2022 Volume 91 Issue 4 Pages 531-540
2022 Volume 91 Issue 4 Pages 531-540
Postharvest blossom-end enlargement (BEE) in summer cucumber has a serious economic effect in Japan. Previous studies suggested that BEE is influenced by cultivation conditions and temperature during transportation. In this study, the relationship between postharvest BEE and growth parameters in plants under various sink–source balances created by defoliation and fruit thinning was determined using a growth analysis technique. Cucumber (Cucumis sativus L. ‘Taibo I’) seedlings were transplanted into an outdoor field at Ibaraki University in 2019, and the harvested fruits were stored in plastic bags at 27°C. The occurrence of BEE, which was scored six days after harvest, was positively correlated with the field air temperature and cumulative duration of sunshine exposure from flowering to harvesting. Furthermore, high temperatures and extended sunshine exposure reduced the time from flowering to harvesting. However, excessive defoliation reduced BEE occurrence, most likely because of the increased time from flowering to harvesting, suggesting that early fruit maturation may be one of the causes of BEE. Therefore, principal component analysis was performed to understand the relationship between growth parameters and the occurrence of BEE in cucumber. The occurrence of BEE increased when the sink–source balance was inclined towards the source. Collectively, these findings indicate that high temperatures, extended exposure to solar radiation, and large leaf area with respect to the number of fruit set increase the occurrence of BEE, with pre-harvest fruit maturity probably related to its onset.
Post-harvest deformation in baby- and regular-sized cucumber fruits was first reported as “apex enlargement” by Okabayashi (2001) and Igarashi et al. (2005), respectively. However, Nishizawa et al. (2018) referred to the deformed portion as the “distal portion”. In the present study, we used the term “blossom-end enlargement (BEE)” for this symptom to avoid confusion.
For the past two decades, BEE has been a serious problem in terms of outdoor cucumber cultivation during the summer in Japan. Nonetheless, Okabayashi (2001) and Igarashi et al. (2005) suggested modified atmospheric packaging and precooling as effective measures to prevent BEE. Although harvested cucumber fruits may appear normal at harvest time, their blossom-ends undergo enlargement when transported in sealed containers (Fig. 1A). In addition, this leads to an unusual odor and etiolation of the enlarged part, resulting in reduced market value.

Symptoms of blossom-end enlargement in cucumber. (A) Cucumbers in a shipping case on the seventh day after harvesting. (B) Severity scores of blossom-end enlargement.
BEE occurrence in cucumbers varies with the cultivation area; BEE is less likely to occur in cucumbers cultivated in areas where modified atmosphere packaging and precooling have already been established. During the summer, cucumbers from neighboring prefectures with underdeveloped systems are cheaper at the Tokyo Central Wholesale Market than those from technologically advanced regions [Tokyo Metropolitan Central Wholesale Market. Market statistics (monthly/annual report), <https://www.shijou-tokei.metro.tokyo.lg.jp/>, accessed: September 19, 2021]. Multiplying the dramatic price difference per kg by the shipment volume results in a loss of millions of dollars per season.
Recently, the above-mentioned packaging technology has been introduced in some areas; however, its implementation has been difficult as complementary co-selection and co-marketing systems have not yet been established in several small production areas. Prior to this study, a monitoring survey in a cucumber-producing area in Iwate Prefecture revealed that the degree of BEE occurrence not only varied over the years, but also differed based on the shipping procedure. Furthermore, some farmers reported that the BEE incidence increased after the introduction of a drip fertigation system. Moreover, BEE occurrence increased at high temperatures (Igarashi et al., 2005; Okabayashi, 2001), indicating a temperature dependency. However, if BEE occurrence was affected only by the weather, there would be no variations in production among different farms.
Pre-harvest BEE fruit is often referred to as “bottle-gourd-type fruit” (BG; Kato and Oda, 1977) or “bottle-gourd-shaped fruit” (Hirama et al., 2007). BG exhibits symptoms similar to BEE; however, BG often occurs during later periods of cultivation, when the translocation of photosynthetic assimilates is insufficient and soil water content is high (Kato and Oda, 1977). Okabayashi (2001) reported that BG was more likely to develop into BEE. This contradicts the reports of farmers, because drip fertigation is highly effective and increases crop yield by improving plant vigor (Tashiro et al., 2021). Therefore, the source–sink balance may play a role in determining BEE occurrence.
