2022 Volume 91 Issue 4 Pages 448-452
2022 Volume 91 Issue 4 Pages 448-452
To determine the effects of drought stress, especially light drought stress, on flower number in passion fruit, one-year-old passion fruit plants grown in 7.5 L plastic pots were subjected to different soil water content treatments, namely wetness, light drought, and heavy drought for two months. Average, maximum, and minimum soil water contents (v/v) were 44, 47, and 41% in the wetness treatment, 23, 40, and 11% in the light drought treatment and 11, 33, and 6% in the heavy drought treatment. Flower number decreased as the strength of drought stress increased, although the number of nodes and flower buds did not. Flowering periods were from June 27 to July 19 in the wetness treatment and June 26 to July 16 in the light drought treatment with three peaks around July 1, 6, and 13. In the heavy drought treatment, the flowering period was from July 11 to 18 with one peak. The flower bud number was not affected by drought stress. Light drought stress did not suppress vegetative growth, such as vine length, leaf number, leaf length, or photosynthetic rate, although heavy drought stress did. Stomatal conductance was suppressed by light drought only at 12:00PM and by heavy drought throughout the day. Leaf water potential was decreased by heavy drought at 3:00PM, but not by light drought. In the wetness and light drought treatments, visible wilting was not observed, and in the heavy drought treatment the plants wilted before irrigation, although they recovered about 15 min after irrigation. In conclusion, even light drought stress, which did not suppress vegetative growth, reduced the flower number in passion fruit. Drought stress suppressed flower bud development but not differentiation.
Passion fruit (Passiflora edulis Sims) is a subtropical fruit which is native to Latin America, and is now widely cultivated in tropical and subtropical regions (Morton, 1987). In Japan, the cultivation area and the production has been increasing mainly for fresh consumption (Kumamoto et al., 2017). High quality fruit with low acidity and good appearance for fresh consumption are mainly cultivated in greenhouses. In greenhouses, irrigation systems, heaters, and artificial lights make it possible to produce high quality fruit year-round, but the cost is generally high. Therefore, cultivation methods to increase yield are now required.
Using artificial pollination, the fruit set rate was more than 90% (Akamine and Girolami, 1959; Ishihata, 1993), and so an increase in flower number may lead to increased yield. Therefore, effects of cultivation conditions on flower number were determined recently; strong light (Menzel and Simpson, 1988, 1989; Shimada et al., 2019), air temperature lower than 30°C (Matsuda et al., 2005), and NaCl application (Kondo and Sato, 2022) all increased the flower number. However, these findings are not so useful to establish new cultivation methods; increasing light intensity artificially and lowering air temperature below 30°C in summer are costly and NaCl application in greenhouses carries a risk of salinity. On the other hand, to maintain the soil water content is relatively inexpensive and easy to practice because irrigation systems installed to produce high quality passion fruit are often used in greenhouses. Therefore, the effects of soil water content on flower number in passion fruit would be useful information for farmers. Effects of drought stress on flower number varied with the intensity of the stress and crop; light drought stress without photosynthetic reduction reduced the flower number in blueberry (Guo et al., 2021), 12 days of drought stress induced flowering in pummelo (Phadung et al., 2011), while severe drought stress with −3.94 MPa of leaf water potential did not induce floral morphogenesis, but did induce earlier floral budbreak, in mango (Nunez-Elisea and Davenport, 1994). Therefore, for each crop the effects of drought stress on flower number should be determined, paying attention to the intensity of the stress.
In passion fruit, heavy drought stress, which suppressed vegetative growth significantly reduced the flower bud and flower number (Menzel et al., 1986; Shimada et al., 2018; Staveley and Wolstenholme, 1990). Findings regarding the detrimental effects of heavy drought stress are not useful to establish new cultivation methods in greenhouses with irrigation systems because such heavy drought stress seldom occurs. On the other hand, a high soil water content reduced the flower number drastically (Iida, 2019) as well as vegetative growth due to root rot (Shimada et al., 2018). Therefore, farmers often reduce the irrigation amount and frequency because they are afraid of those risks, and so light drought stress may sometimes occur. Therefore, in this study the effects of drought stress, especially light drought stress, on flower number were determined.
