2022 Volume 91 Issue 4 Pages 431-436
2022 Volume 91 Issue 4 Pages 431-436
Akebia trifoliata is cultivated locally in few areas of Japan. Artificial pollination is considered indispensable for stable fruit production, as Akebia spp. exhibit self-incompatibility (SI). However, little is known about the reproductive physiology required for effective artificial pollination. In this study, we investigated the effects of self-pollen contamination on the fruit set of ‘Fuji Murasaki’ (A. trifoliata), one of the main lines in Yamagata Prefecture, Japan. Both the self- and cross-pollen tubes reached the base of the ovary and penetrated the ovules at 10 days after pollination, indicating that A. trifoliata exhibits late-acting SI. Self-pollination one day before cross-pollination completely inhibited fruit set, while self-pollination one day after cross-pollination did not inhibit fruit set in both 2016 and 2020, indicating interference in cross-pollination by self-pollen contamination. Fruit set rates for artificial pollination using a 1:1 mix of self- and cross-pollen were significantly lower than those that used non-contaminated cross-pollen in both 2016 and 2019, but were comparable when using a 1:1 mix of lycopodium powder and cross-pollen. The seed number of fruit resulting from mixed pollination was not significantly lower than that of fruit resulting from cross-pollination. Interestingly, 1:1 mixed pollination of self- and cross-pollen sometimes yielded abnormal fruits with an apex that remained pale and did not soften. These results suggest that excessive self-pollen contamination in artificial pollination may not be desirable for A. trifoliata fruit production.
Akebia spp., a genus of the Lardizabalaceae family, is a woody vine distributed in East Asia. A. trifoliata, A. quinata, and their putative hybrid A. pentaphylla grow wild in Japan (Christenhusz, 2012). Sweet arils in the fruits are generally eaten, and their bitter purple pericarp is used locally for cooking. A. trifoliata is cultivated and bred by selection in several areas of Japan, especially in Yamagata Prefecture. A. trifoliata is monoecious and has a raceme with few female flowers at the base and multiple male flowers at the tip (Fig. 1). Its flowers lack petals and have three petaloid sepals that attract flower-visiting insects (Kawagoe and Suzuki, 2003). Female flowers have three to eight cylindrical, style-less pistils, each of which becomes a fruit when successfully pollinated and fertilized.

Raceme inflorescence (left) and pistils (right) of A. trifoliata ‘Fuji Murasaki’. Black and white triangles indicate female and male flowers, respectively.
Instability of fruit set is recognized as a major problem in the cultivation of A. trifoliata, and is thought to be related to self-incompatibility (SI; Matsumoto et al., 2020). SI is a genetic mechanism by which pistils recognize and reject pollen from the same or related individual plant to avoid inbreeding and to promote outcrossing (de Nettancourt, 2001). In the cultivation of fruit trees exhibiting SI, it is generally recommended to promote cross-pollination by planting pollinators, as well as by artificial pollination or the introduction of flower-visiting insects. However, little is known about the reproductive physiology of A. trifoliata, including its cross-compatibility and effective pollination period. As a result, effective artificial pollination methods have not yet been established. In A. trifoliata orchards, farmers have attempted artificial pollination based on experience, such as by using a feather duster or rubbing male flowers by hand. Although we also conducted artificial pollination with a feather duster in 2019 and 2020, the fruit set rates were less than 0.5% in both years (unpublished data).