When cucumbers grow vigorously, multiple fruits simultaneously develop on the same plant. According to Murakami et al. (1982) and Marcelis (1993a), there is strong competition among developing fruits, with one fruit tending to monopolize assimilation products. Additionally, in cucumber cultivation, the number of new shoots increases each time upon squeezing. Therefore, the leaf area and fruit quantity, which represent the sink–source balance, rapidly and constantly fluctuate after squeezing (Sato et al., 2004, 2005, 2007a, b). As a result, although the harvested fruits may appear normal, the time required by the fruits to attain full size and the quantity of assimilation products may vary. Furthermore, owing to variations in fruit age and quality, only certain fruits exposed to specific conditions may develop BEE. Marcelis (1993a, b) and Marcelis and Gijzen (1998) proposed a hypothesis regarding cucumber cultivation in a greenhouse in the Netherlands based on growth analysis. However, there have been no reports on BEE occurrence in the Netherlands, and the environmental conditions, cultivation methods, cucumber varieties, and harvested fruit sizes in Japan notably vary from those in the Netherlands. Therefore, in this study, defoliation and fruit pruning were used to maintain the sink–source balance in cucumber plants, and plant destructive analyses were periodically conducted. Furthermore, a growth analysis technique was used to determine the relationship between pre-harvest plant conditions and BEE occurrence. Understanding the relationship between different growth parameters and BEE occurrence may help devise effective strategies to reduce BEE incidence in cucumbers.
All experiments were conducted in an experimental field at the Center for International Field Agriculture, Research and Education, College of Agriculture, Ibaraki University. In 2019, the following six treatments, comprising leaf defoliation or fruit thinning, were used to determine the effects of sink–source balance on BEE occurrence in cucumber:
These treatments were conducted weekly in July and twice a week in August. Three replicates with five seedlings per replicate were planted for each treatment. In 2020, we conducted all treatments except for LL and FSLS, for which we used the results in 2019.
Cultivation conditionsSeedlings of Cucumis sativus L. ‘Taibo I’ grafted on the rootstocks of Cucurbita moschata DUCH ‘Tokiwa power Z2’ were transplanted on May 31, 2019, and May 25, 2020. The planting density was 0.84 plants per m2 with an average row width of 3.40 m and plant distance of 0.7 m, and the total number of plants including spares was 180 in 214 m2. The plant bed was covered with a black mulch film, and the seedlings were arranged in two rows in a zigzag pattern. A drip tube (Ram17; Netafim Japan, Tokyo, Japan) was placed along each row. Weather data were obtained using the Automated Meteorological Data Acquisition System (AMeDAS). A commercial liquid fertilizer (Kumiaiekihi 2, N:P2O5:K2O = 10:4:8; Katakura & Co-op Agri Corp., Tokyo, Japan) was applied with water using the drip tube. Pesticides and fungicides were applied weekly as needed primarily to prevent downy mildew, anthracnose, aphids, and thrips towards the end of the harvest season. The total amount of fertilizer was determined every 14 days using the leaf-count method, as described by Sato et al. (2007b) and Tashiro et al. (2021). Foliage was trained using a net, which was expanded along arch-shaped stakes. The primary stem and first branches were pinched at the fifteenth and first nodes, respectively, and the remaining branches were left undisturbed. Over the study period, 1 L·m−2 water was used for irrigation per day, except on rainy days. Other management practices were performed according to local guidelines. Field management was completed on September 5, 2019, and August 20, 2020.
Yield evaluation, storage test, and growth estimationYield evaluation was conducted for two periods from June 13 to September 5, 2019, and from June 26 to August 20, 2020. Marketable fruits weighing over 70 g were harvested, and the yield was recorded twice daily. To mitigate the influence of temperature on the harvest, harvesting was carried out between 6:00 AM and 8:00 AM, and the harvested fruits were moved immediately to an experimental room maintained at 27°C, which was higher than the outdoor temperature. Fruits harvested in the afternoon were not used for experiments. Six fruits per plot were used for the storage test, and the remaining fruits were dried at 80°C to measure their water content. Unmarketable fruits, such as deformed fruits, were removed from the field, and their numbers and dry weights were recorded.
In 2019, fruits from all replicates were pooled for yield evaluation and storage testing, whereas in 2020, the storage test was independently conducted for each replicate. All pistillate flowers were first identified using paper tags (25 × 30 mm, Heiko No. 17; Shimojima Co., Ltd., Tokyo, Japan) with their flowering date, and then collected during harvest to record the fruit growth period. A total of 1,473 tags were collected from the harvested fruits, and environmental parameters were estimated for each fruit for each harvest period and treatment.
