, and 1-heptanol (Meethaworn et al., 2019). Accumulation of these volatiles was associated with the increase in activities of enzymes from the LOX pathway in young coconut fruit after harvest (Meethaworn et al., 2019). Similar results were reported in cucumber (Mao et al., 2007; Yang et al., 2012) olive fruit (Padilla et al., 2014) and walnut (Piccirillo et al., 2006) during storage at low temperatures. Downstream in the LOX pathway, hydroperoxide lyase (HPL) hydrolyzes in hydroperoxides into aldehyde. Subsequently, alcohol dehydrogenase (ADH) dehydrogenizes aldehyde into alcohol, followed by alcohol acyltransferase (AAT), which esterifies alcohol to esters (Zhang et al., 2011). Genes with functions associated with the production of volatiles derived from the LOX pathway have been identified from several fruit species, including kiwifruit (Zhang et al., 2006), peach (Zhang et al., 2011), olive (Padilla et al., 2014), tomato (Shen et al., 2014), apple (Salas et al., 2016), and strawberry (Zorrilla-Fontanesi et al., 2012). Moreover, during cold storage of tomato off flavors developed along with the decreasing in aroma (Maul et al., 2000). For young coconut, the LOX pathway genes associated with off-flavor related volatile formation have not been investigated.
Besides the development of off-flavor caused by cold storage, this practice also results in aroma loss of postharvest fruit. In the case of young coconut, the Nam-Hom cultivar used in our study has a distinct aroma akin to cooked Jasmine rice (Yong et al., 2009). The responsible compound for this characteristic aroma was suggested to be 2-acetyl-1-pyrroline (2-AP) (Luckanatinvong et al., 2018). During the shipment of young coconut under low temperatures to distant markets, loss of this aroma was reported (Siriphanich et al., 2011). Production of 2-AP is synthesized via the l-proline metabolic pathway. In jasmine rice, 2-AP biosynthesis starts from either glutamate or ornithine. They are metabolized by Δ-1-pyrroline-5-carboxylate synthase (P5CS) and transaminase, respectively, to Δ-1-pyrroline-5-carboxylate (P5C). P5C is then reduced to proline by Δ-1-pyrroline-5-carboxylate reductase (P5CR). After that, proline is spontaneously converted to γ-aminobutyraldehyde. At this step, γ-aminobutyraldehyde is used as the substrate for betaine-aldehyde dehydrogenase (BADH2) to generate γ-aminobutyric acid (GABA). However, if BADH2 is limited or non-functional, γ-aminobutyraldehyde is spontaneously converted to 1-pyroline and 2-AP, respectively (Wakte et al., 2016). On the other hand, an increase in BADH2 activity or Badh2 expression obstructs 2-AP production (Wakte et al., 2016). Badh2 expression was reported in aromatic coconut kernel (Saensuk et al., 2016), and the expression pattern of Badh2 correlated negatively with the 2-AP content in coconut (Jaroonchon, 2017). However, the Badh2 transcript level and its association with the production of 2-AP during postharvest cold storage of young coconut fruit is not known.
The studies mentioned above indicated that changes in the volatile profile mainly cause flavor quality loss of young ‘Nam-Hom’ coconut fruit during postharvest cold storage. Firstly, cold storage induced the production of volatiles from the LOX pathway, which caused off-flavor development. Secondly, chilling postharvest treatment reduced the content of 2-AP, limiting its aromatic nature. Understanding the pathways associated with the formation of volatiles has provided a framework to further investigate the molecular mechanism of flavor quality loss in young coconut fruit during cold storage. Also, postharvest modified atmosphere treatment by wrapping fruit with films was shown to reduce the oxygen concentration in the mesocarp, and most likely, in the coconut endosperm, leading to the suppression of LOX and HPL activity, resulting in less off-flavor (Meethaworn et al., 2019). Therefore, the present study evaluated the flavor quality of young coconut fruit during postharvest cold storage with different film wrapping, determined the content of flavor related volatiles, enzyme activities, and transcription levels of genes associated with volatiles formation.
Young coconut (Cocos nucifera ‘Nam-Hom’) was harvested at seven months after full bloom (MAFB) from 7–10-year-old coconut trees in the Damnoen Saduak District, Ratchaburi province, Thailand. Uniform fruit was selected with an average fruit weight of 1.3 kg ± 0.2 kg. At harvest, the selected fruit were found to have 7–8% soluble solids content with light green skin. For the first season, whole young coconut fruit was used to investigate the effect of cold storage on fruit flavor quality. One hundred and twenty fruit were divided into two groups, the first group was kept at 25°C as the control, and the second group was stored at 4°C as the cold storage treatment. The fruit was stored for 15 days and were sampled every three days when a total of approximately 300 g of coconut kernel was scooped from each fruit. The collected kernel was divided into two portions, the first 100 g was collected separately for off-flavor sensory evaluation and determination of electrolyte leakage. The remaining sample was chopped with a sharp knife, frozen with liquid nitrogen and stored at −80°C until use. Thirty grams of frozen samples were freeze-dried. The freeze-dried samples were analyzed for malondialdehyde (MDA), volatile compounds, enzyme activity, quantitative real-time PCR analysis (qPCR), and RNA sequencing. The aims in the first season were to identify the volatiles and enzymes along with their gene expressions that related to off-flavor in young ‘Nam-Hom’ coconut fruit under cold storage and to verify the nature of aroma loss in young Nam-Hom coconut in terms of 2-AP generation.