One-year-old hybrid passion fruit (Passiflora edulis Sims × P. edulis f. flavicarpa Deg. ‘Summer Queen’) plants were grown in a greenhouse at Kyoto University (35.0°N, 135.8°E). Fifteen plants were propagated by cutting in October 2020 and transplanted to 7.5 L plastic pots in December. Decomposed granite soil and bark compost were mixed at a ratio of 4:3 by volume for the pot soil. A pumice stone was placed at the bottom of the pots at a depth of 5 cm to improve drainage. The pots were placed in three rows with five pots per row. The pots were spaced in rows at 90 cm between pots and 90 cm between rows. CaCO3 and MgCO3 were applied at 2.5 g each to prevent a pH decline and Ca and Mg nutrients were added on the transplanting day and in April 2021. Chemical fertilizer at 15 g per plant containing N:P2O5:K2O = 10:10:10 was applied weekly. In the greenhouse, the minimum temperature was kept at 10°C during the winter and the maximum temperature was not regulated. The plants were hedge trained, as described by Kondo and Higuchi (2011), with a height of 140 cm and four fruit-bearing vines per plant.
The 15 plants were divided into three groups of five plants. Each group was subjected to a different soil water content treatment, namely wetness, light drought, and heavy drought from May 31 to August 4. For the wetness treatment, a bottom-irrigation tray of 45 cm diameter and 14.5 cm height was placed the bottom of the pot, and the water level was maintained at a depth of around 8 cm to keep the soil water content high (Fig. 1). Water in the bottom-irrigation tray was replaced every three days. Fifteen 1 cm diameter drain holes per pot were made at 10 cm above the bottom of the pot. To supply oxygen, a rectangular airing stone 10 cm long and 2 cm wide (Best Bio AIR 100; GEX. Co., Ltd., Osaka, Japan) was placed the bottom of the pot with an air pump (e-AIR 6000WB; GEX. Co., Ltd.). In addition to bottom-irrigation, from the top of the soil surface 700 mL of water was supplied once a day from May 31 to June 9 and three times a day from June 10 to July 1. After that, 1,400 mL of water was supplied three times a day. On rainy days, water was not supplied. For the light and heavy drought treatments, water was supplied only from the top of soil surface without bottom-irrigation. The amount and frequency of irrigation from the top pot soil in the light drought treatment were the same as in the wetness treatment. For the heavy drought treatment, water was supplied every three days from May 31 to June 9 and after that once a day, keeping the amount the same. Soil water content (v/v) at a 6–12 cm depth and air temperature were measured once every 15 min during the experiment using a soil moisture sensor (EC-5; METER Group, Inc., WA, USA) and a thermometer (VP-4; METER Group, Inc.). These data were recorded on a data logger (EM50; METER Group, Inc.). After the cultivation experiment, water content (v/v) and water potential of the soil used for the experiment were measured by the soil moisture sensor and a soil water potential sensor (TERSO-21; METER Group, Inc.) to determine the relationship between the two variables. Then, the soil water potential during the experiment was calculated. Soil water content (v/v) is more informative for evaluating soil wetness than water potential, while water potential is an indicator of soil dryness regardless of soil type. Thus, both water content (v/v) and water potential of the soil are described in present study.
