2022 Volume 91 Issue 2 Pages 131-139
2022 Volume 91 Issue 2 Pages 131-139
Parthenocarpic apple cultivars have been recognized with simultaneous homeotic floral organ mutations. The mutations included replacements of petals to sepals and stamens to carpels, which were same as the class B mutations of floral organs from Arabidopsis and snapdragon. For apple, the parthenocarpy and class B mutations were tightly inherited and MdPISTILLATA (MdPI) gene deficiency caused homeotic mutations. However, the relationship between the suppression of MdPI and parthenocarpy was unclear. Transgenic apples with suppressed MdPI expression using an anti-sense or co-suppression method were found to have the same homeotic floral organ mutations as parthenocarpic cultivars. Further, the transformants with co-suppression showed parthenocarpy and overexpression of MdPI in fruits prevented normal fruit growth. Other apple MADS genes were analyzed for parthenocarpy. MdMADS13 is classified as another class B gene, which plays a role in floral organ formation together with MdPI. In addition, MdMADS1/8 and MdMADS9 are classified as class E genes, which are could function like SEP1 and 2 genes from Arabidopsis. The MdMADS1/8 and MdMADS9 gene-suppressed apple transformants showed strong inhibition of fruit enlargement, supporting the idea that MdMADS1/8 and MdMADS9 contribute to hypanthium development. It is possible that the MdPI, MdMADS13, MdMADS1/8, and MdMADS9 proteins formed a heterotetramer as a transcriptional regulator and were involved in fruit development. Other plant species such as tomato and grape also showed the respective class B genes affected fruit development. The suppression of tomato class B genes led to class B floral organ mutations and parthenocarpy, and a grape mutant with class B gene expression ectopically inhibited fruit flesh development. Both class B genes seemed to prevent fruit development similar to the apple MdPI. This suggests that the class B genes play not only a role in forming the identities of petals and stamens, but also a pivotal role in fruit development.
First, here is a brief overview regarding the discovery and history of parthenocarpic apple cultivars. Apples are classified as pome and belong to the accessory fruit group. Apple fruit is epigynous, and the hypanthium tissue surrounding its ovary develops into the edible part. Apples are usually self-incompatible and do not achieve fruit set with their own pollen. This gametophytic self-incompatibility is regulated by S allele genes (Janssens et al., 1995; Kitahara et al., 2005). Therefore, mixed planting with tree-supplied pollens or artificial pollination is recommended to ensure stable fruit set. Seedless fruits are important for commercial production because they can be readily eaten. Grapes, mandarin oranges, bananas, and persimmons without seeds are popular worldwide for their easy consumption. Parthenocarpy is defined as seedless fruit that achieve fruit set without pollination or fertilization. Furthermore, it has been reported that some apple cultivars with parthenocarpy have a common mutation in floral organs (Yao et al., 2001). The mutation replaces petals to sepals and stamens to carpels, so the flowers have no petals and stamens. As a result, the flowers are unattractive to pollinators, which are unattracted to flowers without petals. However, the fruits of these cultivars develop normally, even without pollination or fertilization.
Fine observation revealed that apple parthenocarpic flowers have double sepals and many carpels (Fig. 1B). Parthenocarpic cultivars have double ovaries, which each have ovules (Fig. 1B). When the parthenocarpic cultivars are artificially pollinated, seed formation occurs in normal and ectopic carpels (Fig. 1D). The fruits with seeds grew larger than the seedless fruits. Tobutt (1994) reported that the carpels replaced from stamens formed sound seeds by artificial pollination. Furthermore, it was demonstrated by crossing experiments that the phenotype of flower organ mutation was single recessive inheritance. This homeotic mutation of flower organs can be explained by flower morphogenesis research in recent years. From studies using flower morphological variants, the ABCE model has emerged as a unified explanation for flower organogenesis (Jack, 2001; Theiβen et al., 2016). This is a simple model in which the expression positions of the four ABCE genes determine each floral organ identity of the sepals, petals, stamens, and carpels in four concentric whorls. Most ABCE-corresponding genes belong to the MADS-box family, except for the APETALA2 (AP2) gene, which plays a role as a class A gene. In Arabidopsis thaliana, APETALA1 (AP1) and AP2 belong to class A, APETALA3 (AP3) and PISTILLATA (PI) belong to class B, AGAMOUS (AG) belongs to class C, and SEPALLATA (SEP) belongs to class E. The MADS-box genes are widely conserved in animals, fungi, insects and plants as developmental regulators or signal transducers and have a common and conserved domain, which is composed of about 60 amino acids involved in DNA binding and protein-protein interactions as a transcriptional regulator (de Bodt et al., 2003). The domain binds DNA elements known as CArG-boxes (consensus CC[A/T]6GG) and facilitates nuclear localization as dimers. (Kaufmann et al., 2005; Theiβen et al., 2016). The A and C genes are expressed antagonistically between whorl regions; the A gene expresses in whorl one and two and the C gene expresses in whorl three and four. However, the B gene functions in whorl two and three and is involved in organ determination of petals and stamens. It was speculated that parthenocarpic apple cultivars with homeotic changes in floral organs lacked gene function of the corresponding class B gene. The apple class B gene, which is thought to be responsible for parthenocarpy, was discovered as Malus domestica PI (MdPI) (Yao et al., 2001). The MdPI gene of the parthenocarpic cultivars had a retrotransposon-like sequence of a 10 to 11 kb insertion in the genome. It was clarified that the insertion suppressed the expression of MdPI, while MdPI deficiency caused homeotic flower mutations.