Storage tests to evaluate BEE occurrence were conducted from July 12 to September 5, 2019, and from June 26 to August 6, 2020. For the tests, six fruits (80–100 g) were packed in a plastic bag made of biaxially oriented polypropylene film (300 × 150 × 0.025 mm3, New Bodon #25; Daiko Corporation, Miyagi, Japan), with four air holes (diameter: 5.5 mm) on either side, and stored at 27°C under dark conditions. In 2019, packages with fruits from three replicates were tested daily, whereas in 2020, three packages with fruits from each replicate were tested daily. We only recorded the occurrence of BEE in 2019, along with the BEE index in 2020. For indexing, the following BEE scores were given to each fruit based on visual observations six days after storage: 0 = normal fruit, 1 = slight enlargement, 2 = apparent enlargement, and 3 = extreme enlargement (Fig. 1B). Thereafter, the BEE index was calculated as follows:
Data on maximum, minimum, and mean temperatures, diurnal range, precipitation, and duration of sunshine exposure were obtained using AMeDAS from the Tsuchiura observation point, located 7.9 km north of the experimental field (Japan Meteorological Agency, <http://www.jma.go.jp/jma/en/Activities/observations.html/>, accessed: September 14, 2021). Furthermore, cumulative temperatures and cumulative duration of sunshine exposure from flowering to harvesting were calculated for each fruit.
We conducted growth analysis in 2020; a sampling survey was conducted four times every fortnight from July 9, 2020, with three replicates and one plant per treatment. Subsequently, leaf area, partial dry weight of organs (roots, stems, lamina, petioles, pistillate flowers, staminate flowers, and fruits), relative growth rate (RGR), net assimilation rate (NAR), leaf area ratio (LAR), specific leaf area (SLA), and leaf weight ratio (LWR) were calculated. Leaves and stems removed by defoliation, and thinned and aborted fruits were dried at 80°C and used to estimate growth parameters during this period. LIA for WIN32 ver. 0.378 (Yamamoto, 1998) was used to determine the leaf area.
Statistical analysisStatistical analyses were conducted using StatFlex ver. 7 (Artec Corporation, Osaka, Japan). To analyze the results of the storage tests, each bag containing six fruits was treated as a sample in Tukey–Kramer’s multiple comparison test because of the lack of replicates in 2019. In 2020, the mean values of all the sampled bags in each replicate were used for analysis. Principal component analysis (PCA), an approach to develop a comprehensive index of several variables, was conducted using a correlation matrix. A total of 36 samples, including three replicates of average growth data of four treatments and three periods, were used for analysis.
Weather data for 2019 and 2020 are shown in Figure 2A and 2B, respectively. Although continuous rainfall was observed from June 7 to July 28, 2019, there were many sunny days in early August, which increased air temperature. However, rainfall resumed after mid-August. In 2020, the rainy season continued until late July, after which high temperatures and extended sunshine exposure were observed in August; however, cultivation concluded earlier than in 2019 because of outbreaks of anthracnose disease and melon yellow spot virus towards the end of July.

Variations in air temperature and sunshine duration at the Tsuchiura observation point using the Automated Meteorological Data Acquisition System of the Meteorological Agency, Japan. (A) 2019, (B) 2020.
In 2019, the number of leaves linearly increased up to 300 per plant after transplantation until July 12 (42 days after transplantation; DAT, hereafter), exhibiting enhanced growth (Fig. 3A). However, the amount of nitrogen supplied, based on the increase in the number of leaves, did not vary until August 9 (70 DAT).