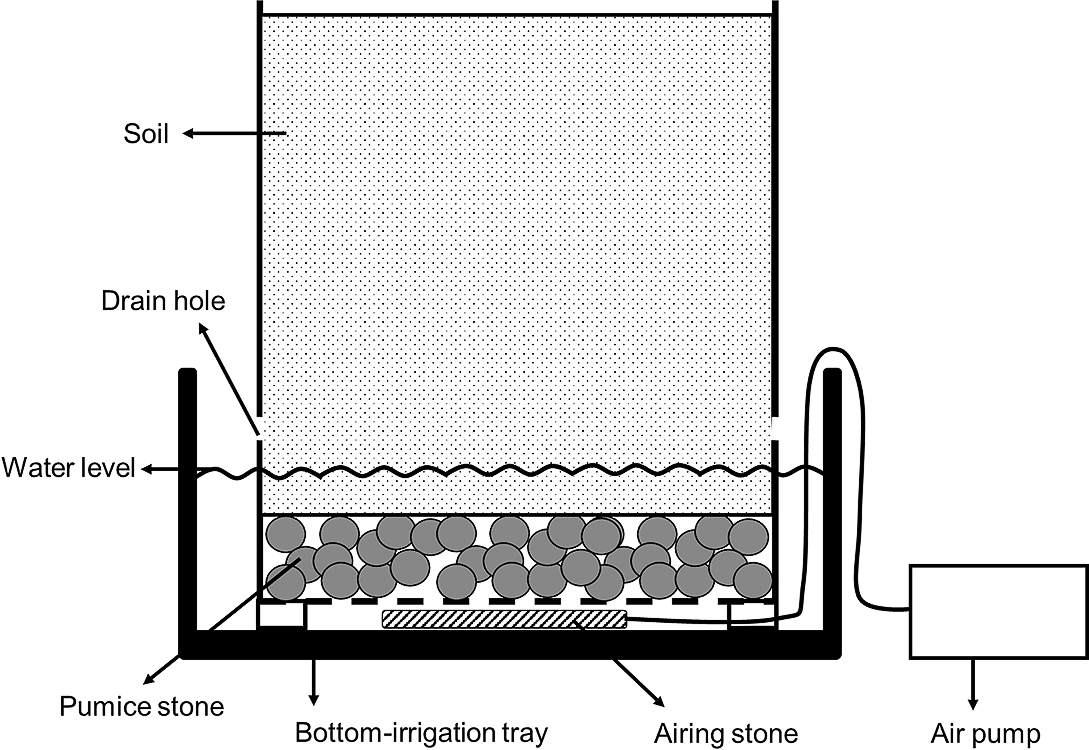
Diagram of the experimental pot in the wetness treatment.
Flower buds 2–3 mm long and bloomed flowers were counted. After flower counting, artificial pollination was conducted. One week after pollination, we regarded enlarged ovules as fruiting and counted the fruit number. Leaf number, length of fruit-bearing vines, and length of the largest leaf were measured weekly. Plant appearance was observed before and after irrigation. Stomatal conductance of the largest leaf on fruit-bearing vines was measured at 6:00AM, 9:00AM, 12:00PM, 3:00PM, and 6:00PM on July 19 using a leaf porometer (SC-1; METER Group, Inc.). The photosynthetic rate of the largest leaf, well exposed to sunlight, on fruit-bearing vines was measured at 10:00–11:00AM and 1:30–2:30PM on July 19 using a photosynthetic rate measurement device (MIC-100; Masa International Co., Ltd., Kyoto, Japan). The measurement conditions were as follows: photosynthetically active radiation (PAR) 1200 μmol·m−2·s−1, stabilization time 3 s, measurement start CO2 concentration 400 ppm, and measurement CO2 span 10 ppm. Leaf water potential was also measured at 6:00AM, 9:00AM, 12:00PM, 3:00PM, and 6:00PM on July 19 using a pressure chamber (Model670; PMS Instrument Co., OR, USA).
The data were analyzed by analysis of variance, and the statistical differences among treatments were subjected to further analysis using Tukey’s test.
Average, maximum, and minimum soil water contents were 44, 47, and 41% in the wetness treatment, 23, 40, and 11% in the light drought treatment and 11, 33, and 6% in the heavy drought treatment (Fig. 2). A water content higher than 18% represented a higher soil water content than −0.1 kPa, while 11 and 6% water contents represented −39.3 and −298.8 kPa, respectively (data not presented). The flower number decreased as the drought stress increased, although the number of nodes and flower buds did not (Table 1). The fruit number decreased only under heavy drought stress. Flowering periods were from June 27 to July 19 in the wetness treatment and June 26 to July 16 in the light drought treatment with three peaks around July 1, 6, and 13 (Fig. 3). In the heavy drought treatment, the flowering period was from July 11 to 18 with one peak. The fruit-bearing vine length in the light drought treatment was longer than that in the wetness treatment after June 28 (Fig. 4). Heavy drought suppressed vine elongation. Leaf number was not affected by light drought, except for July 5 and the leaf number in the light drought treatment was higher than that in the wetness treatment (Fig. 5). The increase in leaf number was suppressed by heavy drought, although at the end of the experiment there was no statistical difference. Leaf length was not affected by light drought, but was reduced by heavy drought throughout the experiment (Fig. 6). In the wetness and light drought treatments, visible wilting was not observed, and in the heavy drought treatment the plants wilted before irrigation but recovered about 15 min after irrigation. Stomatal conductance was suppressed by light drought only at 12:00PM and by heavy drought throughout the day (Fig. 7). Light drought did not suppress the photosynthetic rate, but heavy drought did (Table 1). Leaf water potential was reduced by heavy drought at 3:00PM, but not by light drought (Fig. 8).