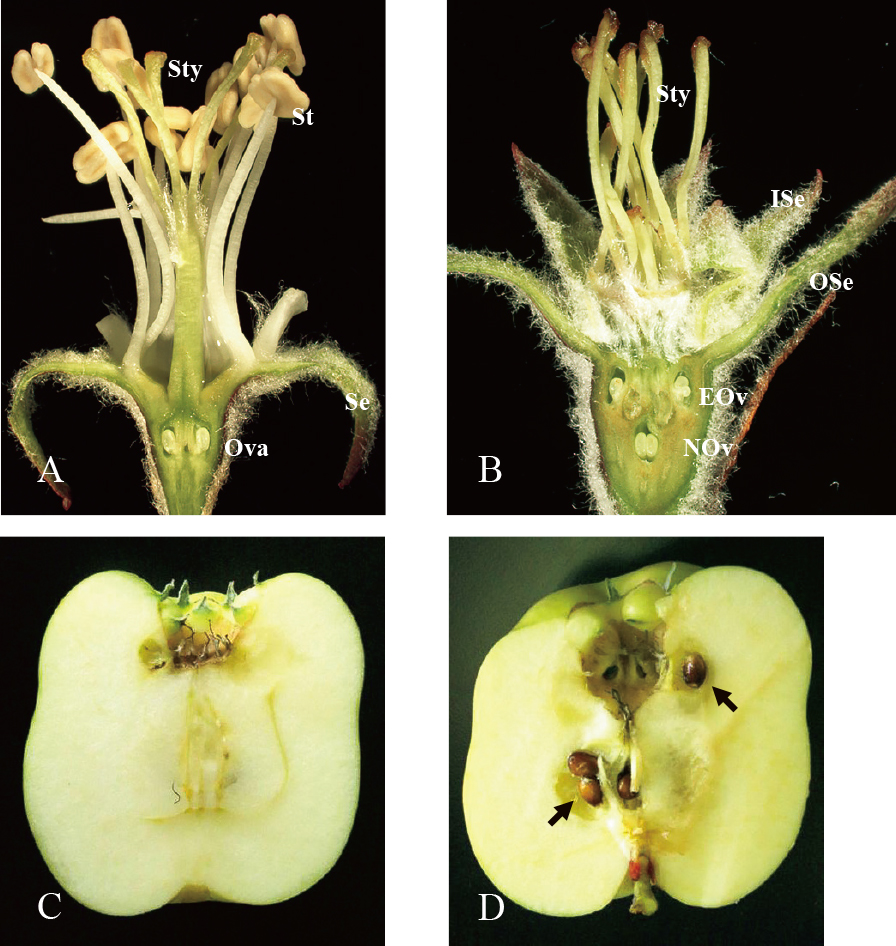
Normal and parthenocarpic flowers and apple fruit. A. Longitudinal section of normal ‘Fuji’ flower petals shows sepals, styles, stamens, and an ovary with ovules. B. Longitudinal section of a parthenocarpic ‘Spencer Seedless’ flower shows no petals and no stamens, but double sepals (outer sepals in whorl one and inner sepals in whorl two) and more styles, having bases with double ovaries and set ovules (ectopic ovary and normal ovary). C. Unpollinated fruit of parthenocarpic cultivar ‘Noblow’. D. Artificially pollinated fruit of ‘Noblow’. Black arrows indicate seeds in each carpel. Se; sepal, St; stamen, Sty; style, Ova; ovary, ISe; inner sepal, OSe; outer sepal, EOv; ectopic ovary, NOv; normal ovary.
According to Stout (1929), parthenocarpic apple cultivars have been known since ancient Greek and Roman times, but it is speculated that parthenocarpy and flower organ mutations have been studied since the 16th century. In this report, two cultivars without petals and stamens, ‘Wellington Seedless’ and ‘Spencer Seedless’, are introduced with photographs. It is suggested that parthenocarpic cultivars have been maintained by grafting rather than by breeding. In a paper by Sturtevant (1890), there was a description of ‘Noblow’ and it was introduced as a parthenocarpic cultivar with a flower organ mutation. The parthenocarpic cultivars, ‘Spencer Seedless’, ‘Wellington Bloomless’, ‘Noblow’, and ‘Wickson’, are currently conserved at the Apple Research Station, Institute of Fruit Tree and Tea Science, NARO in Morioka, Japan. According to Yao et al. (2001), the retrotransposon insertions are located in different sites of the MdPI genome in ‘Spencer Seedless’, ‘Wellington Bloomless’, and ‘Rae Ime’. ‘Noblow’ is also inserted in a different site of the MdPI gene from ‘Spencer Seedless’ and ‘Wellington Bloomless’. It is thought that crossings of heterozygous cultivars with insertional mutations produced the parthenocarpic cultivars. There is a record from before 1900 (Sturtevant, 1890), and we have looked for the genomes of 116 popular cultivars around the world. It was discovered that five cultivars had undergone retrotransposon insertion of the MdPI gene heterogeneously (Table 1). When these five cultivars as pollen parents were crossed with ‘Spencer Seedless’, individuals homozygous for the retrotransposon insertion of the MdPI gene were obtained. We are currently investigating whether these F1 individuals have parthenocarpy with floral organ mutations and the relation between MADS genes and parthenocarpy in several plants will be discussed in this article. Research on parthenocarpic apple, tobacco, tomato, and grape are listed in Table 2.