Artificial pollination methods for SI fruit trees have been established mainly based on rosaceous species. However, the SI mechanism in A. trifoliata is unclear and is assumed to be different. It is not known whether the same approach will be effective for Akebia SI. SI is classified into three types according to the site of self-pollen rejection, that is, stigmatic, stylar, and ovarian inhibition (de Nettancourt, 2001). The latter is also called late-acting SI (LSI). While the SI of rosaceous fruit trees is stylar type, the SI of A. quinata is reported to be LSI (Kawagoe and Suzuki, 2005). A few fruit trees are also known to exhibit LSI, such as chestnut (Castanea mollissima) (Fagaceae), durian (Durio zibethinus) (Malvaceae), and feijoa (Acca sellowiana) (Myrtaceae), although the detailed mechanisms are still unknown (Finatto et al., 2011; Honsho et al., 2004; Lo et al., 2007; Xiong and Zou, 2019). The characteristic feature of LSI is that self-pollen competes with cross-pollen for ovule and/or fruit set in several species (Gibbs, 2014; Seavey and Bawa, 1986). Since LSI includes various mechanisms by which self-pollen is rejected near ovules, at the micropyles, at syngamy, and at embryo development, the results of the competition between self- and cross-pollen differ among plant species. In Cryanthus breviflorus (Amaryllidaceae) and Ceiba spp. (Bombacaceae), simultaneous pollination of self- and cross-pollen has been reported to result in a significant decrease in fruit set and seed number compared to cross-pollination (Gibbs et al., 2004; Kiepiel and Johnson, 2014; Vaughton et al., 2010). In Adenocalymma peregrinum (Bignoniaceae), pollination by a mixture of self- and cross-pollen was reported to result in a significant decrease in fruit set, but not in seed number (Duarte et al., 2017). In stylar SI, self-pollen does not inhibit the elongation and fertilization of cross pollen tubes, and little attention has been paid to self-pollen contamination in artificial pollination. In A. quinata, simultaneous pollination with self- and cross-pollen was reported to inhibit the fruit set rate (Kawagoe and Suzuki, 2005). The influence of self-pollen contamination on artificial pollination is also a concern in A. trifoliata.
In this study, we investigated the effects of self-pollination on fruit set using ‘Fuji Murasaki’, a major line in the Asahi-machi region of Yamagata Prefecture, in order to understand the reproductive physiology of A. trifoliata.
A. trifoliata ‘Fuji Murasaki’ trees planted in a commercial orchard in the Asahi-machi region of Yamagata Prefecture were used for the study. Pollen collected from A. trifoliata ‘Shuka’ planted in the same field was used as compatible pollen with ‘Fuji Murasaki’ (Matsumoto et al., 2020). The orchard was managed following the practices of a commercial orchard, except for artificial pollination. Ten or 11 weeks after pollination, fruit thinning was conducted to leave less than three fruits per fruit cluster.
Collection and germinability testing of A. trifoliata pollenIn 2016, anthers were collected from racemes in which the anthers of only a few male flowers at the tip were dehisced. The collected anthers were incubated on weighing paper with silica gel at room temperature for one to two days. The dried anthers were gently milled with a pestle to release pollen and sieved with a mesh size of 106 μm to collect pollen from the anthers. Racemes similar to those in 2016 were collected in 2019 and 2020. After all female flowers were removed, racemes were incubated in a similar manner to dehisce anthers. Pollen was shaken off from the dried raceme, and impurities were removed with tweezers. Each year, pollen was divided into small portions and stored in polyethylene bags with silica gel at 4°C until use.
The germination rate of the collected pollen was determined by incubating the pollen on 10% sucrose agar medium (1% agar) on glass slides for 12 h at 20°C. After incubation, the pollen was observed using a microscope (ECLIPSE Ni; Nikon Instec Co., Ltd.), and those with tubes elongated more than the diameter of the pollen grain were considered to have germinated. More than 200 pollen grains were observed.
Artificial pollination and fruit set ratesChase pollination tests were conducted in 2016, 2019, and 2020 and mixed pollen pollination tests were conducted in 2016 and 2019. In the chase pollination tests, we conducted prior self-pollination one day before cross-pollination and subsequent self-pollination one day after cross-pollination. In the mixed pollen pollination tests, cross- and self-pollen were mixed at ratios of 1:1, 3:1, and 9:1 (w/w), and cross-pollen and lycopodium powder were mixed at a ratio of 1:1 (w/w). For each pollination, 14–16 female flowers were used in 2016, 20–26 in 2019, and 20–25 in 2020. Four to seven days before artificial pollination, racemes with unopened female flower buds were selected and bagged after male flowers were removed. On the day of artificial pollination, the bags were removed, the calyx was cut from female flowers, and flowers were pollinated with a spatula. In the chase pollination tests, a second pollination was carried out the following day.