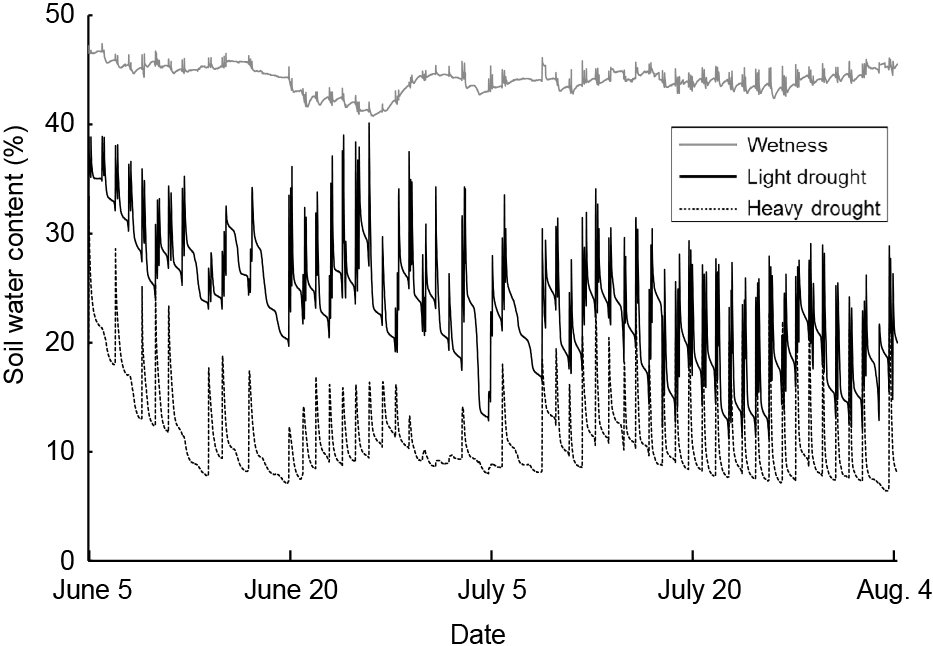
Changes in soil water content (v/v) during the experiment.
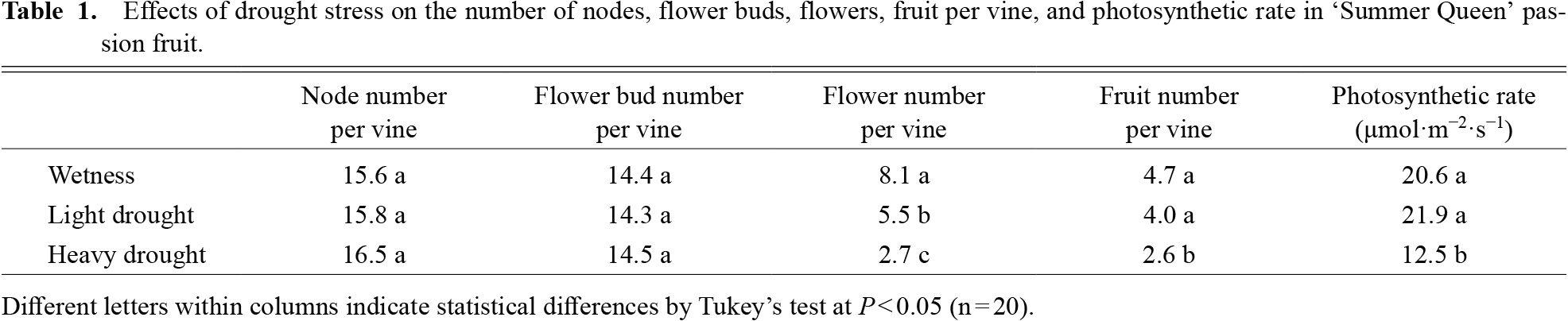
Effects of drought stress on the number of nodes, flower buds, flowers, fruit per vine, and photosynthetic rate in ‘Summer Queen’ passion fruit.