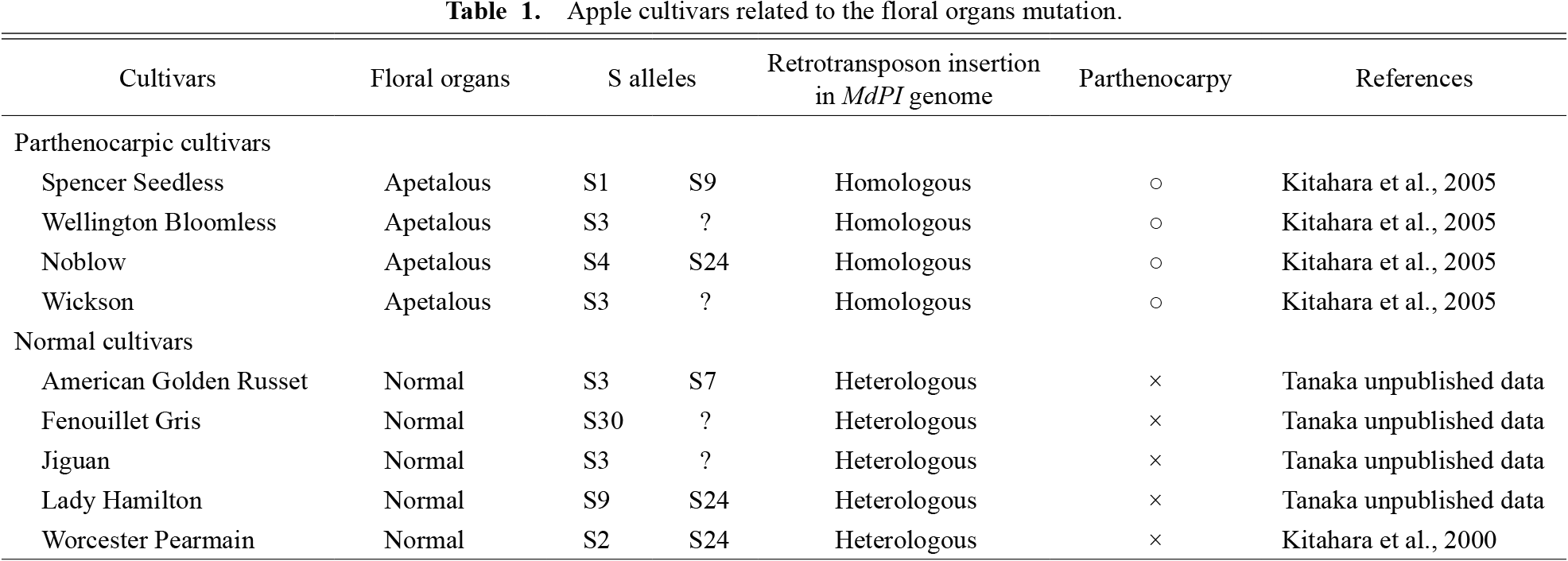
Apple cultivars related to the floral organs mutation.
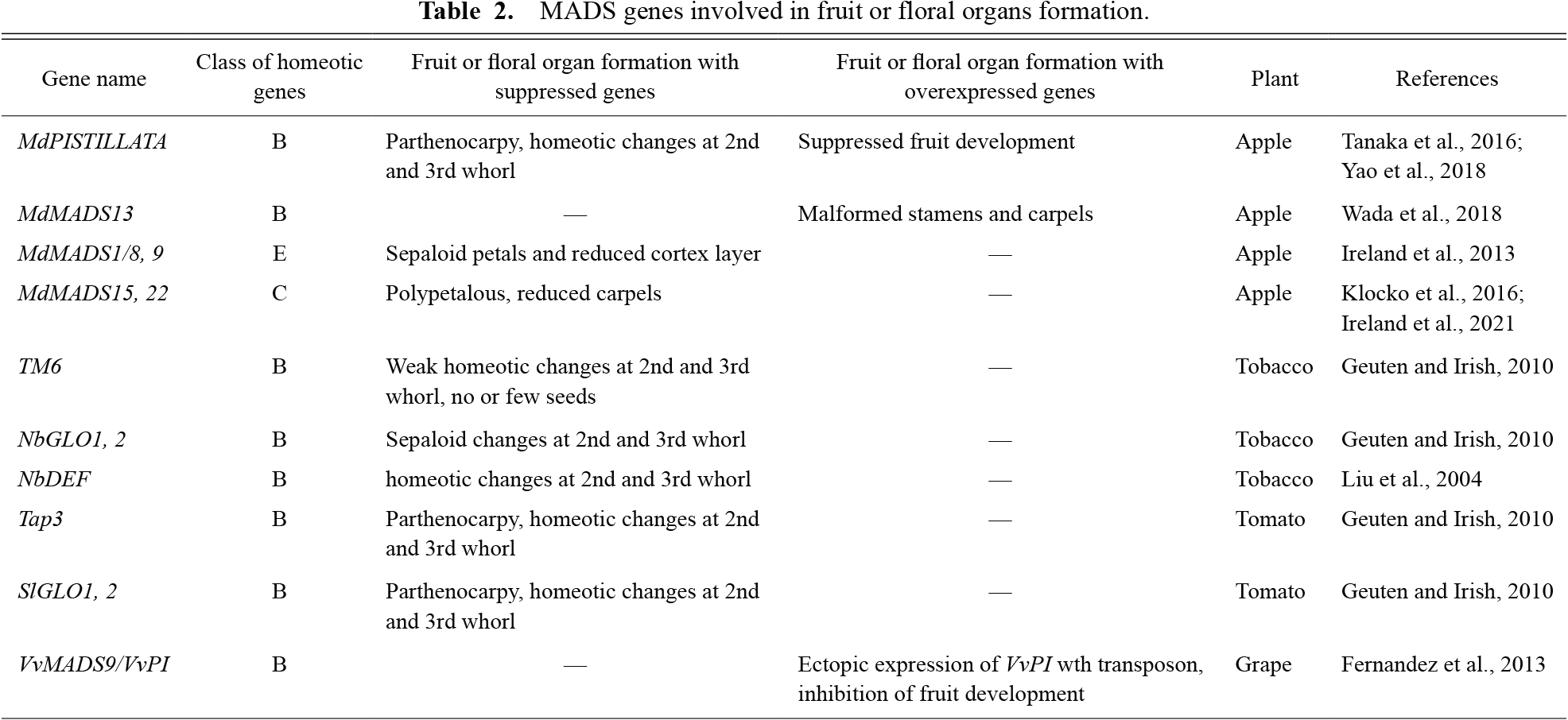
MADS genes involved in fruit or floral organs formation.