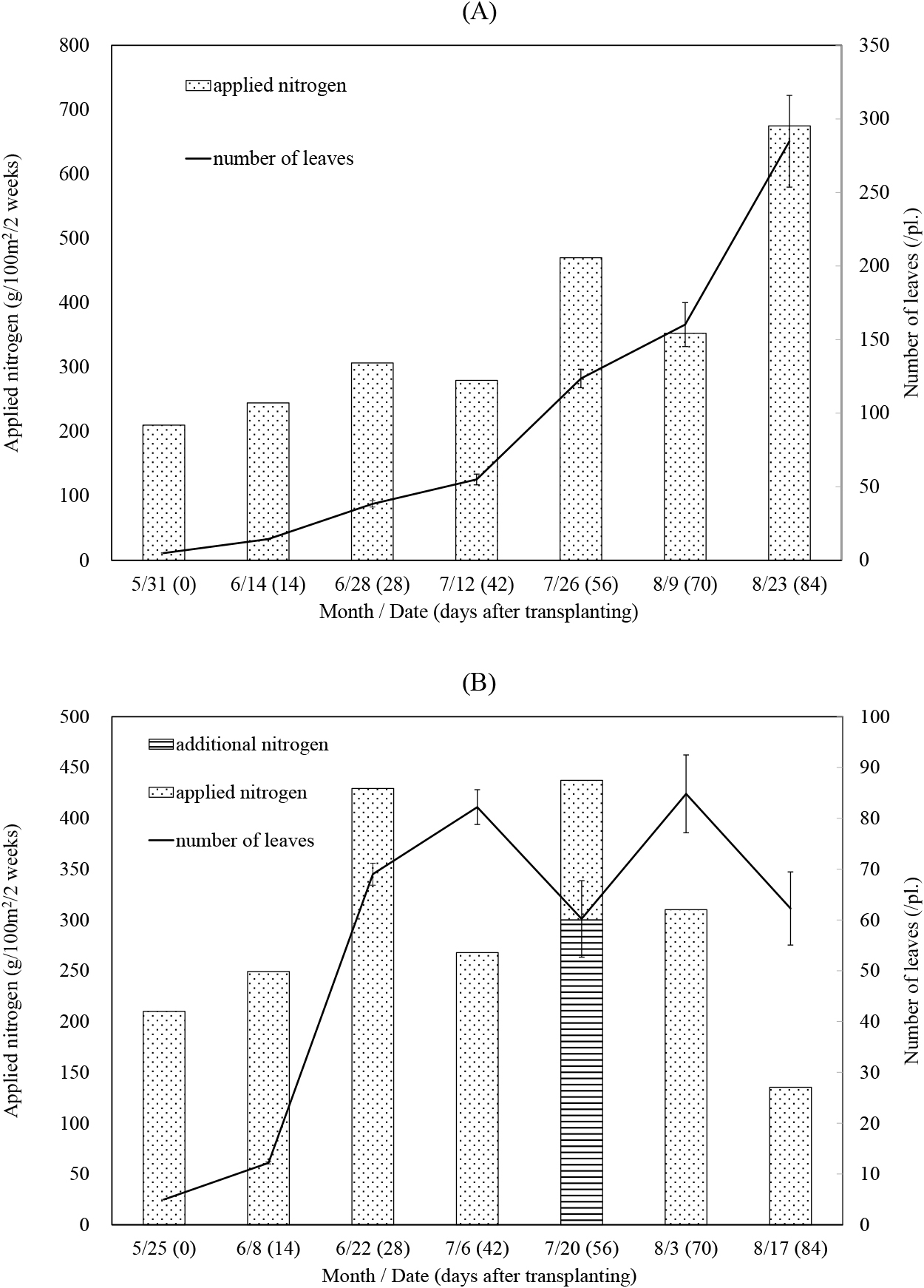
Chronological variations in leaf number and amount of applied nitrogen. (A) 2019, (B) 2020. Vertical bars indicate SE (n = 5).
In 2020, the transplantation date was six days earlier than that in 2019. Although the number of leaves rapidly increased until July 6 (42 DAT) (Fig. 3B), it decreased on July 20 (56 DAT) following intensive defoliation to prevent the spread of anthracnose disease. Furthermore, as liquid fertilizer effused because of continuous rainfall, additional fertilizer was applied on July 20. The total amount of nitrogen applied was 2.53 and 2.04 kg/100 m2 in 2019 and 2020, respectively. Even so, cucumber growth resumed after weather conditions improved in August; however, the number of leaves was < 90 per plant until the end of the cultivation period. The total marketable yield in the NT group was 6.91 and 5.10 kg per plant in 2019 and 2020, respectively (Fig. 4). Although statistical analyses were not performed owing to a lack of replicates, there was no marked difference in the yield among treatments in 2019. In 2020, no significant difference was observed among treatments, probably due to an insufficient number of samples.

Effects of defoliation and fruit thinning on cucumber yield. (A) 2019, (B) 2020. NT, LL, LS, FL, FS, FSLS indicate non treatment, defoliation light, defoliation severe, fruit thinning light, fruit thinning severe, and fruit thinning severe and defoliation severe, respectively.
