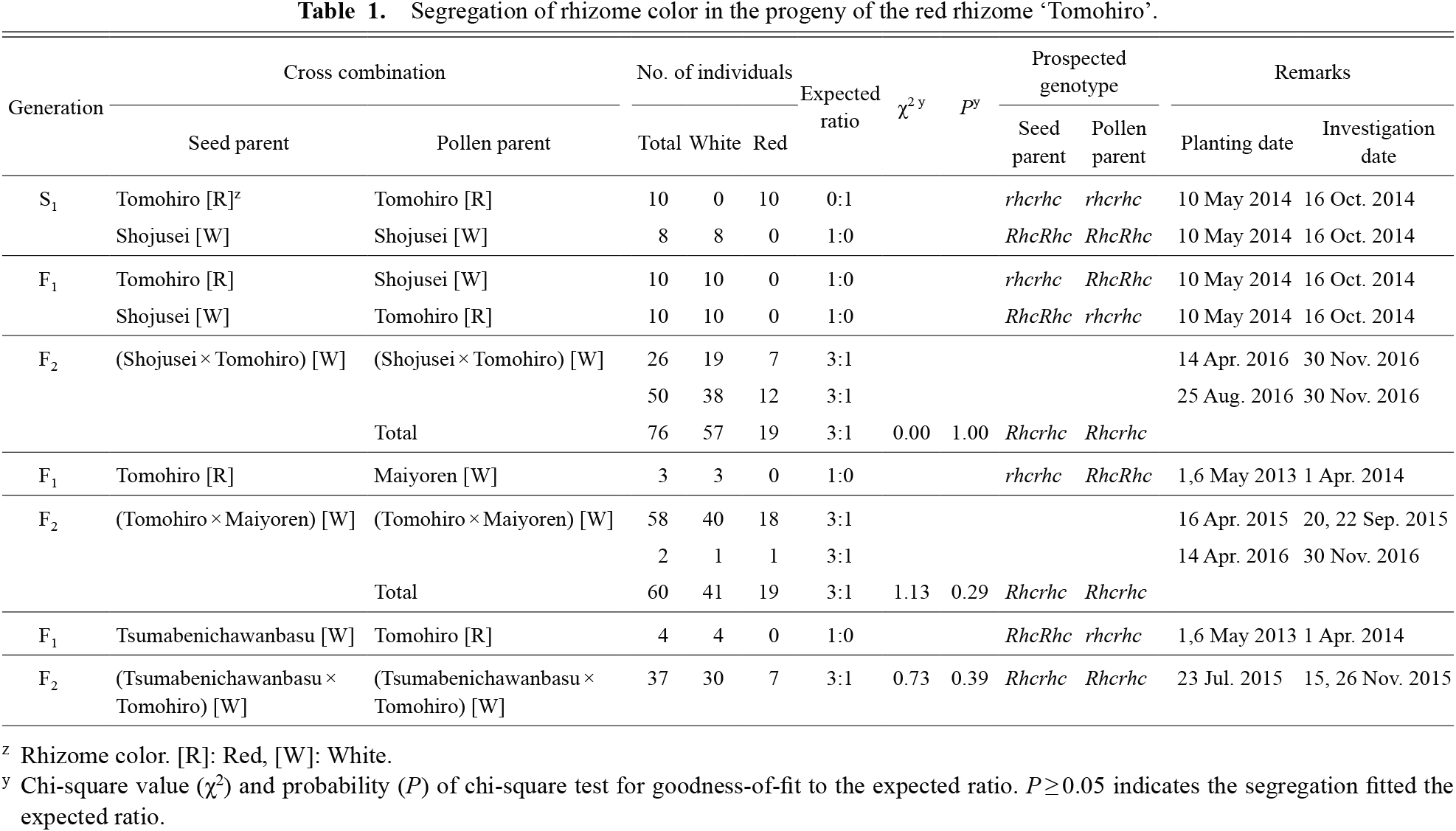
Abstract
Characteristics and inheritance of rhizome coloration in the red rhizome lotus (Nelumbo nucifera) ‘Tomohiro’ were investigated to achieve stable production and breeding of red rhizome cultivars. Rhizome coloration proceeded from the basal internodes to distal ones during the growing stage, and coloration of enlarged distal rhizomes was completed in October. No individuals with both red and white rhizomes in one plant were found, and there were no rhizomes with a mottled pattern or an intermediate color between red and white. The coloration of the skin and flesh was synchronized in red rhizome plants. It was therefore estimated that the skin and flesh coloration of rhizomes was controlled by the same gene. Rhizomes of all selfed plants of ‘Tomohiro’ and white rhizome cultivars were red and white, respectively, and all F1 plants generated by crossing ‘Tomohiro’ and white rhizome cultivars were white. Rhizome color in F2 plants generated by self-pollination of F1 plants divided into white and red individuals with a 3:1 ratio. These results suggested that red rhizome coloration is governed by a single locus, designated RHIZOME COLOR (RHC), with dominant white (Rhc) and recessive red (rhc) coloration alleles.