MdPISTILLATA (MdPI) is a homologue of the class B gene PISTILLATA (PI) of Arabidopsis (Goto and Meyerowitz, 1994; Yao et al., 2001). Apple parthenocarpic cultivars with double sepals and carpels have a transposon insertional mutation in the MdPI gene that suppresses MdPI expression. To identify the MdPI function, an Arabidopsis pi-1 mutant was selected for a complementary experiment. The pi-1 mutant has a homeotic mutation (background; Landsberg erecta, Ler) (Goto and Meyerowitz, 1994), with no petals or stamens that have been replaced by sepals and carpels, respectively. Apple MdPI cDNA from the cultivar ‘Jonathan’ was linked to the CaMV 35S promoter and constructed for the binary vector. First of all, the MdPI vector was transduced into Arabidopsis Columbia (Col, genotype; PI/PI) by the floral dip method because the pi-1 mutant could not be transformed directly. The resulting transformant flowers with antibiotic resistance (genotype; PI/PI + MdPI) showed the sepal margins became white like petals. This result was consistent with the phenotype of transformant flowers by 35S:PI from Arabidopsis (Krizek and Meyerowitz, 1996; Lamb and Irish, 2003; Yang et al., 2003). It was noted that other floral organs did not change in either case (Tanaka et al., 2007). The 35S:MdPI (Col) pollens were crossed with the pi-1 mutant (Ler). The F1 genotypes were PI/pi + MdPI, and the F1 was selfed to obtain F2 (pi/pi + MdPI) transformants. Specific primers for the Arabidopsis pi gene and apple MdPI cDNA were used for PCR reactions and it was confirmed that the expected transformants were obtained. The F2 flowers (pi/pi + MdPI) had four normal petals at whorl two and carpeloid stamens at whorl three. The fine structure of the petals was observed with a scanning electron microscope and recovered completely. The carpeloid stamens were filamentous at the proximal part and had unclosed carpels with ovules at the distal part. This chimeric recovery at whorl three has been reported in the introduction of 35S:PI into the pi-1 mutant (Lamb and Irish, 2003). These results supported the idea that MdPI can act as a PI substitute for floral organ formation. The regulation of MdPI expression was studied with a promoter and reporter system. A 1.0 kb upstream region of the MdPI genome has specific repeated sequences, such as the PI promoter, so the GUS reporter gene was linked to the 1.0 kb promoter (p1MDPI.1G). Transgenic Arabidopsis with p1MDPI.1G was analyzed with GUS staining, and it was observed only at petals and stamens (Tanaka et al., 2007). These results demonstrated that the MdPI promoter specified the expression at petals and stamens under transgenic Arabidopsis. It was concluded that MdPI is an orthologue of Arabidopsis PI, playing a role in floral organ identities similar to the class B genes. Apple parthenocarpic cultivars lost MdPI function following insertion of a transposon into the genome (Yao et al., 2001). The apetalous flowers and parthenocarpic fruit sets were inherited as a recessive trait (Tobutt, 1994). However, the relationship between the loss of MdPI and parthenocarpy was still ambiguous (Wada, 2008). Apple flowers in bloom were dissected into four organs, sepals, petals, stamens, and carpels, with a hypanthium that covered the ovaries. The sepals, petals, and stamens are located on the hypanthium in pome fruits such as apples. MdPI expression was detected at petals and stamens and faint expression was detected at carpels with hypanthium from the dissected flowers (Wada, 2008; Yao et al., 2001). MdPI expression was detected more accurately by histochemical staining, and flower bud development was analyzed from the primordia to maturation of floral organs by in situ hybridization (Wada, 2008). Apple flower buds started to develop from the summer of the previous year’s bloom. Petal and stamen primordia staining was detected from September, and vascular bundles of stamens were also stained (Wada, 2008). The stains were observed on petals and stamens throughout development stages, but no signals in other tissues were detected. The vascular bundles of stamens were stained from the primordia to mature organs (Fig. 2). The cross-sections of mature flowers indicated that 20 vascular bundles were stained, and the signals continued to the top of the ovaries and faded out at the distal part (Fig. 2C–F). This means that no MdPI expression signals were detected in hypanthium tissues; in other words, it is hard to confirm a direct link between parthenocarpy and MdPI expression.