The results of the storage tests performed in 2019 are shown in Figure 5. There was no significant difference in BEE occurrence among the six treatments during the three periods: July 12–25, July 26–August 8, and August 9–22. However, FL and FS treatments resulted in a higher occurrence of BEE than the LSFS treatment from August 23–September 5.

Effects of defoliation and fruit thinning on the occurrence of blossom-end enlargement in 2019. Vertical bars indicate SE (n = 26–80). Different letters above the bars indicate significant difference (P < 5%) determined using Tukey-Kramer’s multiple range test. NT, LL, LS, FL, FS, and FSLS indicate non treatment, defoliation light, defoliation severe, fruit thinning light, fruit thinning severe, and fruit thinning severe and defoliation severe, respectively.
The correlation coefficients between BEE occurrence and environmental parameters evaluated using an average of 24 values are shown in Table 1. The maximum, minimum, and mean temperatures, duration of sunshine exposure, and cumulative duration of sunshine exposure after flowering were positively correlated with BEE occurrence. Moreover, the diurnal range reflected the daily maximum temperature because fluctuations in maximum temperature tended to be larger than those in minimum temperature in the summer season. However, the cumulative temperature from flowering to harvesting showed no significant correlation with BEE occurrence. This suggested that high temperatures and extended duration of solar radiation were probably related to BEE occurrence.
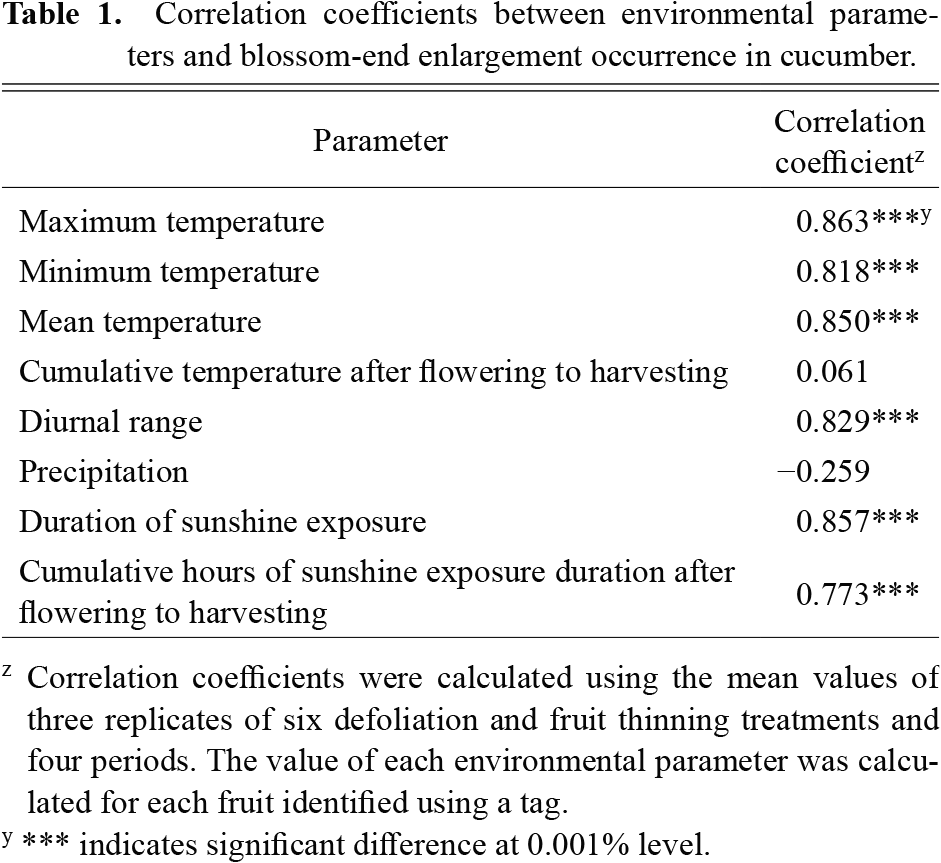
Correlation coefficients between environmental parameters and blossom-end enlargement occurrence in cucumber.