Introduction
Lotus is a perennial aquatic and vegetative propagation plant belonging to the family Nelumbonaceae, and the plants are classified into two species, Nelumbo nucifera Gaertn. and N. lutea (Willd.) Pers., originating in India to China and the United States, respectively (Sakamoto, 1977; Toyoda, 1981). These species show wide variations in flower color and shape, and have been used as ornamental plants. The plants have also been widely used for food by consuming enlarged rhizomes and seeds, for tea made from the leaves and petals, and for woven fabrics using cellulose fibers that can be taken from the petioles (Kitamura, 2000; Ogasawara, 2005). Especially in China, the plant has been prized as a panacea since ancient times and is still used in traditional medicine (Guo, 2009). According to a recent study, lotus has attracted a lot of attention as a plant good for health because it is effective at improving allergy symptoms (Kaneyasu et al., 2019).
Ornamental lotus plants exhibit easy flowering and insufficient rhizome enlargement, whereas the edible lotus exhibits hard flowering and a strong, hypertrophic rhizome. Edible lotus has been cultivated as a traditional vegetable for more than 1000 years in Japan, and the enlarged rhizomes are eaten boiled or raw. Edible lotus plants generally require a long period of approx. three to four years from true seed sowing to flowering, and it is impossible for them to flower unless they are cultivated in a large field. Breeding of edible lotus, therefore, is not advanced as compared to other vegetables because of the labor required and long juvenile stage. The major objectives of breeding are limited to high-yield, disease resistance and early-harvesting characteristics, and rhizome color is not considered (Minamikawa and Saito, 1962; Sawada, 2010). However, the red rhizome lotus ‘Tomohiro’ (Fig. 1) was found in a cultivation field in which the white rhizome lotus ‘Bicchu’ was cultivated in Tokushima Prefecture, Japan a few decades ago. It is therefore thought that the red rhizome lotus is a mutation of the white rhizome cultivar ‘Bicchu’. Compared to ‘Bicchu’, ‘Tomohiro’ has a reddish color not only in the rhizome, but also the flower, back of the leaves and petiole (Fig. 2). Potatoes and sweet potatoes show color variation in the edible underground part, e.g., red or purple colors, and it was reported that these red and purple pigments are anthocyanins (Li et al., 2019; Rodriguez-Saona et al., 1998). There is, however, no report of the red rhizome characteristic in lotus, except for ‘Tomohiro’. It has been reported that anthocyanins have various benefits on health such as antioxidant, antiinflammatory, antihypertensive and antidiabetic effects (Blesso, 2019; Cassidy et al., 2011; Khoo et al., 2017; Sancho and Pastore, 2012; Wang et al., 1999). Purple-fleshed sweet potatoes containing anthocyanin are used as a raw material in many processed foods (Suda et al., 2003; Yasuda et al., 2014) because the anthocyanin in purple sweet potatoes is a good color and shows excellent stability against light and heat (Odake et al., 1994). Vegetables containing anthocyanin have a vivid in appearance and contain many functional ingredients, so they are preferred by consumers and command high market value. Analysis of the pigments extracted from the red rhizome of ‘Tomohiro’ showed that it contains six types of anthocyanins, the major ones being delphinidin 3-glucoside and cyanidin 3-glucoside (unpublished). Therefore, it is expected that ‘Tomohiro’ has high potential as a cultivar with high value in the market. However, the yield of this cultivar is very low, as low as half as much, compared to other common white cultivars (personal communication). If a high-yielding red rhizome cultivar could be bred, it would achieve high market value and good productivity for farmers.