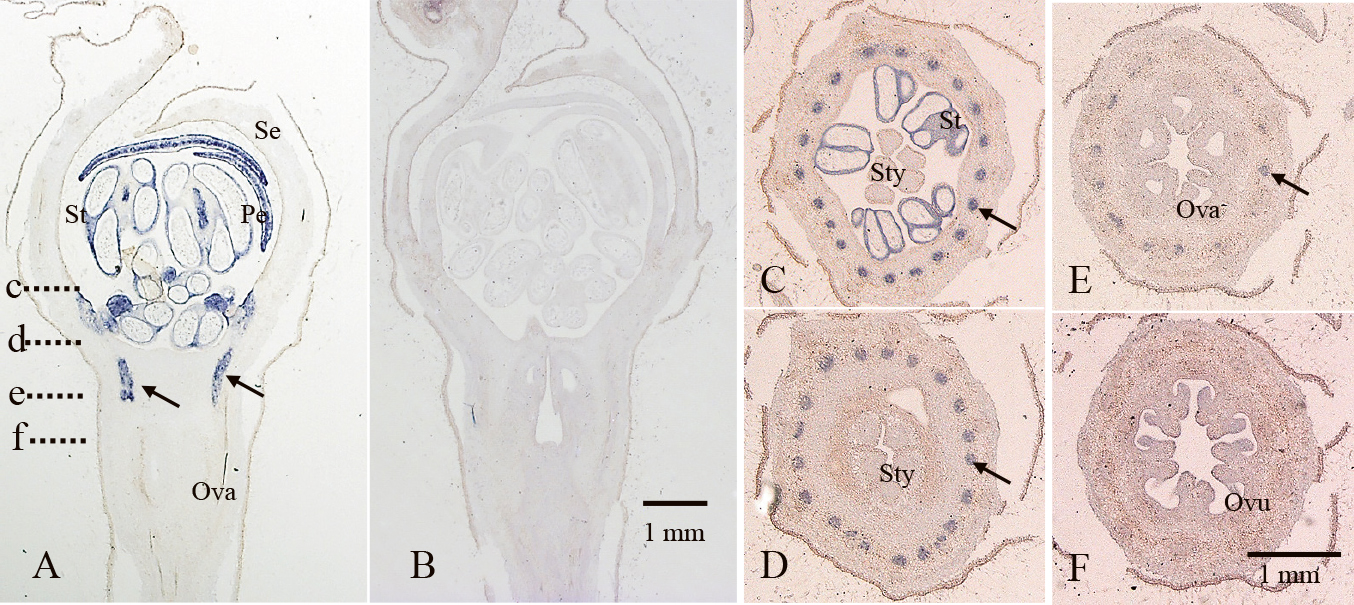
Staining images of MdPI expression. A. Longitudinal section of a ‘Fuji’ mature flower in April with an anti-sense MdPI probe. Blue staining represents MdPI expression. The stamens, petals, and vascular bundles (black arrows) were stained. B. Serial section of A with a sense MdPI probe. There were no signals. C–F. Cross sections of a mature flower with an anti-sense MdPI probe. Each small letter in A indicate a cross position corresponding to images with capital letters. The vascular bundles of stamens (black arrows) were stained. Se; sepal, Pe; petal, St; stamen, Sty; style, Ova; ovary, Ovu; ovule. A and B, or C, D, E, and F are on the same scale.
The next investigation was to see whether transgenic apples without MdPI expression would have the same traits as the parthenocarpic cultivars. Two reports analyzed MdPI-repressed transgenic apples (Tanaka et al., 2016; Yao et al., 2018). Tanaka et al. (2014) made a unique vector for the MdPI repressive transformant, composed of the MdPI promoter linked to anti-sense directional cDNA of MdPI and rolC:AtFT to accelerate flowering. The 37% of antibiotic resistant transformants flowered six months after transformation. There were fewer transformants with apetalous flowers compared with flowering ones. The transgenic apples, which had decreased endogenous expression of MdPI, had apetalous flowers, and the floral organs revealed that petals changed to sepals and stamens changed to carpels, the same as the parthenocarpic flowers. The 35S promoter linked to anti-sense MdPI was attempted, but no transformants were obtained. This suggested that the repression of many MADS genes could be harmful to the development or redifferentiation of apple culture. Yao et al. (2018) made MdPI-repressive transformants in another way. They used the 35S:MdPI vector for transformation, and two transformant phenotypes were obtained. One showed overexpression of MdPI lines, and the other had co-suppression of MdPI lines. The co-suppression lines had apetalous flowers identical to the parthenocarpic cultivars. This demonstrated that the suppression of MdPI led to the homeotic replacement of floral organs, even if a different approach was used. However, each anti-sense and co-suppression transformant indicated a clear difference in terms of parthenocarpy. The transformants with anti-sense repression had no parthenocarpy, while co-suppression showed parthenocarpy the same as the parthenocarpic cultivars. Therefore, the endogenous quantities of MdPI expression from each transformant may be different. It could not be compared exactly, but it is possible that a small amount of MdPI expression remained on the anti-sense side. Thereby, parthenocarpy may have been inhibited. However, it is a novel finding that an excessive amount of MdPI affected the fruit development of apples (Yao et al., 2018). The MdPI overexpression lines produced flattened fruit shapes and had basipetal and longitudinal grooves, which were undetected in the WT fruits. This is because cell expansion at the basipetal part close to the grooves was inhibited from the beginning of fruit development. In ordinary fruit development, MdPI and other PI orthologues hardly express in the fruiting stage after pollination. This indicated that the ectopic expression of MdPI affected the cell expansion and division of fruits; however, is not yet clear how MdPI disturbs fruit development.
Tomato (Solanum lycopersicum) is one of the model plants for studying fruit development and ripening. Plant hormones, MADS genes, and parthenocarpic mutants were analyzed for their involvement in tomato fruit development (Molesini et al., 2020). The class B genes from tomato were reported to be duplicated orthologs: TAP3 and TM6 are a homolog of APETALA3 or DEFICIENS, and SlGLO1 and SlGLO2 are homologs of PISTILLATA or GLOBOSA, respectively (Geuten and Irish, 2010; Poupin et al., 2007). These genes play a key role in petal and stamen identities, the same as the corresponding genes from Arabidopsis and Snapdragon (Weigel and Meyerowitz, 1994). However, the TAP3 mutant (tap3) and downregulation of a TAP3 transgenic tomato with RNAi silencing produced homeotic changes, petals changed to sepals and stamens changed to carpelloid structures, along with parthenocarpic fruit formation (de Martino et al., 2006; Okabe et al., 2019). The mutant and the RNAi lines developed fruit by cell expansion rather than the cell number increasing in the pericarp; this pattern of fruit development was different from pollinated wild-type fruit. The parthenocarpic fruit had increased phytohormone gibberellin (GA) contents in the ovary after anthesis. This suggested that a decrease in TAP3 expression in petals and stamens led to an increase in active GA in the wild type ovary. There are several reports that active GA induced parthenocarpy in apple, pear, and Arabidopsis (Galimba et al., 2019; Liu et al., 2018; Vivian-Smith and Koltunow, 1999; Watanabe et al., 2008). In apples, the enlargement of the fruit core and hypanthium were not distinct between pollinated and parthenocarpic fruit. Active GA was effective for both fruits. Other class B gene PISTILLATA or GLOBOSA orthologues, SlGLO1 and SlGLO2, were also investigated with downregulation experiments using RNAi (Geuten and Irish, 2010). The resulting transformants repressed SlGLO genes had homeotic changes of petals to sepals and stamens to carpels in floral organs and developed fruit without seeds, similar to the TAP3-silenced transgenic tomato. Two types of class B genes in tomato function as organ identity genes for the petals and stamen, the same as PI and AP3 of Arabidopsis. These tomato experiment results demonstrated that the suppression of tomato class B genes caused parthenocarpy.