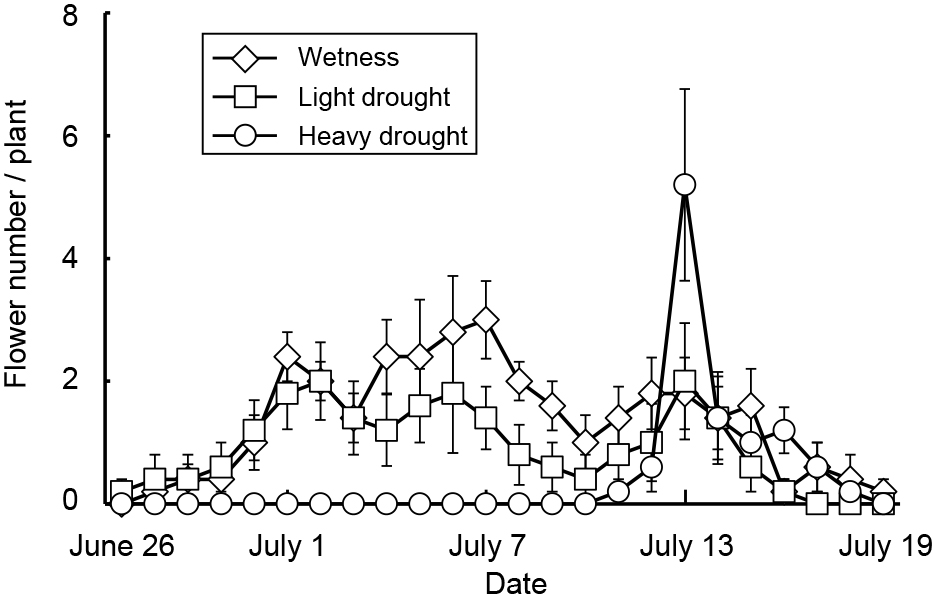
Effect of drought stress on the flower number in ‘Summer Queen’ passion fruit. Bars indicate SE (n = 5).
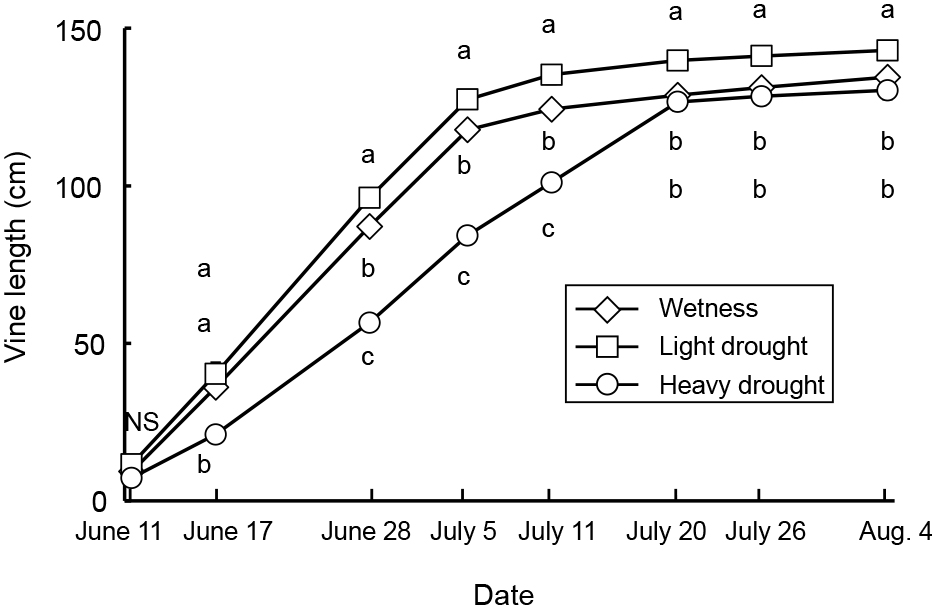
Effect of drought stress on vine length in ‘Summer Queen’ passion fruit. Bars indicate SE (n = 20). Different letters within the same day indicate statistical differences by Tukey’s test at P < 0.05.