We observed that the fruit growth rate was markedly different among treatments, survey periods, and the number of fruits per plant. Moreover, a negative correlation was observed between the duration from flowering to harvesting and the occurrence of BEE (Fig. 6), suggesting that early maturing fruits exhibited BEE symptoms. Unexpectedly, high temperatures and increased solar radiation were the primary causes of an increased fruit growth rate. Defoliation and fruit thinning may also affect bud formation and fruit abortion for a certain period and alter the duration between flowering and harvesting, particularly the sink–source balance, which may be associated with the fruit growth rate. Furthermore, as reported by Murakami et al. (1982) and Marcelis (1993a), fruits compete for source commutation, and the predominant fruit assimilates a large proportion of photosynthates and rapidly grows, increasing the growth period of inferior fruits.

Relationship between fruit growth period and occurrence of blossom-end enlargement in cucumber. NT, LL, LS, FL, FS, and FSLS indicate non treatment, defoliation light, defoliation severe, fruit thinning light, fruit thinning severe, and fruit thinning severe and defoliation severe, respectively.
In addition, plant conditions were related to fruit growth. Figure 7 illustrates the duration from flowering to harvesting for the four study periods. From July 12–25, each treatment took approximately seven days or more from the flowering day to the harvest stage and no difference among treatments was observed. A rapid increase in the number of leaves (Fig. 3) without a substantial increase in yield (Fig. 4) suggested that the slowing of fruit growth due to excessive vegetative growth masked the treatment effect in all treatments. From July 26–August 8, the over-foliage in NT from the previous growth period possibly resulted in the low assimilation efficiency of the plant community and a tendency towards vegetative growth. Defoliation may contribute to the improvement of light-intercepting characteristics and promote reproductive growth. In addition, fruit thinning may contribute to the growth of remaining fruits by altering the sink-source balance. In contrast, from August 9–22, no significant difference was detected among treatments except for the LSFS treatment. Fruit thinning was not effective to change the number of days from flowering to harvesting, even though defoliation may extend it in this period. From August 23–September 5, we did not find any probable cause for the varying values. Possibly, accumulated differences in the sink-source balance, vegetative-reproductive balance, or light-intercepting characteristics may become prominent towards the end of cultivation.

Effect of defoliation and fruit thinning on the duration between flowering to harvesting in 2019. Different letters above bars indicate SD at P < 5% (n = 11–14). Vertical bars indicate SE (n = 26–80). Different letters above bars indicate significant difference (P < 5%) determined using Tukey–Kramer’s multiple range test. NT, LL, LS, FL, FS, and FSLS indicate non treatment, defoliation light, defoliation severe, fruit thinning light, fruit thinning severe, and fruit thinning severe and defoliation severe, respectively.
For PCA, each replication of the treatment was considered as a separate case, and each case was ranked by the degree of BEE occurrence rather than whether or not BEE had occurred. Therefore, the validity of the BEE index was examined. Results of the storage tests performed in 2020 revealed no notable differences between the BEE appearance rate (Fig. 8A) and BEE index (Fig. 8B); however, the difference was more notable in the BEE appearance rate than in the BEE index from July 24–August 6. Overall, most fruits exhibited BEE symptoms with near-saturated occurrence throughout the experimental period; the BEE index was more applicable for PCA. However, no rational explanation was found for the significant difference in FS incidence.

Effects of defoliation and fruit thinning on the occurrence of blossom-end enlargement in 2020. (A) Blossom-end enlargement (BEE) occurrence ratio. (B) BEE index. BEE index = (Σ(n × v)/N × Z) × 100. n: BEE score class; v: number of samples in the score class; N: highest score; Z: total number of samples. Vertical bars indicate SE (n = 3). Different letters above bars indicate significant difference (P < 5%) determined using Tukey–Kramer’s multiple range test. Harvest period: June 26–August 6, 2020. NT, LL, LS, FL, FS, and FSLS indicate non treatment, defoliation light, defoliation severe, fruit thinning light, fruit thinning severe, and fruit thinning severe and defoliation severe, respectively.