Another fruit reported with a class B gene mutation is grape, which is has a somatic variant FLESHLESS BERRY (Flb) in the grape cultivar ‘Ugni Blanc’ (Fernandez et al., 2013). The Flb mutant shows normal buds the same as the wild type before anthesis, and cell divisions in the pericarp occurred at a lower level than wild type on the third day after anthesis. The Flb mutant could not continue to increase cell numbers in the mesocarp, resulting in a lack of flesh during fruit development. Interestingly, the Flb mutant seeds were slightly lighter than the wild type, but the germination rate was normal the same as the wild type (Fernandez et al., 2006). To identify the Flb gene, a microarray and suppression subtractive hybridization method (SSH) was used (Diatchenko et al., 1996; Fernandez et al., 2007). The Flb gene is located on chromosome 18, and the VvPI (VvMADS9) gene is closely linked to the Flb mutation, so it could be identified by SSH screening. The VvPI gene has already been reported as a PI homolog from Arabidopsis that specifies petal and stamen identity. It was clearly expressed in the flowers and showed lack of expression in the carpels and fruit of the wild type (Sreekantan et al., 2006). This trait is identical to PI orthologues from other plant species under morphogenesis. The sequencing of VvPI genes in the Flb mutant revealed that the VvPIb allele contained a 1.5 kb transposon insertion 730 bp upstream of the ATG initiation codon. This insertion caused ectopic expression of VvPI during fruit development, while the Flb mutant continues to express VvPI in the carpels and fruit during development. Therefore, it is assumed that the ectopic expression of VvPI prevents flesh development (Fernandez et al., 2013). Putative downstream genes of VvPI were analyzed, and up-regulated genes in Flb fruit were described as a regulator of flowering time, auxin-induced repressors, and transcription factors, and were thought to be involved in the gibberellin and auxin response. These results supported the idea that there is a certain regulation linkage between class B genes and fruit development via signal transduction of phytohormones, but the causal relationship is still unclear as in apple parthenocarpic cultivars.
The MdMADS13 is another candidate class B gene, along with APETALA3 (AP3) of Arabidopsis (Irish, 2009) and it expresses mostly in petals and stamens (van der Linden et al., 2002). MdMADS13 can form a heterodimer with MdPI (Wada et al., 2018), as in AP3 and PI. Additionally, MdMADS13 is a close orthologue of AP3 in apple. MdMADS13 was also analyzed using in situ hybridization in terms of flowers and fruit development. However, staining images were unclear in normal flowers during developmental stages (data not shown). MdPI expression was found in vascular bundles of stamens by a histological method (Fig. 2), but it was impossible to determine any subtle expression of MdMADS13 in normal flowers. MdMADS13 expression in normal flowers and fruits decreased similar to MdPI expression after anthesis (Wada et al., 2018; Yao et al., 2018), but interestingly, the MdMADS13 expression in the parthenocarpic cultivars was retained in flowers and fruits (Wada et al., 2018). The MdMADS13 expressions in parthenocarpic cultivar flowers were observed in ovules and the hypanthium by in situ hybridization (Fig. 3). This suggested that the ectopic expression of MdMADS13 in the hypanthium after anthesis affects the fruit enlargement of parthenocarpic cultivars. It is necessary to investigate what kind of genes regulate MdMADS13. Further, it is important to reveal how MdMADS13 influences fruit enlargement. The overexpression of MdMADS13 under the 35S promoter was attempted to produce transgenic Arabidopsis (Wada et al., 2018). Some transformants exhibited severe phenomena, including malformed stamens and carpels. Several stamens changed to carpeloids, with anthers partially replaced by papillae. The carpels were unclosed with naked ovules and the number was slightly increased. These homeotic changes due to MdMADS13 differed from the effects caused by 35S:AP3 in Arabidopsis (Jack et al., 1994). The ectopic expression of AP3 in whorl four caused replacement of carpels with stamens. AP3 expression also induced PI expression in whorl four and that is why the carpels changed to stamens according to the ABC model theory. In 35S:MdMADS13, it seemed the MdMADS13 product interfered with the binding of AP3 and AG protein in whorl three. As a result, the transformants with 35S:MdMADS13 exhibited carpeloid stamens in whorl three as in class B mutants. It was not clear whether MdPI and MdMADS13 influenced mutual expressions in apples. The loss of MdPI due to a retrotransposon in flower development seemed to cause ectopic expression of MdMADS13, resulting in fruit enlargement. To analyze how the MdMADS13 overexpression affected fruit enlargement in apple, we attempted to obtain transformants with 35S:MdMADS13. However, the resulting plants had antibiotic resistance, did not grow and died in vitro (data not shown). For MdMADS13, a MADS-box transcriptional factor, its expression in all tissues affected too many genes and could have inhibited plant growth.