In order to breed a new cultivar efficiently, it is effective to clarify the inheritance and expression patterns of the required traits. Although the inheritance of anthocyanin accumulation has been clarified in many vegetables, such as onion, radish, tomato, and basil (Asako et al., 2011; Hoshi et al., 1963; Jones et al., 2003; Khar et al., 2008; Phippen and Simon, 2000), the inheritance of red rhizome coloration in lotus has not been clarified. Moreover, the color of ‘Tomohiro’ rhizomes at the young elongation stage is white, and they turn red as they mature (based on our preliminary observation). Therefore, it’s necessary to establish the coloration and stability of rhizome coloring at harvest time.
In this study, we investigated rhizome coloration and inheritance of rhizome coloring in the progeny of ‘Tomohiro’ in an attempt to breed new red rhizome lotus cultivars.
Materials and Methods
Elucidation of coloring time for red rhizome lotus
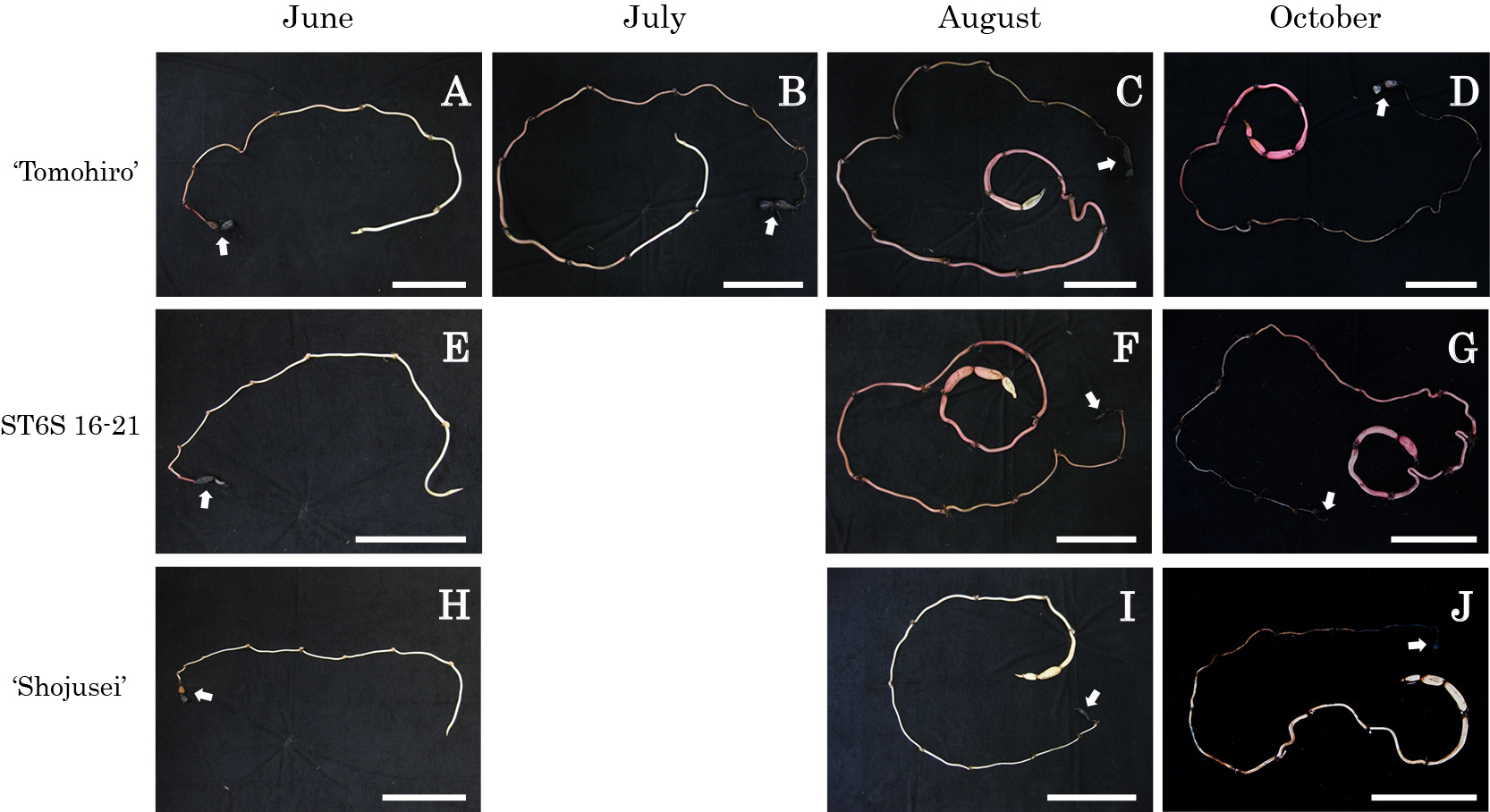
Red rhizomes of ‘Tomohiro’ and ST6S 16-21, a red rhizome strain generated by self-crossing of an F1 individual (‘Shojusei’ × ‘Tomohiro’), and white rhizomes of an ornamental lotus ‘Shojusei’ as a control were used in this study. The rhizomes were planted in 30 cm-diameter plastic pots containing mixed soil (paddy field soil:horticultural soil = 2:1, v/v) with 20 g slow-release fertilizer (N:P:K = 14:12:14%). They were cultivated in an open field of the University Farm, School of Agriculture, Kyushu University and the pots were filled with water during cultivation. Rhizomes of ‘Tomohiro’ were planted on 15 April 2019, and five, three, four and five plants were harvested on 23 June, 13 July, 25 August and 20 October, respectively. Rhizomes of ST6S 16-21 and ‘Shojusei’ were planted on 14 April 2018 and harvested on 17 June (ST6S 16-21: four plants, ‘Shojusei’: two plants), 18 August (ST6S 16-21: five plants, ‘Shojusei’: two plants) and 21 October (ST6S 16-21: five plants, ‘Shojusei’: three plants). The color of each main internode was examined using the Royal Horticultural Society (RHS) Colour Chart. In addition, rhizome flesh color was investigated with cross sections of the ‘Tomohiro’ rhizome.