A total of 189 parameters were measured or calculated in each of the 36 cases derived from the four treatments (NT, LS, FL, and FS) x three replications x three periods (June 26–July 9, July 10–July 23, and August 6). After performing a PCA for all datasets, parameters that did not contribute to the results were removed. Finally, we determined 24 parameters that reflected the growth situation (Table 2). The contributions of the first and second components were 0.375 and 0.182, respectively, and they explained 55.7% of the total variation (Table 2). The eigenvectors of the first principal component (z1) with large positive values (> 0.200) were marketable yield, number of aborted and harvested fruits, total number of fruit set, fruit water content, and dry weight. However, the eigenvectors with large negative values (< −0.200) included leaf area, ratio of leaf area to the number of harvested fruits, ratio of leaf area to the total number of fruit set, number and dry weight of pistillate and staminate flowers, number of leaves, and dry weight of lamina and of petioles. Therefore, the first principal component reflected the assimilation potential of fruits. When the assimilation potential was high, that is, when the sink–source balance was inclined towards the sink, the score of the first principal component was a large positive value. Correspondingly, when the assimilation potential was low, that is, when the sink–source balance was inclined towards the source, the principal component score was negative.
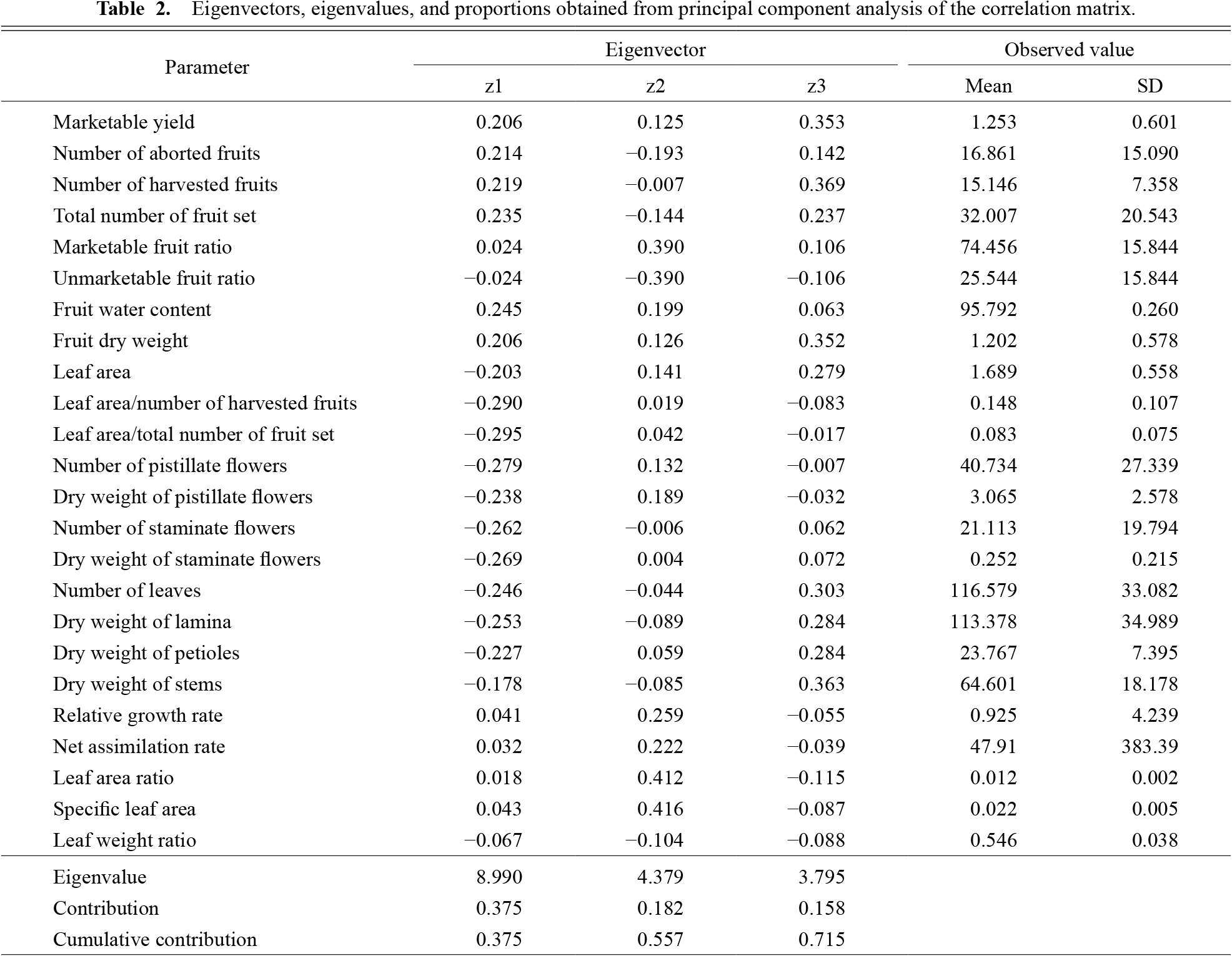
Eigenvectors, eigenvalues, and proportions obtained from principal component analysis of the correlation matrix.