In the second season experiment, trimmed fruit were produced by partly shearing off the fruit exocarp, and then trimming them to a tapered cylinder form with a cone-shaped top and flat base. These were immersed in 3% sodium metabisulfite for 5 min following the Thai Agricultural Commodity and Food Standard (2007). This trimmed fruit was then wrapped with two different films, polyvinyl chloride (PVC) film [M wrap, 11 μm thickness, 15,000 cm3 (m2·day)−1 oxygen transmission rate (OTR); BASF] and polyethylene (PE) plastic [(50 μm thickness, oxygen transmittance rate (OTR) 3,000 cm3 (m2·day)−1), developed by the National Metal and Material Technology Center (MTEC), Thailand], stored at 4°C for 15 days and sampled every three days. The coconut samples were collected and analyzed for MDA, volatile compounds, enzyme activity, and quantitative real-time PCR analysis (qPCR) as in the first experiment. The aims of the second season were to confirm the results from the first season by using a different approach (using a different plastic wrapping) and to recommend the best procedure to reduce or prevent off-flavor.
Fruit quality determination Sensory evaluationFive panelists were trained to evaluate the taste of coconut kernel from young intact or trimmed fruit and then rated the samples for the presence of off-flavor or aromatic (2-AP) score, using a 9 point hedonic scale as followed: Off-flavor score, 1 = no off-flavor, 3 = slight, 5 = moderate, 7 = high, and 9 = extreme off-flavor presence. Aromatic (2-AP) score, 1 = no aroma, 3 = slight, 5 = moderate, 7 = high, and 9 = extreme aroma presence. Even number scores were given for intermediate levels.
Electrolyte leakageThe coconut kernels were cut into 0.5 × 0.5 × 0.5 cm cubes, rinsed with distilled water, then immersed in 0.3 M mannitol for one hour before being measured for electrical conductivity. A duplicate sample of coconut kernel was boiled for 10 min and measured. The percentage of electrolyte leakage in the coconut kernel was determined using the method of Meethaworn et al. (2019).
Malondialdehyde (MDA) contentTBA (thiobarbituric acid) reactive compounds were determined by using a modified method of Mao et al. (2007). A sample of 0.2 g of the freeze-dried kernel was homogenized (on ice) in 5 mL 10% trichloroacetic acid, including 0.1 g of polyvinylpyrrolidone (PVPP). Next, it was filtered through four layers of cheesecloth, and centrifuged at 12,000 × g at 4°C for 15 min. A 1 mL of the mixture was transferred to a test tube containing 4 mL of 0.5% thiobarbituric acid in 20% trichloroacetic acid solution and mixed well. The mixture was boiled at 100°C for 15 min and allowed to cool. Absorbance was determined at 532 and 600 nm with a microplate reader (Bio-Tek, model synergy H1; Bio-Tek instrument). The MDA content was calculated using a molar extinction coefficient of 155 mM−1·cm−1.
Enzyme activity Lipoxygenase (LOX) activityThe extraction and assay followed Meethaworn et al. (2019) except 250 mg of freeze-dried coconut kernel was homogenized (on ice) in 5 mL of extraction buffer containing 100 mM phosphate buffer, pH 7.5, 2 mM dithiothreitol (DTT), 1 mM ethylenediaminetetraacetic acid (EDTA), 0.1% (v·v−1) Triton X-100, and 1% (w·v−1) polyvinyl polypyrrolidone (PVPP). This extract was centrifuged at 10,000 × g at 4°C for 30 min. The activity was assayed by mixing 140 μL of 0.1 M phosphate buffer, pH 8.0, 30 μL substrate solution [8.6 mM linoleic acid, 0.25% (v·v−1) Tween-20, and 10 mM NaOH in 100 mM phosphate buffer, pH 8.0] and 30 μL of crude enzyme extract. One activity unit was defined as the increase of one-unit absorbance at 234 nm·min−1. The result was represented by specific activity (U·mg−1 protein). Protein content was determined following the method of Bradford (1976).
Hydroperoxide lyase (HPL) activityHPL extraction method was modified from Padilla et al. (2014). Two hundred milligrams of the freeze-dried coconut kernel were homogenized in 5 mL of extraction solution containing 50 mM HEPES-NaOH (pH 7.5), 20 mM KCl, 2 mM MgCl2, 2 mM Na2S2O5, 2 mM EDTA, 7 mM DTT, 0.1% ascorbate, 0.5% Triton X-100, and 2% PVPP, then centrifuged at 20,000 × g at 4°C for 20 min. The supernatant was recovered and set on ice as a crude enzyme extract. HPL activity was monitored by the decrease in absorbance at 234 nm with a microplate reader (Bio-Tek, model synergy H1; Bio-Tek instrument). The assay was modified from Patui et al. (2010). The mixture consisted of 200 μL of 100 mM sodium phosphate buffer (pH 8.0), 10 μL of 1.6 mM substrate solution, and 5 μL of enzyme solution. The result was reported as specific activity (U·mg−1 protein). One unit of activity was the decrease in one unit of absorbance at 234 nm·min−1. The 13-HPOD substrate was purchased from Cayman Chemical (USA). Protein determination followed Bradford (1976).