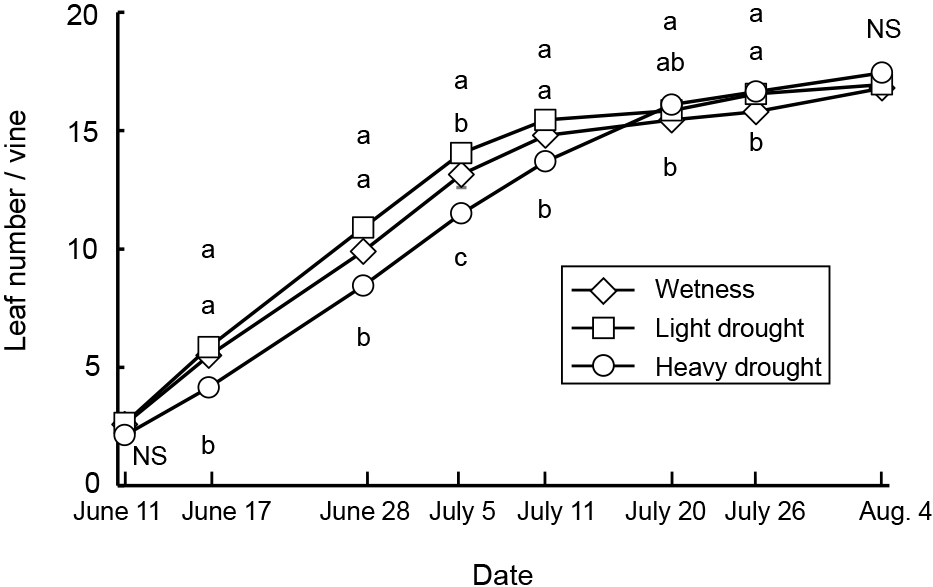
Effect of drought stress on the leaf number in ‘Summer Queen’ passion fruit. Bars indicate SE (n = 20). Different letters within the same day indicate statistical differences by Tukey’s test at P < 0.05.
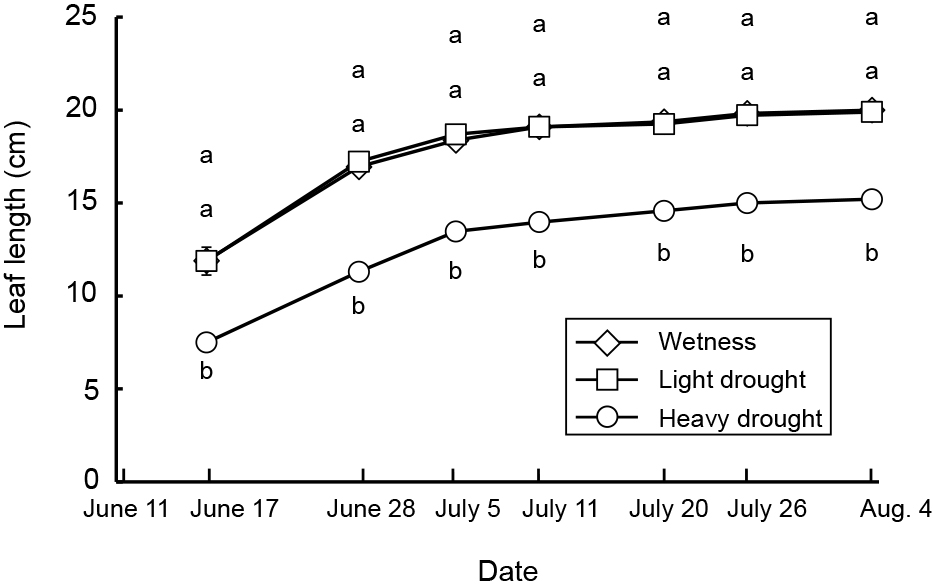
Effect of drought stress on leaf length in ‘Summer Queen’ passion fruit. Bars indicate SE (n = 20). Different letters within the same day indicate statistical differences by Tukey’s test at P < 0.05.