Nine weeks after pollination, fruit set rates were determined for each female flower by dividing the number of fruits with the number of pistils of the same female flower on the pollination day. In early September, 15 random fruits, or all fruits when there were fewer than 15, were harvested, and the number of normal, empty, and small ovule-like seeds less than 1 mm in diameter were counted.
Observation of pollen tube elongation in pollinated pistilsTen pistils each were self- or cross-pollinated as described above, collected 10 d after pollination, and stored in FAA solution (formalin: glacial acetic acid: 50% ethanol = 5:5:90). Before observation, the pistils were rinsed three times with distilled water and immersed in 0.1% aniline blue solution for 12 h. Then, using a razor blade, the pistils were cut into three pieces parallel to the suture and the central piece was observed under a fluorescence microscope (ECLIPSE Ni).
Statistical analysisStudent’s t-test or Dunnett’s multiple comparison test against cross-pollination were performed using SAS software. Arcsine-transformed values were used for the statistical analysis of the fruit set rate.
In all the years of study, self-pollinated pistils started to abscise around three weeks after pollination (WAP), and none of them were left 5 WAP, indicating that ‘Fuji Murasaki’ exhibited SI. Pollen tubes in both self- and cross-pollinated pistils had reached a point close to the base of locules 10 days after pollination (DAP, Fig. 2). Some of them were observed to penetrate ovules, indicating that A. trifoliata exhibited LSI, as in the case of A. quinata (Fig. 3). In Crotalaria juncea with LSI, callose accumulation in the nucellus cells of self-pollinated pistils has been reported (Thimmaiah et al., 2018). However, callose accumulation was not observed even 14 DAP in A. trifoliata (data not shown).
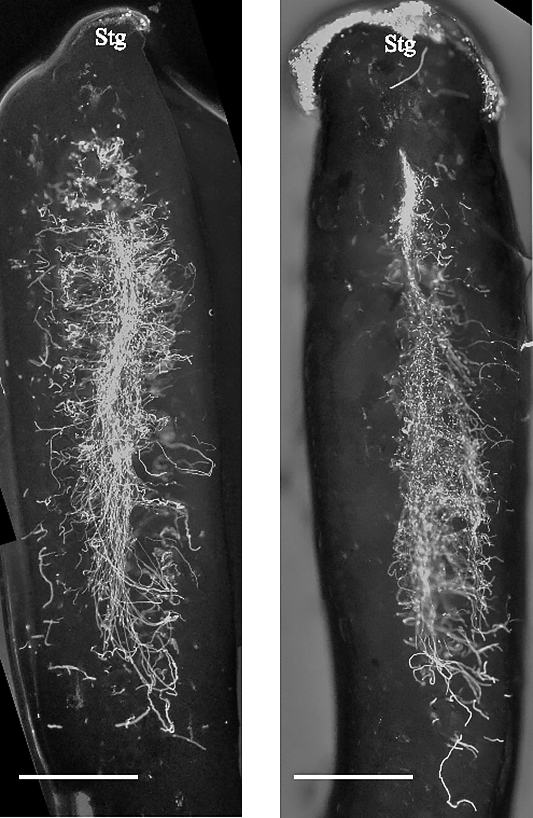
Fluorescent microscopic observation of pollen tubes in self- (left) and cross- (right) pollinated pistils of A. trifoliata ‘Fuji Murasaki’ 10 days after pollination. The bars indicate 1 mm. The images were made by connecting multiple photos. Stg: stigma.

Fluorescent microscopic observation of ovule penetration in self-pollinated pistils 10 days after pollination. The bar indicates 500 μm. Triangles indicate pollen tube tips penetrating the ovule.
The germination rates of ‘Fuji Murasaki’ and ‘Shuka’ pollen were 44% and 36% in 2016, 45% and 54% in 2019, and 50% and 55% in 2020, respectively. Pollen collected in 2016 were highly contaminated with anther debris, while there were fewer contaminants in pollen collected in 2019 and 2020.