Characteristics of red rhizome coloration in lotus
Back crossed seeds (BC1) of [‘Tomohiro’ × ‘Maiyoren’ (white rhizome cultivar)] × ‘Tomohiro’ were used in this study. The seeds were prepared for germination on 8 April 2015 according to the method of Masuda et al. (2006). They were soaked in conc. H2SO4 for three to four hours and rinsed with water. After rinsing, they were soaked in water for one day at 25°C and softened seed coats were removed. The peeled seeds were incubated in water at 25°C under a continuous fluorescent light condition until germination. The seedlings were transplanted to 21 cm-diameter plastic pots containing mixed soil (paddy field soil:horticultural soil = 2:1, v/v) with 20 g slow-release fertilizer (N:P:K = 14:12:14%) on 16 April. Nine seedlings were grown in an open field of the University Farm, School of Agriculture, Kyushu University and plastic pots were filled with water during cultivation. The skin color of all internodes was categorized as red or white by appearance on 20 and 22 September.
Self-pollinated seeds (F2) of an F1 individual (‘Shojusei’ × ‘Tomohiro’) were provided to investigate the relationship between the skin and flesh colors of rhizomes. Germination treatment was carried out on 14 June 2020, and the seedlings were transplanted to 30 cm-diameter plastic pots on 21 June. These were cultivated in the same field and with the same method as above, and 5 g additional fertilizer (N:P:K = 16:16:16%) was applied on 19 July. Rhizomes were harvested on 23 November, and the skin and flesh colorations of their 2nd internodes from the tip were judged. The skin color was determined using the RHS Colour Chart, and the flesh color was judged by appearance.