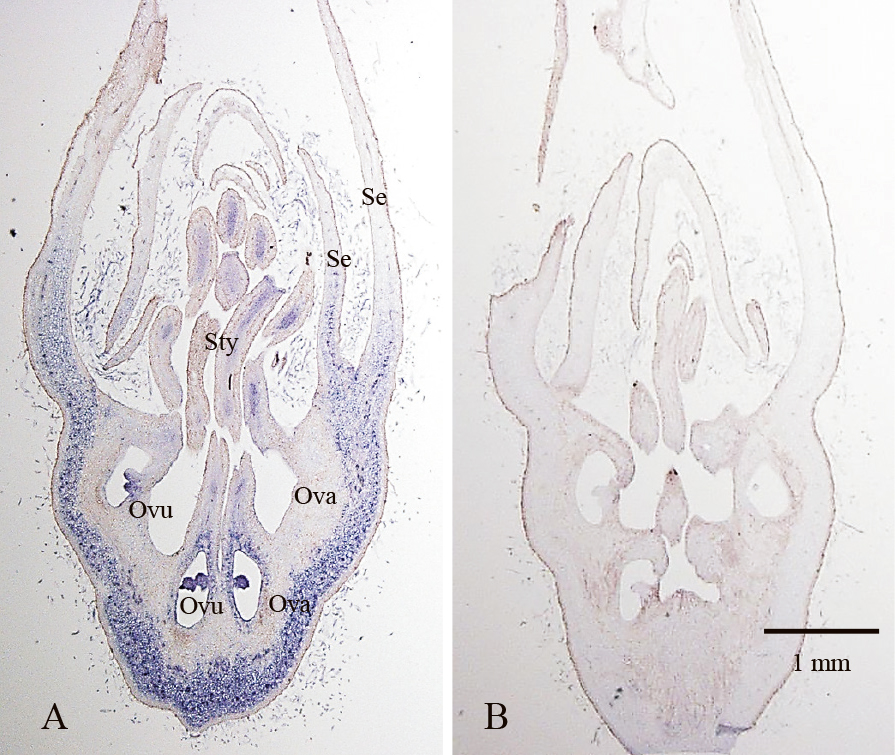
Staining images of MdMADS13 expression. A. Longitudinal section of an apetalous flower (parthenocarpic cultivar: ‘Spencer Seedless’) in April with an anti-sense MdMADS13 probe. Blue staining represents MdMADS13 expression. In addition, the ovules and hypanthium were stained. B. Serial section of A with a sense MdMADS13 probe. Se; sepal, Sty; style, Ova; ovary, Ovu; ovule. A and B are on the same scale.
Another class B gene is the MdTM6 gene in apple, which belongs to the tomato MADS-box gene 6 (TM6) lineage (Kitahara et al., 2004) that is another sub-lineage of AP3 (Kramer et al., 1998). The phylogenetic tree indicated that MdTM6 was close to MdMADS13, which belongs to the euAP3 lineage. However, Arabidopsis lacks this TM6 homolog. On the other hand, grape, petunia and tobacco have the TM6 homolog, and have been studied regarding the expression time and tissues. For the results on apple and tomato, each TM6 homolog expressed a different time and tissue profile (Geuten and Irish, 2010; Poupin et al., 2007). The homologs seemed to be divided in each lineage and obtained new functions. The suppression transformants of NbTM6 from tobacco (N. benthamiana) exhibited a weak phenotype of the class B mutation and set fruits with zero or very few seeds. The heterodimer formations with other MADS box proteins were studied in apple and tobacco, and the MdTM6 protein did not bind to MdPI or MdMADS13 protein either (Wada et al., 2018). NbTM6 protein binds to NbGLO1 and NbGLO2 (PI homologs) proteins, but not to NbDEF (AP3 homolog) protein in tobacco (Geuten and Irish, 2010). These results suggest that the MdTM6 proteins are not composed of a MADS box protein heteromultimer (Honma and Goto, 2001; Smaczniak et al., 2012) and did not play the main role in flower organ identities and fruit formation in apples.
The SEPALLATA (SEP) genes also have MADS-box and act as class E genes in the ABCE model. SEP plays an essential role with ABC genes to identify each floral organ in the four whorls (Jack, 2001). There are four SEP genes in Arabidopsis that show partial redundancy, so the SEP1/2/3/4-deficient quadruple mutant replaced floral organs with leaf-like organs (Ditta et al., 2004; Honma and Goto, 2001; Pelaz et al., 2000). Overexpression of SEP4 transformants changed the inflorescences into terminal flowers (Ditta et al., 2004). Apples have eight genes homologous to the SEP genes (Sung and An, 1997; Sung et al., 2000; Yao et al., 1999). Apple MdMADS1/8 and MdMADS9 are located in the SEP1/2 clade, MdMADS18/118 in the SEP3 clade, and MdMADS4 in the SEP4 clade by phylogenetic analysis. MdMADS3/7 and MdMADS6 are located in a separate clade to SEP1/2, SEP3, and SEP4, homologous to petunia (Petunia hybrida) SEP genes (PhFBP9 and PhFBP23) (Ireland et al., 2013). The eight SEP-like genes from apple were studied for their spatial and temporal gene expressions, respectively. MdMADS1/8 and MdMADS9 showed high expressions at the core and cortex during fruit flesh development in the wild type. The expression profile of MdMADS1/8 and MdMADS9 suggested that the genes are involved in fruit development. An apple transformant with MdMADS1/8 suppression was produced by the anti-sense method and investigated in terms of the flower and fruit formation (Ireland et al., 2013). It was noted that the MdMADS1/8-suppressed apple flowers have sepaloid petals and altered cell walls in the hypanthium. However, the stamens and carpels seemed to have normal numbers and sizes. Further, the fruits had a reduced cortex layer and lacked hypanthium enlargement (Ireland et al., 2013). A study to examine the interaction factors binding to the MdMADS13 protein was conducted by a yeast two-hybrid system. We obtained two clones, which were identical to MdMADS1/8 and MdMADS9 (unpublished data). This suggested that the MdPI, MdMADS13, MdMADS1/8, and MdMADS9 proteins could form a heterotetramer and act as a transcription factor for the identity of floral organs as in the floral quartet model of Arabidopsis (Theiβen et al., 2016).