Alcohol dehydrogenase (ADH) activityThe method for ADH extraction and analysis followed Lara et al. (2003) with modification. Briefly, 250 milligrams of the freeze-dried coconut kernel were homogenized in 5 mL of extraction solution containing 85 mM 2-(N-morpholino) ethane-sulfonic acid (MES) buffer, pH 6.0, 5 mM DTT and 2% (w·v−1) PVPP, and centrifuged at 20,000 × g for 15 min at 4°C. The supernatant was collected on ice as a crude enzyme extract. The ADH activity was assayed by mixing 2.25 mL NADH solution (150 mM NADH in 85 mM MES buffer, pH 6.0), 150 μL of acetaldehyde solution (80 mM acetaldehyde in 85 mM MES buffer, pH 6.0), and 300 μL of crude enzyme extract. The decreasing absorbance measured oxidized NADH at 340 nm over time by using a microplate reader (Bio-Tek, model synergy H1; Bio-Tek instrument). One unit of activity was the decrease in one unit of absorbance at 340 nm·min−1; the result was reported as specific activity (U·mg−1 protein). Protein determination followed Bradford (1976).
Alcohol acyl transferase (AAT) activityThe method for AAT extraction and analysis followed Lara et al. (2003) with modification. Briefly, two hundred milligrams of the freeze-dried coconut kernel were homogenized in 5 mL of extraction solution containing 100 mM phosphate buffer, pH 8.0, 1 mM EDTA, 0.1% (v·v−1) Triton X-100, and 1% (w·v−1) PVPP. After that, the homogenate solution was centrifuged at 20,000 × g for 20 min at 4°C. The supernatant was recovered and set on ice as a crude enzyme extract. AAT activity was assayed by mixing 2.5 mL of MgCl2 solution (5 mM MgCl2 in 100 mM phosphate buffer, pH 8.0), 150 μL of 2.5 mM acetyl CoA, 200 μL of crude enzyme extract and 100 μL of butanol solution (200 mM butanol in 100 mM phosphate buffer, pH 8.0). The mixture was incubated at 35°C for 15 min, and then 100 μL of 10 mM 5,5'-dithiobis(nitrobenzoic acid) (DTNB), was added and it was allowed to stand at room temperature for 10 min. Total volume was 3.05 mL. One unit of activity was defined as an increase of one unit of absorbance at 412 nm·min−1. Protein determination followed Bradford (1976).
Fruit volatiles analysisThe volatile analysis was carried out according to Marchi et al. (2015) with modifications. One gram of powdered freeze-dry sample was placed into a 10 mL screw-cap vial. Five milliliters of saturated NaCl solution were added together with 10 μL of 0.8 g·L−1 of 2-octanol as the internal standard. The sample was equilibrated at 60°C for 10 min with constant stirring, then a solid-phase micro extraction (SPME) fiber assembly of 50 μm of polydimethylsiloxane, divinylbenzene, and carboxen (PDMS-DVB-carboxen) stable flex (Supelco) was used to absorb the volatiles in the vial for 20 min. SPME fiber was inserted into the GC inlet for desorption. These processes were automatically done by a GC auto-sampler (Agilent 120; Agilent technology). The temperature of the gas-inlet (injector) was 220°C in the splitless mode. A chromatograph analyzer (GC 7890A; Agilent Technology) interfaced with a mass detector operating in the electron ionization (EI) mode, with a scan range of m/z 35–350 was used (MS 5975C; Agilent technology). The separation was carried out by using an HP-5MS column (30 m × 0.25 mm i.d. × 0.25 μm film thickness; Agilent Technology) at 40°C after injection and maintained for 2 min. The temperature was increased at a stable rate of 3°C·min−1 to 150°C, then increased to 250°C at 6°C·min−1 and maintained for 15 min. The flow rate of the helium carrier gas was 1.5 mL·min−1. Volatile compound identification was carried out according to the NIST 08 library. The quantitative determination of compounds was expressed in mg·kg−1 equivalent to the internal standard.
Electronic nose measurementsChanges in fruit overall volatile profiles during storage were analyzed by using an electronic nose (e-nose) FOX 4000 (AlphaMOS), according to Zhang et al. (2011). A frozen sample was transformed into a 10 mL glass tube containing 5 mL saturated sodium chloride solution and kept on ice. Two milliliters of the mixed solution were transferred into 10 mL capped vials and heated to 40°C for 30 min. Two milliliters of the headspace gas were collected and injected into the e-nose. Signal acquisition time was 2 min, followed by 6 min for baseline recovery.
Gene expression analysis by RNA sequencing and real-time quantitative PCRIn this research, the total RNA was extracted according to Zhang et al. (2006). For sequencing, the total RNA was isolated from freeze-dried coconut kernel. Then, the quality of RNA was checked by using a NanoPhotometer spectrophotometer (IMPLEN). The integrity and quantity of the total RNA were assessed by using a BioAnalyzer (Agilent Technologies). The RNA-Seq was performed with LC-bio (China) on an Illumina HiSeq 4000 platform. The raw reads obtained from the RNA-Seq were pre-processed, adapters were trimmed, and low-quality and shorter reads were removed. Elaeis guineensis (69.73%), Phoenix dactylifera (17.34%), Anthurium amnicola (1.62%), Musa acuminata (0.86%), Beta vulgaris (0.64%), Oryza sativa (0.57%), and other (9.24%) genomes were selected as the references for young coconut. Gene expression level was evaluated by fragments per kilobase of exon model per million mapped reads (FPKM). Three different RNA isolations were used as replicates for library construction and RNA sequencing.