Chase pollination tests were conducted in 2016 and 2020 to clarify whether incompatible pollen interferes with fruit set by compatible pollination. In both years, all of the pistils that were self-pollinated 1 d before cross-pollination abscised by 5 WAP, whereas subsequent self-pollination 1 d after cross-pollination resulted in fruit set (Fig. 4). The fruit set rates at 9 WAP were compared between subsequent self-pollination and cross-pollination, and the fruit set by cross-pollination rarely abscised. In 2016, the fruit set rate in cross-pollination was 32.7% (n = 14), whereas that of subsequent self-pollination was significantly lower at 7.2% (n = 16, P < 0.05). In 2019, only subsequent self-pollination was conducted; 57.8% (n = 26) of fruit set was achieved by cross-pollination and 45.2% (n = 21) by subsequent self-pollination, which were not significantly different (P > 0.05). In 2020, the fruit set rate for cross-pollination was 42.6% (n = 23), while that of subsequent self-pollination was 43.9% (n = 22), comparable to cross-pollination (P > 0.05). These results indicate that prior self-pollination inhibits fruit set through cross-pollination, whereas subsequent self-pollination does not affect fruit set.

Fruit set rates in chase pollination of A. trifoliata ‘Fuji Murasaki’ nine weeks after pollination (A, 2016; B, 2019; C, 2020). Data represent the average ± SE of replicates, the number of which is shown above the bars. Asterisks indicate significant differences in Dunnett’s multiple comparison test compared to cross-pollination (P < 0.05).
Pollination using a mixture of self- and cross-pollen was conducted in 2016 and 2019 to examine the quantitative effects of self-pollen contamination. In 2016, the fruit set rate for cross-pollination was 32.7% (n = 14), whereas that for mixed pollination using 1:1, 3:1, and 9:1 mixtures of cross- and self-pollen were 1.6% (n = 16), 7.1% (n = 15), and 15.1% (n = 16), respectively (Fig. 5A). Fruit set rates for all of these mixed pollination techniques were significantly lower than those for cross-pollination (P < 0.05). Cross-pollination using 2-fold diluted pollen with lycopodium powder was also conducted, and the fruit set rate was 4.4% (n = 16), comparable to that of 1:1 mixed pollination (P > 0.05 in Student’s t-test). In 2019, the fruit set rate by cross-pollination was 60.2% (n = 26), whereas that by mixed pollination using 1:1, 3:1, and 9:1 mixtures of cross- and self-pollen were 27.9% (n = 21), 46.7% (n = 20), and 50.7% (n = 20), respectively (Fig. 5B). Among these, fruit set rates for 1:1 mixed pollination were significantly lower than those for cross-pollination (P < 0.05). The fruit set rate for cross-pollination using 2-fold diluted pollen was 33.5% (n = 20), which was comparable to that of 1:1 mixed pollination (P > 0.05 in Student’s t-test).

Fruit set rates in mixed pollination of A. trifoliata ‘Fuji Murasaki’ nine weeks after pollination (A, 2016; B, 2019). Data represent the average ± SE of replicates, the number of which is shown above the bars. Asterisks indicate significant differences in Dunnett’s multiple comparison test compared to cross-pollination (P < 0.05).
A few fruits set by mixed pollination were harvested in 2016, and among these 1:1 mixed pollination yielded only one fruit (Table 1). The number of seeds in fruits obtained by 3:1 and 9:1 mixed pollination were not statistically significant compared to that obtained by cross-pollination (P > 0.05). An adequate number of fruits was set by mixed pollination in 2019. There was no significant reduction in seed number by either type of mixed pollination. In addition, several fruits obtained from 1:1 mixed pollination were abnormal (Fig. 6). Only one fruit in 2016 and six of 15 fruits in 2019 had hard and green apices, whereas such abnormal fruits were not produced by the other pollination methods. Among the fruits obtained by 1:1 mixed pollination in 2019, the number of seeds in abnormal fruits (182.8 ± 14.3) was not statistically significant compared to that of normal fruits (173.4 ± 12.6, P > 0.05).