AGAMOUS (AG) is one a MADS-box family and class C gene that specifies stamens and carpels from Arabidopsis (Dreni and Kater, 2014; Yanofsky et al., 1990). MdMADS15 and MdMADS22 are close homologs of AG and are located in the AG clade of the phylogenetic tree (Ireland et al., 2021; Klocko et al., 2016). Transformants suppression of MdMADS15 was achieved by an RNAi method and the flower and fruit development were studied. The transformants showed reduced expression of MdMADS15 and MdMADS22 and polypetalous flowers, with stamens replaced by petals and petaloid stamens. Further, the number of carpels was decreased, and sepals were normal. Thus, the phenotype of transformants was similar to the ag mutant of Arabidopsis. The apple cultivar ‘Galaxy’ used in the transformation exhibits parthenocarpy. However, it is unclear whether MdMADS15 and MdMADS22 influenced fruit development, but the transformants showed the same fruit enlargement as control fruits. Therefore, suppression of the class C genes does not prevent fruit enlargement in apples.
ConclusionApple parthenocarpic cultivars with a class B mutation of floral organs had an insertion of the MdPI genome leading to a lack of MdPI expression (Yao et al., 2001). The homeotic changes in floral organs were elucidated by suppression of MdPI, which was demonstrated to be an orthologue of the Arabidopsis PI gene (Tanaka et al., 2007). However, it is possible that the retrotransposon affected other genes, causing parthenocarpy. Transgenic apple research involving co-suppression or the antisense of MdPI revealed that suppression of MdPI expression caused the same homeotic changes in floral organs (Tanaka et al., 2016; Yao et al., 2018). Further, co-suppression of the MdPI transformants showed parthenocarpy, demonstrating that MdPI is the responsible gene for parthenocarpy. The inhibitory trait of MdPI was demonstrated by overexpression of MdPI in fruit, of which the shape became flattened due to cell expansion inhibition at the basipetal fruit tissues (Yao et al., 2018). These results indicated that MdPI expression prevented fruit development. The grape fleshless mutant Flb was identified as having ectopic expression of VvPI in fruit, which prevented flesh development (Fernandez et al., 2013). In both apple and grape, repression of the class B genes of MdPI and VvPI after fertilization was necessary for normal fruit development. It is assumed that the class B floral genes have some kind of inhibitory effects on the ovaries and hypanthium in fruit development. In situ hybridization of apple flowers with an MdPI probe clarified that MdPI was not expressed in the hypanthium, which largely contributed to fruit enlargement. It is therefore hypothesized that such signals transmitted cell to cell act as development repressors, and the signals were diffused from the class B floral organs, petals and stamens (Fig. 4). Emasculation of stamens and removal of petals in apple flowers did not induce parthenocarpy, and the vascular bundles of stamens remained in the tissues surrounding ovaries. MdPI expression was detected in the vascular bundles (Fig. 2), so the signals may be active. Putative candidates for the signals are a micro RNA, a phytohormone, a peptide or a protein (Galimba et al., 2019; Ripoll et al., 2015; Watanabe et al., 2008; Yao et al., 2015). The class B genes have an inhibitory role in fruit development as a kind of regulator, acting to avoid wasting nutrition and seeds when fertilization is unsuccessful. It is possible MdMADS13 has a similar inhibition to MdPI. Genome editing for the exact deletion of MdPI, MdMADS13 and MdTM6 expression could clarify their roles in flower and fruit formation. It was demonstrated clearly that MdMADS1/8 and MdMADS9 are involved in hypanthium development (Ireland et al., 2013). How the class B genes regulate the SEP genes and how they control the metabolism of GA in fruit development are the targets of further research on apple parthenocarpy.
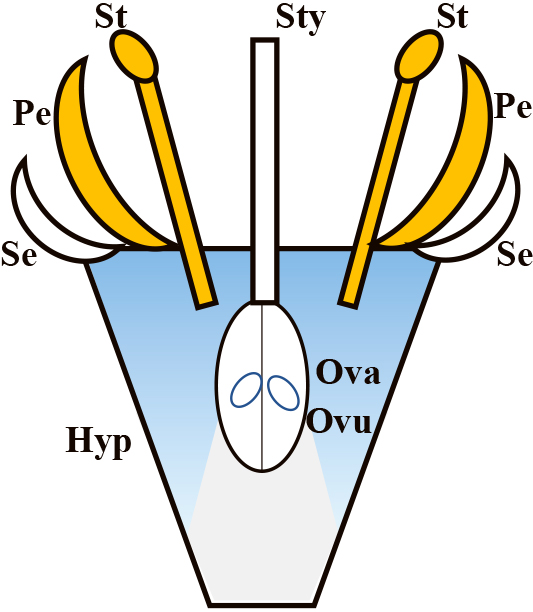
Model of putative signals for inhibition of fruit development. The picture represents a simplified apple flower. The stamens and petals are colored yellow and putative signals for inhibition are colored blue with gradation. Se; sepal, Pe; petal, St; stamen, Sty; style, Ova; ovary, Ovu; ovule, Hyp; hypanthium.