Real-time quantitative PCR (qPCR) was performed for gene expression analysis, including CnLOX1, CnHPL1, CnADH2, and CnAAT1 genes in the lipid oxidation pathway, and the CnBadh2 gene of the 2-AP synthesis pathway. The oligonucleotide primers were designed based on the 3'-UTR sequence, with the size of sequence being 90 to 150 bp (Table S1). The CneIF4 gene of the coconut fruit was used as a housekeeping gene. At least three different RNA isolations and cDNA syntheses were used as replicates for the RNA-seq and qPCR analysis. Expression levels produced by qPCR were expressed as a ratio relative to the fruit at harvest, which was set at 1.
Oxygen concentrationGas samples were collected from coconut kernel scooped out of the shell, that were then placed in a vacuum chamber filled with water. A vacuum was applied to draw gases from the kernel under water. Oxygen concentrations were determined using a Shimadzu GC 8A gas chromatograph, Japan, equipped with molecular sieve 5A column. The oven temperature was 80°C. Ultra-purified nitrogen (99.999%) was used as the carrier gas, at a rate of 30 mL·min−1.
Statistical analysisAverage data and standard errors (SE) were calculated by using Microsoft Excel, and all the figures were made by using OriginPro 8.0 (Microcal Software). The partial least squares discriminant analysis (PLS-DA), Pearson correlation, and the value of variable importance in projection (VIP) were calculated by using MetaboAnalyst 3.0 <http://www.metaboanalyst.ca/>. PLS-DA model validation was performed by permutation testing in which P < 0.05 (Fig. S1A) and by cross validation (Fig. S1B). Data were normalized using ‘Autoscaling’ in the MetaboAnalyst program. A difference was considered to be statistically significant when P ≤ 0.05 using the SPSS version 24 software package (IBM).
During storage at 4°C, a slightly higher off-flavor score was observed for fruit stored at 4°C for six days, but was not statistically significant from that at 25°C. A significant difference was found after 12 days (Fig. 1A). The off-flavor was described as having a fatty or oily note by the trained panelists. An electronic nose (e-nose) was applied to provide a volatiles overview profile of the fruit treated at different temperatures. A distinct separation between the fruit was observed based on storage temperature, in which fruit stored at 4°C were located on the negative x-axis DF1 while the fruit stored at 25°C were located on the positive side on the discriminant function analysis (DFA) plot (Fig. 1B). A higher percentage of electrolyte leakage was observed for the young coconut fruit stored at 4°C than those stored at 25°C (Fig. S2A). Meanwhile, the content of the lipid oxidation product MDA was statistically significantly higher in fruit during cold storage at 4°C (Fig. S2B).

Kernel sensory evaluation in young intact coconut stored at 4 and 25°C for 15 days. (A) Off-flavor score of the coconut kernel. Data are means ± SE of five replicates. (B) Kernel aroma profile analysis by electronic nose (e-nose). * indicates significant differences between treatments from the same storage duration by t-test at P ≤ 0.05, NS = not significant.
A total of 45 volatile compounds were annotated from the young coconut kernel (Table S2). These volatile compounds consisted of eight acids, two alcohols, one aldehyde, seven alkanes, eight esters, one ketone, three lactones, six terpenes, seven phenyls, and two other compounds. Ester was the quantitatively predominant volatile class, accounting for 40% of the total volatiles on harvest day (day 0), followed by acid at 27%, and lactones at 15% (Table S3). The content of total volatiles was higher at 25°C than in fruit stored at 4°C. However, a few volatile compounds showed an opposite trend. Higher levels of benzaldehyde, decane, 1-octanol, 3-ethyl-2,5-dimethylpyrazine, 1-heptanol, and nonanal were observed in the young coconut fruit stored at 4°C than that stored at 25°C (Table S3).
In this study, the purpose of the PLS-DA was to reveal changes in volatile(s) related to off flavor and lipid peroxidation, so the storage time was considered as a minor factor. However, to determine whether storage duration has any influence on the volatile profile, PLS-DA analysis at each time point was conducted. The results showed that volatile profile of young coconut stored at 4°C changed between time points, but not at 25°C (Fig. S3). However, at 4°C the profile of volatiles related to lipid peroxidation, including 1-octanol, nonanal, and 1-heptanol at different time points, remained similar (Fig. S3F–J). The profiles of other volatiles such as decanoic acid, benzaldehyde, decane and ethyl decanoate at 4°C did change with time. Regarding PLS-DA analysis, the scores and correlation loadings of all the volatile compounds were obtained for the first two components of the acquired PLS-DA model (Fig. 2A). The two primary components accounted for 38% of the total variance among the samples. The volatiles of the young coconut stored at 25°C and 4°C are shown at different locations on the PLS-DA plot, where fruit stored at 4°C had volatiles located on the negative side and the fruit stored at 25°C had them on the positive side.