Seed numbers in fruit developed by mixed pollination.

Abnormal ‘Fuji Murasaki’ fruits with hard and green apices developed by 1:1 mixed pollination (left, normal fruits; right, abnormal fruits in 2019).
Self-pollen tubes of A. trifoliata ‘Fuji Murasaki’ were observed to reach close to the base of locules, similar to those of A. quinata reported by Kawagoe and Suzuki (2005), indicating that Akebia SI is late-acting. LSI is further classified into three types based on the site and stage of self-pollen rejection: prezygotic rejection in the ovary, prezygotic rejection in the ovule, and postzygotic rejection (Seavey and Bawa, 1986). Akebia LSI is one of the latter two types because ovule penetration by self-pollen tubes was observed. Although we did not compare the rates of self- and cross-pollen tube elongation, Kawagoe and Suzuki (2005) reported that there was no difference between self- and cross-pollen tubes. These results suggest that self-pollen may compete with cross-pollen for the ovules.
Prior self-pollination, one day before cross-pollination and self-pollen contamination in artificial pollination at more than a certain level significantly reduced the fruit set rate. In the three years of chase pollination tested, subsequent self-pollination one day after cross-pollination reduced fruit set significantly only in 2016, suggesting the possibility that subsequent pollination did not affect fruit set. Artificial pollination was conducted more carefully in 2016 compared to the other years, since pollen was less cohesive possibly due to contaminated anther debris. The significant fruit set reduction by subsequent self-pollination in the same year could be attributable to pistil damage caused by repeated careful artificial pollination. It is still of interest to determine whether the suppression of fruit set by self-pollen is the result of competition between self- and cross-pollen for ovules. It is noteworthy that the half-diluted cross-pollination also resulted in low fruit set, which was comparable to the 1:1 mixed pollination in the two years tested. These results suggest the importance of the amount of pollinated compatible pollen, rather than that of contaminated self-pollen, for successful fruit set. Fruit set inhibition by prior self-pollination may not be due to competition between pollen, but to insufficient cross-pollen attachment to the stigma as they are already covered by self-pollen.
Although fruit set was reduced by mixed pollination, there was no apparent decrease in the number of seeds. This result indicates that the reduction in fruit set is not simply due to a decrease in fertilized ovules. Assuming that self-pollen is able to compete with cross-pollen for ovules, it is possible that self-pollen also produces seeds in mixed pollination. However, we could not examine that possibility. Narcissus triandrus (Amaryllidaceae) and Theobroma cacao (Malvaceae) that both exhibit LSI, have been reported to produce self-fertilized seeds in mixed pollination (Adu-Ampomah et al., 1991; Glendinning, 1960; Sage et al., 1999). Interestingly, it has been suggested that the LSI of these plant species is a prezygotic reaction. In N. triandrus, as well as N. papyraceus and Ipomopsis aggregate (Polemoniaceae), it has been observed that ovule development was different between self- and cross-pollinated pistils even before pollen tubes reached an ovary (Sage et al., 1999, 2006; Simón-Porcar et al., 2015). In T. cacao, an increase in IAA and a decrease in ABA in cross-pollinated pistils and a slight increase in ABA in self-pollinated ones were detected at 8 h after pollination, when pollen tubes reached the base of styles (Baker et al., 1997). If Akebia LSI also has a prezygotic reaction similar to these plant species, ovules could develop normally in mixed pollination, but not in self-pollination, to allow self-pollen fertilization. It is important for stable fruit set and breeding of A. trifoliata to clarify whether Akebia LSI has a prezygotic reaction and whether it is possible to generate selfed progenies.
In conclusion, we showed that self-pollen-contamination during artificial pollination could reduce the fruit set of A. trifoliata. Excess self-pollen contamination may also be related to the development of abnormal A. trifoliata fruit, which has been occasionally observed in commercial orchards (Fig. 6). Effective pollination management suitable for the reproductive physiology of A. trifoliata, such as rubbing compatible male flowers or compatible pollen by brushing, is necessary for stable fruit production.