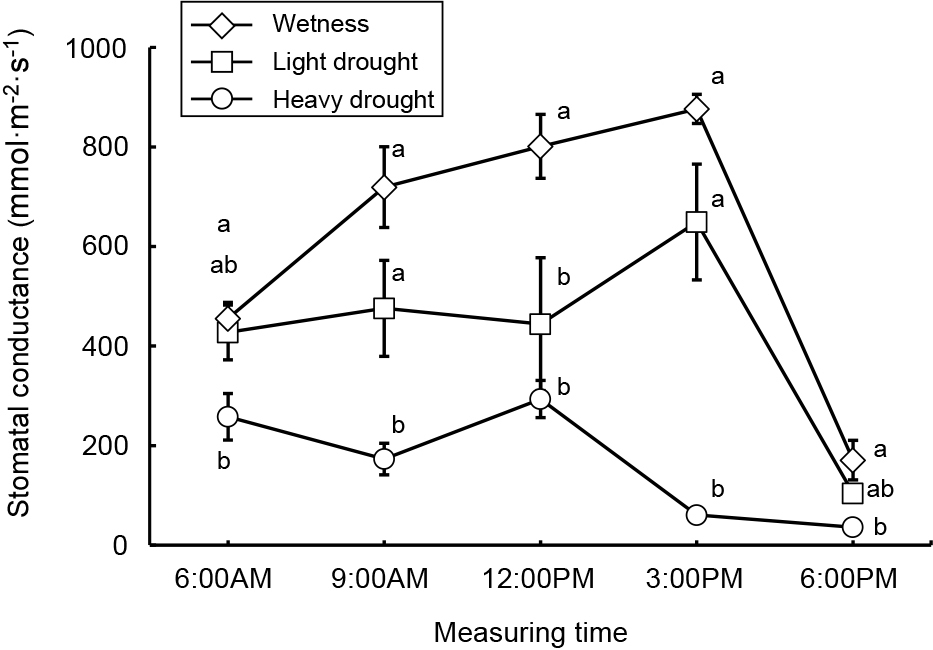
Effect of drought stress on stomatal conductance in ‘Summer Queen’ passion fruit. Bars indicate SE (n = 5). Different letters within the same measuring time indicate statistical differences by Tukey’s test at P < 0.05.
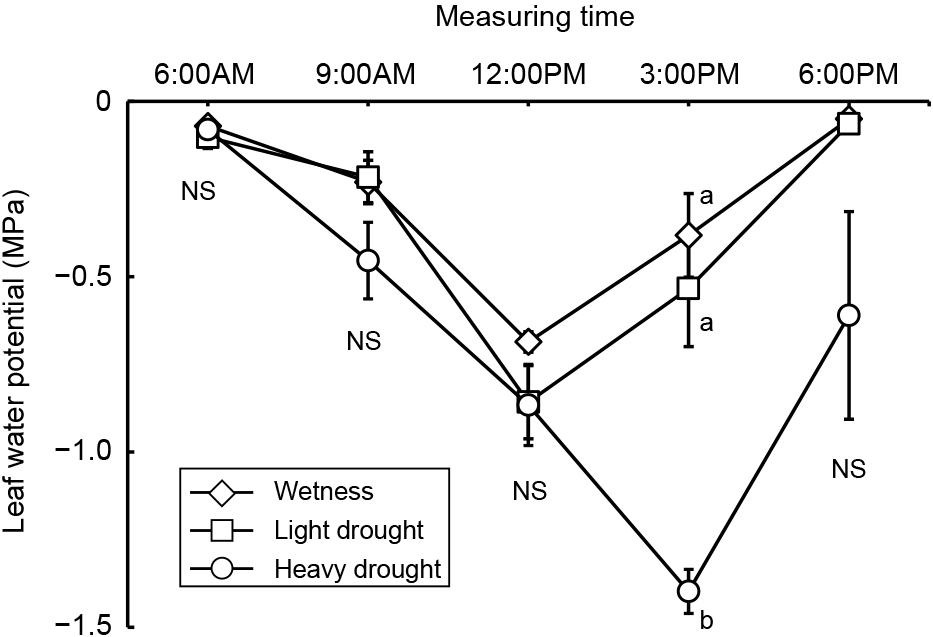
Effect of drought stress on leaf water potential in ‘Summer Queen’ passion fruit. Bars indicate SE (n = 5). Different letters within the same measuring time indicate statistical differences by Tukey’s test at P < 0.05.