Multivariate analysis of volatiles in the coconut kernel using PLS-DA during storage of young intact coconut at 4 and 25°C. (A) PLS-DA score plot of volatiles of young intact coconut fruit during storage at 4°C (blue circle) and 25°C (red triangle) from three days to 15 days. (B) VIP score of volatiles that contributed to separation of young intact coconut when stored at 4 and 25°C. Red solid squares and blue solid squares represent high and low value of volatiles and off-flavor scores.
To identify the volatiles that could explain the separation of the young coconut fruit stored at 4°C and 25°C, the VIP values were calculated. A total of 14 volatile compounds with a VIP score higher than one were identified; seven volatile compounds were associated with 4°C storage including 1-octanol, 3-ethyl-2,5-dimethylpyrazine, benzaldehyde, nonanal, 1-heptanol, decane, and methyl octanoate, while the remaining compounds were correlated to 25°C storage (Fig. 2B). Six compounds had a significant positive correlation (P < 0.01) with the off-flavor score, including nonanal, 1-octanol, benzaldehyde, 3-ethyl-2,5-dimethylpyrazine, decane, and 1-1-heptanol (Table 1). However, only 1-heptanol, 1-octanol and nonanal were considered to be compounds involved in off-flavor development due to their odor description, low odor threshold (Table 1) and the high content levels of these compounds. The contents of these three compounds increased more in the young coconut stored at 4°C than at 25°C (Fig. 3).

Volatile compounds of coconut kernel from young intact coconut found to have a positive correlation with off-flavor at a P-value ≤ 0.05.

Concentration of off-flavor related volatile compounds in the kernels of young intact coconut stored at 4 and 25°C for 15 days. 1-heptanol (A), 1-octanol (B), and nonanal (C). Data are means ± SE of three replicates. * indicates significant differences between treatments for the same storage duration by t-test at P ≤ 0.05, NS = not significant.
An average of 44 million clean reads (Q30 > 96.16%) were obtained in a total of 33 samples in the RNA sequencing study (Table S4). There were 73 significant differential expression genes (DEGs) between coconut stored at 4°C compared to 25°C at all time points, as shown in Figure S4. Only 21 genes were up-regulated at 4°C (Table S5). Calmodulin and small heat shock protein genes are two examples, which have been reported to be involved in plant responses to cold stress (Ding et al., 2001; Yuan et al., 2018). This information from the RNA-seq study reveals a cold response in young coconut; however, it was beyond the scope of our work.
It is known that volatiles such as heptanol and nonanal are derived from fatty acids. Therefore, genes associated with the lipoxygenase pathway were screened using RNA-seq. Homology genes were cloned and their expression patterns were analyzed. qPCR was also performed to confirm the expression patterns of these candidate genes associated with volatile formation. In this study, 13 members of the LOX gene family were identified in young coconut (Table S6). From these members, CnLOX 1 (DN 45067 c1 g1) had the highest transcript levels at harvest day and was up-regulated during low-temperature storage of the young coconut fruit. For the downstream lipid degradation through the LOX pathway, one isoform for each of the HPL, ADH, and AAT gene were found, including CnHPL1 (DN 44642 c4 g8), CnADH2 (DN 44607 c1 g1), and CnAAT1 (DN 43989 c1 g3) (Table S6). During storage at low temperature, the expression patterns of CnLOX1, CnHPL1, and CnADH2 were up-regulated in agreement with the off-flavor development (Fig. 1A), the content of off-flavor-related volatiles (Fig. 3) and chilling injury indicators, including electrolyte leakage and MDA content (Fig. S2), while the expression of CnAAT1 was not. Therefore, qPCR was performed to confirm the expression patterns of these genes.
Regarding qPCR analysis, a high transcript level of CnLOX1 was observed for young coconut fruit stored at 4°C throughout the experimental period (Fig. 4E). The LOX activity at 4°C was 20–25% higher than that of fruit at 25°C during postharvest storage (Fig. 4A). For the transcript levels of CnHPL1 (Fig. 4F), it was also approximately 4-fold higher in 4°C stored fruit as compared to that stored at 25°C. Similarly, HPL enzyme activity increased rapidly after three days of storage at 4°C and maintained a high level for up to nine days, followed by a decrease toward the end of 15 days’ storage. The activity at 4°C was approximately 4-fold higher than that at 25°C after nine days of storage (Fig. 4B). For the expression of CnADH2, the transcript level increased during storage at 4°C and was approximately three times higher than that stored at 25°C (Fig. 4G). An increase in ADH activity was observed in fruit stored at 4°C for three days; it was approximately 4–5 fold higher than in fruit stored at 25°C (Fig. 4C).

Lipid oxidation was activated via the LOX pathway. LOX (A), HPL (B), ADH (C), and AAT (D) enzyme activities and their transcription of CnLOX1 (E), CnHPL1 (F), CnADH2 (G), and CnAAT1 (H) genes in young intact coconut kernels stored at 4 and 25°C for 15 days. Data are means ± SE of three replicates. * indicates significant differences between treatments for the same storage duration by t-test at P ≤ 0.05, NS = not significant.
Conversely, the CnAAT1 expression was up-regulated at 25°C from three days in storage onward. At 4°C storage, the expression was 2–3 times lower than that at 25°C (Fig. 4H). The AAT activity at both temperatures was reasonably stable during the first six days of storage, and after that the enzyme activity at 25°C increased sharply, but slightly decreased at 4°C (Fig. 5D).