Inheritance of red rhizome coloration in lotus
Seeds of S1, F1, and F2 plants generated by crossing among the red rhizome lotus ‘Tomohiro’, three white rhizome lotuses, ‘Maiyoren’, ‘Tsumabenichawanbasu’ and ‘Shojusei’, and their progeny as shown in Table 1 were collected. The seeds were prepared for germination and the seedlings were transplanted to 21 cm or 30 cm-diameter plastic pots containing mixed soil and using the same method as described before. They were cultivated as mentioned in the above experiment. After cultivation, the color of 2nd distal internodes of enlarged rhizomes was categorized as red or white by appearance. Segregation of the rhizome color was analyzed by chi-square test for goodness-of-fit to expected ratio, and P ≥ 0.05 indicates the rhizome color fitted the expected ratio.
Results
Elucidation of coloring time for red rhizome lotus
In ‘Tomohiro’, rhizomes grew from 8 to 10 internodes in June, and the 1st to 3rd internodes from the mother rhizomes were well colored (Fig. 3; Table 2). Insufficient and no/less coloring was, however, observed in the middle and upper internodes, respectively. In July, rhizomes grew from 11 to 13 internodes, and the basal and middle (5th to 9th) internodes were colored, but a few distal internodes were less colored. In August, rhizomes grew from 14 to 19 internodes, and deep coloring was recognized, except for a few distal internodes. Rhizome girth enlargement was observed in a few distal internodes in most of the plants in August. In October, rhizomes grew from 15 to 18 internodes, and all of them were colored deeply. Rhizome girth enlargement was observed in a few distal internodes in all individuals. The results of ST6S 16-21 were similar to those for ‘Tomohiro’ (Table 2). In contrast, almost all the ‘Shojusei’ internodes were white to cream in all periods, and no internodes with colored rhizomes were observed except for withered browning internodes observed in the lower nodes in June. Colorations of the skin and flesh were synchronized (Table 2; Figs. 3 and 4). Basically, no flesh coloration was observed in the internodes in which the skin was not colored. The more the skin coloration of the internodes, the greater the flesh coloration. In addition, it was found that the coloration progressed in parts of the vascular bundle as compared to those around vent holes (data not shown).
There were six and three BC1 plants with red- and white-colored rhizomes of (‘Tomohiro’ × ‘Maiyoren’) × ‘Tomohiro’, respectively (Table 3). No individuals with both red and white color rhizomes in one plant were observed, and there were no rhizomes with a mottled pattern or an intermediate color between red and white.
In F2 plants generated by self-pollination of an F1 individual (‘Shojusei’ × ‘Tomohiro’), there were two types of plants; red rhizome plants with a deep red skin color and white rhizome plants that had white to cream skin color (Table 4). Observation of the rhizome skin and cross section in the second internode proved that colors of the skin and flesh were similar, and there were no plants with different rhizome colors between the skin and flesh (Fig. 5).
Inheritance of red rhizome coloration in lotus
Enlarged rhizomes of ‘Tomohiro’ and ‘Shojusei’ self-pollinated plants were red and white, respectively (Table 1; Fig. 6). Enlarged rhizomes of F1 plants generated by crossing between ‘Tomohiro’ and white rhizome cultivars were white. In contrast, segregation of individuals with white and red rhizomes was observed in F2 generations. The segregation ratio of individuals with white and red rhizomes in the F2 generations of an F1 individual (‘Shojusei’ × ‘Tomohiro’) fitted the expected 3:1 ratio (white:red = 57:19, P = 1.00). F2 generations of the F1 individual (‘Tomohiro’ × ‘Maiyoren’) (white:red = 41:19, P = 0.29) and (‘Tsumabenichawanbasu’ × ‘Tomohiro’) (white:red = 30:7, P = 0.39) also fitted with the expected 3:1 ratio.
Discussion
It was found that the rhizome coloration in the red rhizome ‘Tomohiro’ proceeded from the basal internodes to distal ones during the growing stage, and coloration of enlarged distal rhizomes was completed in October. Observation of internode cross sections also proved that the skin and flesh coloration of rhizomes proceeded simultaneously. In Japan, edible lotus is generally harvested from the end of the July to May of the following year in open field cultivation. The demand for edible lotus increases particularly from late July to early August for the Bon Festival, and from middle to late December for New Year’s Day. Although the rhizomes harvested in the winter season are fully-matured, those harvested in the summer season are still growing. This study revealed that the coloration of distal internodes in red rhizome cultivars/strains was insufficient in August. It is thought that the coloring of enlarged red rhizome varieties is not completed at the time of early harvesting. Elucidation of the factors accelerating rhizome coloration, e.g. amount of fertilizer applied, soil pH, light quality, solar radiation flux, and presence or absence of ultraviolet rays, will provide useful information to establish a stable cultivation method for early-harvesting red rhizome lotus.