The flower number in the light drought treatment was lower than that in the wetness treatment, although vegetative growth, such as vine length, leaf length, and the number of nodes and leaves, did not decrease and no visible wilting was observed. There were no differences in leaf water potential and photosynthetic rate, although stomatal conductance was slightly lower in the light drought treatment. In conclusion, even light drought stress that did not suppress vegetative growth, reduced the flower number in the present study, even though a decrease in flower and flower bud number due to strong drought stress that suppressed vegetative growth has been reported (Menzel et al., 1986; Shimada et al., 2018; Staveley and Wolstenholme, 1990). On the other hand, maintaining a high soil water content and high leaf water potential resulted in a drastic reduction in flower number to about 10% (Iida, 2019). Iida (2019) concluded that a temporary decrease in leaf water potential was necessary to promote flowering. In the present study, leaf water potential decreased to −0.8 MPa in the wetness treatment possibly because of restriction of the root zone due to the pot, and so the flower number may not have been reduced.
The flower bud number was not affected by light or heavy drought stresses. Therefore, drought stress might not affect flower bud differentiation, but caused flower bud drop and reduced the number of flowers. Flower bud development may be more sensitive to drought stress than flower bud differentiation. In contrast, strong drought stress reduced the number of flower buds, so strong drought stress may suppress flower bud differentiation (Menzel et al., 1986; Shimada et al., 2019). Vegetative growth and photosynthetic rate were not suppressed by light drought stress. Thus, the flower bud drop in the light drought treatment was not caused by a reduction in vegetative growth or photosynthetic rate induced by the stress. The flower bud drop may have been caused by changes in the synthesis of plant hormones, such as ethylene, induced by the stress.
In addition to the detrimental effect of drought stress in the light drought treatment, in the heavy drought treatment the reduction and delay of vegetative growth caused by a decrease of photosynthetic rate may also have dropped the flower buds. The photosynthetic rate was suppressed by heavy drought stress, and vegetative growth was delayed. As a result, the flowering period in the heavy drought treatment was also delayed because flowering began in all treatments when the fruit-bearing vine length reached about 100 cm. Passion fruit flowering was suppressed by air temperatures higher than 30°C (Chang and Cheng, 1992). In the present study, the flowering period ended in all treatments when the maximum temperature exceeded 35°C after July 19. In the heavy drought treatment, the delayed flowering period due to delayed vegetative growth may have resulted in a shortened flowering period because the maximum temperature exceeded 35°C immediately after the flowering period began.
The fruit number was not decreased by light drought stress, although the flower number was decreased. In passion fruit, the fruit set rate decreased as the crop load increased (Kondo and Higuchi, 2011, 2021). In the present study, 4 to 5 fruits per fruit-bearing vine would have represented full capacity, beyond which the plants could not set additional fruit because relatively small, 140 cm tall plants were used. As a result, there may have been no difference in the fruit number between the wetness and light drought treatments. Bigger plants than those used in the present study are generally cultivated in commercial orchards, so a reduction in the flower number induced by light drought stress may result in a decrease in yield.
In passion fruit, the flower number was reduced by light drought stress that was not detected visibly. Therefore, to increase the flower number, a high soil water content close to the field water capacity should be maintained. On the other hand, a temporary decrease in the leaf water potential was reported to be necessary for flower formation (Iida, 2019). Therefore, further studies on the relationships among soil water content, leaf water potential, and flower number are needed.