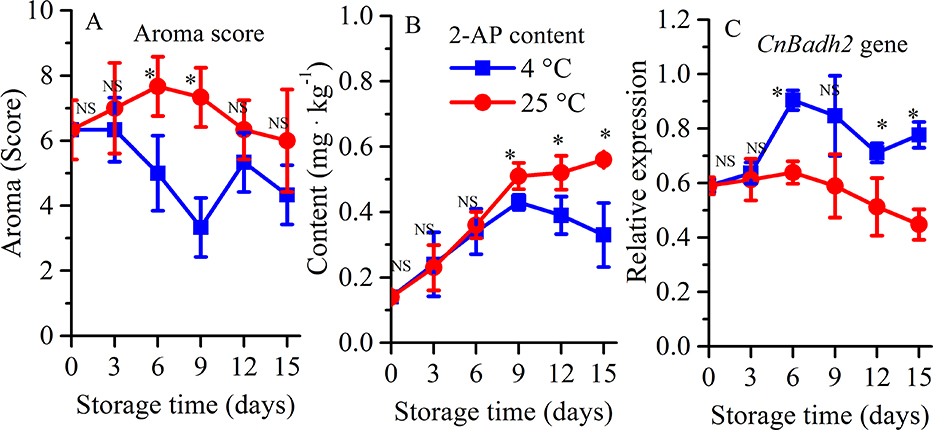
Aroma (2-AP) score (A), 2-AP content (B), and CnBadh2 expression (C) in the kernels of young intact coconut fruit stored at 4 and 25°C for 15 days. Data are means ± SE of three replicates. * indicates significant differences between treatments for the same storage duration by t-test at P ≤ 0.05, NS = not significant.
To identify the function of the LOX pathway gene in young coconut, the sequences of CnLOX1, CnHPL1, CnADH2, and CnAAT1 were aligned with those in different plant species by using the National Center for Biotechnology Information (NCBI) database. It was found that the CnLOX1 gene was similar to the 9-lipoxygenase gene (LOX5) in the palm family [87–92% from 88% of query cover], that is involved in plant response to oxidative stress, lipid peroxidation, and plant defense (Table S7). Concerning aroma production, however, the CnLOX1 gene of young coconut showed 74.9% similarity from 70% of query cover to the LOX 5 in banana, 73.3% similarity from 24% of query cover to the LOX1 in rice and 63.4% similarity from 15% of query cover to TomloxC in tomato (Table S7). CnHPL1 was very similar to the cytochrome P450 gene in the palm plant that involves plant response and tolerance to chemical stress at about 70–80% similarity from 77% of query cover (Table S7). Regarding CnADH2, it was very similar to CnADH2 of the palm family that is associated with the plant defense mechanism at 93–96% from 60–88% query cover. It was also approximately 78.3% like CnADH2, from 41% of the conserved region in tomato that controls alcohol aroma production (Table S7). CnAAT1 showed a 92–96% similarity from 44–45% of query cover to CnAAT1 in the palm family that is associated with a lipid degrading enzyme, but without a known function (Table S7).
Postharvest cold storage reduced the content of aroma-related 2-AP and maintained transcript levels of CnBadh2In addition to off-flavor development, aroma acceptance of young coconut fruit after cold storage by consumers was also investigated. A higher aroma (2-AP) score was detected for the fruit stored at 25°C than 4°C (Fig. 5A). Significantly lower aroma (2-AP) scores for young coconut fruit stored at low temperatures were observed on day six and nine. For an important aroma-related volatile compound, 2 acetyl-1-pyrolline (2-AP), the content was reduced in young coconut fruit during storage at 4°C (Fig. 5B). Significantly lower content of 2-AP was observed at day nine and thereafter during cold storage. Transcript levels of CnBadh2 showed an opposite pattern with that of 2-AP when young coconut was subjected to postharvest cold storage (Fig. 5C).
PE film wrapping altered volatile synthesis and gene expression of young coconut fruit during cold storageThe occurrence of off-flavor was significantly lower for PE when compared with PVC film treatment after 9 days of storage (Fig. S5A). Meanwhile, the level of electrolyte leakage and MDA content was also reduced in young coconut fruit wrapped with PE film (Fig. S6A, B). The content of 1-heptanol in PE was approximately 3.6-fold lower than that in the PVC-treated fruit after three days of storage (Fig. 6A). For 1-octanol, the greatest difference in content was observed in fruit stored for six days, at which point the content in the PE-treated fruit was approximately 3.5 times lower than that of the PVC film-wrapped fruit (Fig. 6B). Approximately 20–30% lower content of nonanal was detected in the fruit treated with PE film relative to the PVC film-treated fruit during cold storage (Fig. 6C). For young coconut fruit wrapped with the PVC film, the concentration of O2 was significantly higher than that in fruit wrapped with the PE film (Fig. S3B).

Volatiles responsible to off-flavor in the kernel that showed a difference between PVC and PE wrapping on young trimmed coconut stored at 4°C for 15 days included 1-heptanol (A), 1-octanol (B), and nonanal (C). Data are means ± SE of three replicates. * indicates significant differences between treatments for the same storage duration by t-test at P ≤ 0.05, NS = not significant.