It was found that all the BC1 progeny had red or white rhizomes, and there were no individuals with red and white internodes within a plant. Investigation of F2 plants generated by a self-pollinated F1 individual (‘Shojusei’ × ‘Tomohiro’) also indicated that the coloration of skin and flesh were similar. In diploid potatoes, there are some strains with different tuber colors of skin and flesh, and it was reported that the skin coloration is controlled by the I gene, while the flesh coloration controlled by I and Pf genes (De Jong, 1987). In sweet potatoes, Mano et al. (2007) reported that expression of the IbMYB1 gene is involved in the flesh coloration of tuberous roots, and other regulatory genes are involved in the skin coloration of tuberous roots. Therefore, in potatoes and sweet potatoes, there are varieties/strains in which different flesh and skin coloration has been recognized, and the genes involved in skin coloration differ from those of flesh coloration. There were, however, no individuals with different coloration between the skin and flesh in lotus plants. As a result, it appears that skin and flesh coloration of rhizomes is controlled by the same gene.
It was found that the rhizomes of all ‘Tomohiro’ selfed plants were red, and the rhizomes of all F1 plants generated by crossing ‘Tomohiro’ and white rhizome cultivars were white. In addition, rhizome color in F2 plants generated by self-pollination of F1 plants divided into white and red individuals with a 3:1 ratio. It is therefore suggested that red rhizome coloration is governed by a single locus with dominant white and recessive red coloration alleles. We designated this locus RHIZOME COLOR (RHC). Assuming that the genotypes of white and red cultivars were RhcRhc and rhcrhc, respectively, those of the plants from self-pollination of the red rhizome ‘Tomohiro’ and from cross-pollination between ‘Tomohiro’ and white rhizome cultivars were rhcrhc (red) and Rhcrhc (white), respectively. In F2 plants generated by self-pollination of F1 plants, segregation of individuals with rhizome color (white:red = 3:1) was also in agreement with the predicted genotypes (RhcRhc:Rhcrhc:rhcrhc = 1:2:1).
The inheritance of anthocyanin pigment accumulation has been investigated in various vegetables. It was reported that accumulation of anthocyanin pigments in tubers is controlled by loci R/Rpw, P and I in cultivated diploid potatoes (De Jong, 1987; Dodds and Long, 1955, 1956). In addition, it was suggested that bulb color is controlled by at least five major loci in onion (Khar et al., 2008). Therefore, in many plants, multiple genes are involved in anthocyanin accumulation in the storage organ. However, in the present study, it was found that the lotus rhizome color displayed simple inheritance controlled by only one locus, RHC, without the complicated inheritance pattern of other geophytes.
The present study demonstrated that coloration of red rhizome cultivars was completed in the autumn, and the rhizome’s red trait was controlled by one locus, RHC. It was reported that the petals of lotus with red or pink flowers contain anthocyanins in common with white rhizome lotus cultivars (Chen et al., 2013; Deng et al., 2013). Anthocyanin biosynthesis is inhibited in the rhizomes of those cultivars, although they have the ability to produce anthocyanins in the petals. In contrast, anthocyanin accumulation is observed in the rhizomes as well as the petals in ‘Tomohiro’. It is therefore assumed that anthocyanin biosynthesis genes, for which expression is suppressed in the rhizomes of common cultivars, are expressed in ‘Tomohiro’; i.e., ectopic expression occurs. Since rhc is a recessive gene, ‘Tomohiro’ appears to have come from the selfing of a heterogenic Rhcrhc plant, a spontaneous mutant from a white rhizome cultivar such as ‘Bicchu’ with the RhcRhc genotype.