The eigenvectors of the second principal component exhibiting large positive values (z2) were marketable fruit ratio, RGR, NAR, LAR, and SLA. In addition, the unmarketable yield showed a large negative value for the second principal component, suggesting that the second component reflected the amount of assimilation. When the amount of assimilation was high (for example, high temperature and increased solar radiation), the score of the second principal component was a large positive value, whereas when the amount of assimilation was low (for example, inclement weather), the value was negative.
Using the computed principal component score, a scatter plot was obtained, wherein the first and second principal components represented the x- and y-axes, respectively (Fig. 9). The BEE index (calculated in 3.3) was then superimposed on the scatter plot. We observed that BEE occurrence was localized; the test plots positioned at the top left of the scatter plot exhibited a higher BEE index, whereas those on the lower right exhibited a lower BEE index. These results suggest that BEE was affected by the sink–source balance in cucumber plants, as well as environmental conditions such as temperature and duration of solar radiation. In this context, it is possible that drip fertigation indirectly causes BEE as mentioned by producers in the field survey because of its growth improvement effect (Tashiro et al., 2021). Furthermore, a low total number of fruit set with respect to leaf area resulted in a high fruit growth rate and post-harvest BEE. In contrast, a high total number of fruit set with respect to leaf area resulted in low fruit growth rates and a lower possibility of post-harvest BEE.

Scatter plot, generated using the first and second principal components, showing the distribution pattern of the severity of blossom-end enlargement in cucumber. BEE index = (Σ(n × v)/N × Z) × 100. n: BEE score class; v: number of samples in the score class; N: highest score; Z: total number of samples.
The symptoms of BEE are similar to those of BG, and thus BG is more likely to develop into BEE (Okabayashi, 2001). However, the former occurs when the fruit growth rate is high, whereas the latter occurs when plant vigor decreases at the end of cultivation. Therefore, we hypothesized that the cause of BEE was hormonal translocation based on the similarity of symptoms between BG and BEE. Ogawa et al. (1990) and Liu et al. (2020) estimated the chronological variations in the equivalents of endogenous cytokinins in fruits after flowering, and reported that their contents rapidly increased between six days and six to nine days after flowering, respectively. Assuming that post-harvest aging rapidly progressed (BEE) because the fruits reached harvest size and were harvested before the increase in zeatin content, reduced fruit growth likely inactivated zeatin production and resulted in pre-harvest aging (BG). Furthermore, BEE and BG may share triggers of partial enlargement that are turned on by signals such as sugar starvation (Tazuke et al., 2017) or reduction in phytohormone influx (Shishido et al., 1990). In the former case, survival of the embryo portion may have been selected for sugar-starved fruits, followed by an influx of nutrients from other portions. In the latter case, abnormal growth may result from the shutting off of the influx of phytohormones into the fruit before the embryos are able to synthesize phytohormones, such as cytokinins. However, although the role of phytohormones, such as cytokinins, has been reported in parthenocarpic fruits (Hikosaka and Sugiyama, 2015; Su et al., 2021a, b), the differences among pollinated fruits remain unclear (Boonkorkaew et al., 2008). Moreover, the role of phytohormones in fertilized fruits has garnered little attention. Therefore, further investigation using exogenous plant growth regulators (Takeno et al., 1992) and expression analysis of related genes (Wang et al., 2021) is necessary to improve the understanding of BEE in cucumbers.
ConclusionsIn this study, we determined the causes of BEE in cucumber. We observed that post-harvest BEE of cucumber fruits was influenced by cultivation conditions, including temperature and duration of sunshine exposure. Furthermore, BEE frequently occurred in rapidly growing fruits when the amount of photosynthates was high, that is, when the sink–source balance was inclined towards the source. Collectively, these findings indicate that BEE is likely to occur when the ratio of leaf area to the number of fruit set is high under high temperatures and extended periods of solar radiation. These results could explain how BEE occurrence depends on farmers or fields. However, further investigations are necessary to determine the effects of fruit maturity and phytohormonal activity during fruit harvest to improve the understanding of the causes of BEE.
We gratefully acknowledge the assistance of all members of the Sato–Tanabata laboratory.