To test whether the LOX pathways were affected by using PE film and thereby alleviating the off-flavor caused by cold storage, enzyme activities and transcript levels were analyzed. The activity levels of LOX (Fig. 7A) and HPL (Fig. 7B) in young coconut fruit wrapped with PE were 1.5 and 2 times lower than the PVC-wrapped fruit after nine days of storage, respectively. No significant difference was observed for ADH or AAT activity (Fig. 7C, D). For young coconut fruit wrapped with PE film, the transcript level of CnLOX1 was lower in relative terms to the fruit wrapped with PVC film (Fig. 7E). For CnHPL1, the fruit treated with PVC film had higher transcript levels than that of the PE-treated fruit during storage at 4°C (Fig. 7F). After wrapping with PE film, significantly lower transcript levels were observed for CnADH2 and CnAAT1 to those of PVC-treated fruit (Fig. 7G, H).

Lipid oxidation was activated via the LOX pathway. LOX (A), HPL (B), ADH (C), and AAT (D) enzyme activity and CnLOX1 (E), CnHPL1 (F), CnADH2 (G), and CnAAT1 (H) expression of young trimmed coconut kernels covered with PVC or PE stored at 4°C for 15 days. Data are means ± SE of three replicates. * indicates significant differences between treatments for the same storage duration by t-test at P ≤ 0.05, NS = not significant.
Regarding the aromatic nature of young Nam-Hom coconut, the aroma score (Fig. 8A), 2-AP content (Fig. 8B) and Badh2 expression (Fig. 8C) were not statistically different between the two wrapping methods during cold storage. However, there was a tendency that in coconut wrapped with PVC film the aroma and 2-AP content were lower, while the expression of the Badh2 gene was higher, than those wrapped with PE film.

Aroma (2-AP) score (A), 2-AP content (B), and CnBadh2 expression (C) in the kernels of young trimmed coconut, wrapped with PVC or PE film and stored at 4°C for 15 days. Data are means ± SE of three replicates. * indicates significant differences between treatments for the same storage duration by t-test at P ≤ 0.05, NS = not significant.
Postharvest cold storage is widely used to extend the shelf-life of agricultural produce. However, this process spoils the flavor quality of fruit, including tomato (Zhang et al., 2016), peach (Zhang et al., 2011), and young coconut fruit (Meethaworn et al., 2019). For young coconut fruit after cold storage, reduced flavor quality is mainly caused by the development of off-flavor related volatiles and by loss of aroma-related volatiles. In the present study, the volatiles related to off-flavor and aroma loss caused by cold storage were identified, and enzymes and genes associated with the formation of volatiles were characterized.
Considering the effects of environmental factors on fruit volatiles (Zhang et al., 2016), a seasonal replication of young coconut fruit during postharvest cold storage was performed. In our previous report (Meethaworn et al., 2019), volatile profiles of coconut kernel and coconut water were similar, but the contents in the water were as much as 10 times lower. This led to similar flavor characteristics in both the kernel and the water, with only half the intensity in the water. Moreover, based on the off-flavor development, it was shown that lipid degradation enzymes were required. However, at seven months after full bloom, coconut water is clear and does not have any cells (Jaroonchon, 2017) to produce enzymes. This information indicates that the source of the off-flavor in coconut was from volatiles produced in the coconut kernel and later released into the water (Meethaworn et al., 2019). Hence, this study was conducted only using the coconut kernel. Moreover, it is very difficult to extract RNA from coconut water for gene expression analysis. Therefore, the kernel was selected in the present study. Previously, we identified 4 key volatiles including heptanal, octanal, nonanal, and 1-heptanol, responsible for the off-flavor in young coconut after low-temperature storage. In the present study, based on VIP score analysis, odor description and low odor threshold, 1-heptanol, 1-octanol, and nonanal were considered to be compounds involved in off-flavor development. Taking both studies together, although only 1-heptanol and nonanal were consistently detected at 4°C, 1-octanol was also likely to be the key compound responsible for the off-flavor in young coconut during storage at low temperature as it had the highest VIP value of 2.2, a relatively low odor threshold and a high content. The reason that 1-octanol was missed in our previous report could be due to the two-fold shorter capillary GC column and different sample preparation employed.
It has been reported that the content of aldehyde and alcohol could be regulated by controlling the oxygen level during fruit storage (Morales et al., 1997). As expected, when PE film was used to wrap the coconut fruit instead of PVC, the off-flavor and lipid oxidation were minimized, and a lower content of 1-heptanol and nonanal volatiles were observed in the PE-wrapped fruit. Similar observations were reported in other fruit such as apple (Salas et al., 2016), avocado (Prabath-Pathirana et al., 2013), guava (Singh and Pal, 2008), pomegranate (Belay et al., 2017), and peach (Zhou et al., 2018).