The low yield of ‘Tomohiro’ is a shortcoming compared to common white rhizome cultivars, and breeding red rhizome cultivars with a high yield is required. The main morphological factors affecting lotus yield are a hypertrophic rhizome and number of branches (Kasumi, 2003). In our preliminary observation, there were variations in rhizome hypertrophy and number of branches among red rhizome individuals in ‘Tomohiro’ progeny. Therefore, there is no relationship between anthocyanin accumulation by rhizomes and rhizome hypertrophy or number of branches, and high yield red rhizome cultivars should be produced by crossbreeding with high yield cultivars. In potatoes, genetic analysis and development of DNA markers are promoted for efficient breeding of potatoes that accumulate anthocyanin pigments (Strygina et al., 2019; Tierno and Ruiz de Galarreta, 2017). In the same way, development of genetic analysis and DNA markers for the red rhizome characteristic will provide useful information to establish more efficient breeding methods for new red lotus cultivars, and their development is expected in the near future.
Acknowledgements
The authors would like to thank Mr. Tomohiro Wakayanagi at Wakayanagi Farm, who found the spontaneous mutant ‘Tomohiro’ and provided us with the plants for the experiments. We also thank Dr. Eiji Sawada at Tokushima Agriculture, Forestry, and Fishers Technology Support Center for valuable information.
Literature Cited
- Asako, Y., Y. Owaki, Y. Ozeki, N. Sasaki, Y. Abe, T. Momose and K. Shimomura. 2011. Parental line “Inuidani” of Japanese radish (Raphanus sativus L.) accumulating pelargonidin as a major anthocyanidin entirely within its underground part and preliminary genetic analysis. Breed. Res. 13: 65–73 (In Japanese with English abstract).
- Blesso, C. N. 2019. Dietary anthocyanins and human health. Nutrients 11: 2107. DOI: 10.3390/nu11092107.
- Cassidy, A., E. J. O’Reilly, C. Kay, L. Sampson, M. Franz, J. P. Forman, G. Curhan and E. B. Rimm. 2011. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 93: 338–347.
- Chen, S., Y. Xiang, J. Deng, Y. Liu and S. Li. 2013. Simultaneous analysis of anthocyanin and non-anthocyanin flavonoid in various tissues of different lotus (Nelumbo) cultivars by HPLC-DAD-ESI-MSn. PLoS One 8: e62291. DOI: 10.1371/journal.pone.0062291.
- De Jong, H. 1987. Inheritance of pigmented tuber flesh in cultivated diploid potatoes. Am. Potato J. 64: 337–343.
- Deng, J., S. Chen, X. Yin, K. Wang, Y. Liu, S. Li and P. Yang. 2013. Systematic qualitative and quantitative assessment of anthocyanins, flavones and flavonols in the petals of 108 lotus (Nelumbo nucifera) cultivars. Food Chem. 139: 307–312.
- Dodds, K. S. and D. H. Long. 1955. The inheritance of colour in diploid potatoes. I. Types of anthocyanidins and their genetic loci. J. Genet. 53: 136–149.
- Dodds, K. S. and D. H. Long. 1956. The inheritance of colour in diploid potatoes. II. A three-factor linkage group. J. Genet. 54: 27–41.
- Guo, H. B. 2009. Cultivation of lotus (Nelumbo nucifera Gaertn. ssp. nucifera) and its utilization in China. Genet. Resour. Crop Evol. 56: 323–330.
- Hoshi, T., E. Takemura and K. Hayashi. 1963. Genetic modification of hydroxylation pattern in radish anthocyanins. Studies on anthocyanins, XLII. Bot. Mag. Tokyo 76: 431–439.
- Jones, C. M., P. Mes and J. R. Myers. 2003. Characterization and inheritance of the Anthocyanin fruit (Aft) tomato. J. Hered. 94: 449–456.
- Kaneyasu, M., M. Nagata, H. Ikeda, K. Ohnuki and K. Shimizu. 2019. Anti-allergic activity of lotus root (Nelumbo nucifera) powder in TDI-sensitized nasal allergy model mice. Food Agric. Immunol. 30: 968–978.
- Kasumi, M. 2003. Method of crossbreeding in edible lotus rhizome (Nelumbo nucifera Gaertn.) Bull. Ibaraki Plant Biotech. Inst. 6: 31–42 (In Japanese).