To identify the genes related to off-flavor development, RNA-seq was performed to screen candidate genes. Then, qPCR was performed to validate the gene expression patterns of young coconut fruit in response to cold storage. We compared the patterns of volatiles and gene expression patterns of young coconut fruit during cold storage and fruit stored at room temperature. The accumulation of these off-flavor related volatile compounds caused by cold storage agreed well with the pattern of CnLOX1, CnHPL, and CnADH2 transcription levels. Wrapping the young coconut fruit with PVC film during cold storage maintained higher transcript levels of CnLOX1 and CnHPL1 than those in fruit wrapped with PE film. Increased LOX pathway gene expression, and the accumulation of alcohol and aldehyde during storage at low temperatures were also reported in mango (Sivankalyani et al., 2017) and olive fruit (Padilla et al., 2014). Moreover, high enzyme activities were observed for LOX and HPL during cold storage of young coconut fruit. The present observation further supports our hypothesis that increased LOX pathway enzyme activities and transcript levels contributed to off-flavor development of young coconut fruit during cold storage (Meethaworn et al., 2019).
Although sequence similarities were observed for CnLOX1, CnHPL1, and CnADH2 to genes associated with volatile production in banana, rice, and tomato (Kou et al., 2006; RoyChowdhury et al., 2016; Shen et al., 2014; Speir et al., 1998), stronger similarities were found for genes associated with response to stress in the palm species (Hanano et al., 2017). Induced expression of fruit LOX gene pathways caused by postharvest cold storage was also observed for kiwifruit (Zhang et al., 2006) peach fruit (Zhang et al., 2011) and mango (Sivankalyani et al., 2017). Transcript levels of CnLOX1, CnHPL1, and CnADH2 increased during cold storage and exhibited a pattern similar to that of the LOX, HPL and ADH activity and MDA content of young coconut. This suggested that specific low-temperature events are triggering LOX gene pathway expression and its related enzyme activity. Earlier studies reported that low temperature storage led to an accumulation of reactive oxygen species in fruit tissues such as banana (Wu et al., 2014) and pineapple (Nukuntornprakit et al., 2015). Therefore, the putative function of induced LOX pathway genes in young coconut stored at low temperature is likely to be associated with lipid metabolism in response to cold stress.
As an oxygen-dependent enzyme, LOX catalyzes the oxidization of unsaturated fatty acids into hydroperoxides. Wrapping young coconut fruit with PE resulted in lower transcript levels of CnLOX1 compared to fruit wrapped with PVC film. The PVC film was 11 μm thick with a 15,000 cm3 (m2·day)−1oxygen transmission rate, while the PE film was 50 μm thick with an oxygen transmittance rate of 3,000 cm3 (m2·day)−1. This lower expression level of the LOX gene may be related to the reduced oxygen concentration of young coconut fruit after wrapping with PE film compared to PVC film (Meethaworn et al., 2019). It was reported that oxygen at 5°C was two times more soluble in aqueous solution than at 25°C (Battino et al., 1983). For young coconut, the water of fruit stored at 4°C had 100–300 nL·L−1 dissolved oxygen, while at 25°C, oxygen could not be detected (Meethaworn and Siriphanich, 2015). These results suggested that decreased LOX gene expression and enzyme activity may be associated with a reduced oxygen level of young coconut after wrapping with PE film during cold storage. Consequently, it is possible that aldehyde and alcohol could be accumulated as products of LOX catalyze lipid oxidation in response to cold storage (Ayala et al., 2014), contributing to off-flavor development of young coconut.
Regarding the aromatic nature of young Nam-Hom coconut that is associated with the 2-AP content (Luckanatinvong et al., 2018), the aroma of young coconut diminished along with the content of 2-AP under cold storage, in agreement with a previous report in which shipping at 2–4°C resulted in a loss of aroma (Siriphanich et al., 2011). In this study, it was shown that the reduced level of 2-AP was due to the up-regulation of CnBadh2, whereas BADH2 activity led to a reduced level of substrate for the synthesis of 2-AP (Wakte et al., 2016). The up-regulation of CnBadh2 during cold storage is suggested to be a cold temperature response and a stress tolerance function, similar to that found in rice (Hashemi et al., 2018).
Regarding the film wrapping study, there was no significant difference in aroma score, 2-AP content or CnBadh2 expression between PVC- and PE-wrapped fruit. However, the aroma score tended to be better in PE-wrapped fruit. This could be the result of lower alcohol and aldehyde accumulation due to a reduction in the LOX pathway caused by PE treatment.
ConclusionThe present study revealed that the effect of cold storage induced the development of off-flavor and aroma loss in young coconut fruit. Off-flavor development was associated with induced transcript levels of CnLOX1, CnHPL1, and CnADH2, along with their respective enzyme activities in the LOX pathway. These changes in gene expression and enzyme activity via the LOX pathway contributed to the accumulation of 1-heptanol, 1-octanol and nonanal, resulting in fruit developing off-flavor during cold storage. At the same time, postharvest cold storage induced expression of CnBadh2 that negatively correlated with the production of 2-AP, the compound responsible for the aromatic nature of Nam-Hom coconut, resulting in a loss of aroma. Modified atmosphere treatment using PE film wrapping instead of PVC wrapping as used in commercial practice, helped maintain the flavor quality of young coconut fruit during postharvest cold storage by limiting the LOX pathway.
This research was supported by the Golden Jubilee Project, Thailand Research Fund grant number PHD/0131/2014, Postharvest Technology Innovation Center Ministry of Higher Education, Science, Research and Innovation, Thailand. Project code PBM.P.1/2016 and the National Key R&D Program of China (2016YFD0400101), Zhejiang Provincial Science and Technology Project (2016C04001), and the 111 Project (B17039).