- Khar, A., J. Jakse and M. J. Havey. 2008. Segregations for onion bulb colors reveal that red is controlled by at least three loci. J. Amer. Soc. Hort. Sci. 133: 42–47.
- Khoo, H. E., A. Azlan, S. T. Tang and S. M. Lim. 2017. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 61: 1361779. DOI: 10.1080/16546628.2017.1361779.
- Kitamura, F. 2000. Hasu—hasu wo tanoshimu—(In Japanese). Net Musashino Press, Tokyo.
- Li, A., R. Xiao, S. He, X. An, Y. He, C. Wang, S. Yin, B. Wang, X. Shi and J. He. 2019. Research advances of purple sweet potato anthocyanins: extraction, identification, stability, bioactivity, application, and biotransformation. Molecules 24: 3816. DOI: 10.3390/molecules24213816.
- Mano, H., F. Ogasawara, K. Sato, H. Higo and Y. Minobe. 2007. Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple-fleshed sweet potato. Plant Physiol. 143: 1252–1268.
- Masuda, J., T. Urakawa, Y. Ozaki and H. Okubo. 2006. Short photoperiod induces dormancy in lotus (Nelumbo nucifera). Ann. Bot. 97: 39–45.
- Minamikawa, K. and H. Saito. 1962. Studies on the edible east indian lotus, Nelumbo nucifera GAERTN. (V) On the breeding methods and instances. Kyushu Agric. Res. 24: 37–39 (In Japanese).
- Odake, K., A. Hatanaka, T. Kajiwara, T. Muroi, K. Nishiyama, O. Yamakawa, N. Terahara and M. Yamaguchi. 1994. Evaluation method and breeding of purple sweet potato “YAMAGAWA MURASAKI” (Ipomoea batatas POIR.) for raw material of food colorants. Nippon Shokuhin Kogyo Gakkaishi 41: 287–293 (In Japanese with English abstract).
- Ogasawara, S. 2005. Lotus fiber—comparison with the lotus fiber weaving at Myanmar and the picture scroll of taima-mandala. Sen’i Gakkaishi 61: 298–302 (In Japanese).
- Phippen, W. B. and J. E. Simon. 2000. Anthocyanin inheritance and instability in purple basil (Ocimum basilicum L.). J. Hered. 91: 289–296.
- Rodriguez-Saona, L. E., M. M. Giusti and R. E. Wrolstad. 1998. Anthocyanin pigment composition of red-fleshed potatoes. J. Food Sci. 63: 458–465.
- Sakamoto, Y. 1977. Hasu (In Japanese). Hosei University Press, Tokyo.
- Sancho, R. A. S. and G. M. Pastore. 2012. Evaluation of the effects of anthocyanins in type 2 diabetes. Food Res. Int. 46: 378–386.
- Sawada, E. 2010. Renkon—saibai kara kakou hanbai made—(In Japanese). Rural Culture Association Japan, Tokyo.
- Strygina, K. V., A. V. Kochetov and E. K. Khlestkina. 2019. Genetic control of anthocyanin pigmentation of potato tissues. BMC Genet. 20: 35–53.
- Suda, I., T. Oki, M. Masuda, M. Kobayashi, Y. Nishiba and S. Furuta. 2003. Physiological functionality of purple-fleshed sweet potatoes containing anthocyanins and their utilization in foods. JARQ 37: 167–173.
- Tierno, R. and J. I. Ruiz de Galarreta. 2017. Characterization of high anthocyanin-producing tetraploid potato cultivars selected for breeding using morphological traits and microsatellite markers. Plant Genet. Resour. 15: 147–156.
- Toyoda, K. 1981. Hasu no Kenkyu (In Japanese). Ariakeshobo Press, Tokyo.
- Wang, H., M. G. Nair, G. M. Strasburg, Y. C. Chang, A. M. Booren, J. I. Gray and D. L. DeWitt. 1999. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 62: 294–296.
- Yasuda, S., N. Taga, K. Honda, T. Murata, Y. Matsuda, T. Shibata, T. Araki and K. Kabata. 2014. Sustainable use of anthocyanins as the functional ingredients from purple sweet potato. J. Brew. Soc. Japan 109: 557–564 (In Japanese).