2023 Volume 92 Issue 3 Pages 269-280
2023 Volume 92 Issue 3 Pages 269-280
Garland chrysanthemum is conventionally ratooned and harvested by selective hand picking. This manually intensive mode of harvesting may be improved by adopting mechanized harvesting practices. In view of incorporating machine harvesting into garland chrysanthemum crops, this study evaluated the effects of plant and cutting height on ratoon crop regrowth. Garland chrysanthemums were horizontally harvested using six combinations of three plant height levels at harvest (20 cm, 30 cm, and 40 cm) and three cutting height levels (5 cm, 10 cm, and 15 cm) over three repeated harvests. Plant height at harvest as well as the cutting height affected the total ratooning yield and regrowth process. If plant heights at harvest were low (20 cm) or cutting height relative to plant height at harvest was excessively high, yields decreased. Higher plant heights at harvest and lower cutting heights resulted in longer regrowth periods. Evaluation of branch structures revealed that low cutting heights decreased the number of branches and nodes remaining for regrowth. If the plant height at harvest was low (20 cm), node numbers tended to decrease. Excessively high cutting heights relative to plant height at harvest potentially results in the harvest of immature leaves and stems. We determined that harvesting at 30 cm plant height with 10 cm cutting height was suitable for ratooning, and resulted in high yields. This combination resulted in a sufficient number of first lateral branches and nodes on the plants, thus enabled the harvest of fully grown leaves.
The agricultural population in Japan is facing a rapid decline and increase in age (Ministry of agriculture, forestry and fisheries, https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00500209&tstat=000001016170&cycle=0&tclass1=000001112708&tclass2=000001112709&stat_infid=000031676785&cycle_facet=tclass1%3Atclass2&tclass3val=0, 2018). Nevertheless, there is a need to enhance the domestic supply of vegetables for commercial use in food processing industries that depend on vegetable imports (Kobayashi, 2018). Agricultural mechanization may resolve labor shortage problems, ensuring food security in the domestic production of vegetables. Although Japan uses a unified mechanized system for rice cultivation (Nakatani, 2018), the use of machinery is only partially introduced in vegetable production. As most vegetables are selectively harvested by hand, introducing automated harvesting will help minimize labor costs. However, harvest mechanization does not only consist of developing new harvesting machines, it also involves the establishment of crop-specific production and harvesting techniques.
Garland chrysanthemum (Chrysanthemum coronarium L.), a member of Asteraceae, is a leafy vegetable which is often cooked in hot pot or used in salads. Although garland chrysanthemum production is declining (https://www.maff.go.jp/j/tokei/kouhyou/sakumotu/sakkyou_yasai/index.html, 2020), we believe that the demand for this leafy vegetable will continue throughout the year for its unique flavor. The cultivars of garland chrysanthemum are divided into large, medium, and small leaf types; the large and medium leaf types are commonly cultivated in Japan (Suzuki, 2004). The medium leaf type cultivar for plucking harvest has traits of internode elongation and branching. After the main stem is harvested, branch stems regrow with allowing several subsequent harvestings. This harvesting process is followed conventionally for garland chrysanthemums and can be considered a type of ratooning.
In ratoon cropping, a part of the plant is left after first harvest and allowed to regrow. The plants are then re-harvested, with this cycle repeated. Ratooning has advantages over traditional cultivation as it lowers seedling costs and shortens growth periods. Ratooning is used in sugar cane and rice production (Marin et al., 2019; Wang et al., 2020). In Japan, vegetables such as Welsh onions and rape blossoms are ratooned and of which cultivation techniques are studied (Muneishi and Itokawa, 2019; Odawara et al., 1995). Suzuki et al. (2018, 2021) studied ratooning of spinach incorporating machine harvesting, where only leaves are harvested by the horizontal motion of a mowing blade, and the plants allowed to regrow. Suzuki et al. (2021) reported that ratoon cropping increased yields, and that the regrowth of spinach was influenced by cultivar and fertilizer management.
Ratooning harvest methods and regrowth processes vary with crop morphological traits. For example, Welsh onions and spinach have short stems and lower situated growing points (Thakur, 2018; Yakuwa, 1953). After the above-ground sections are cut at the lower position, plants regrow from the remaining growing points. In contrast, garland chrysanthemum and rape blossom display internode elongation and branching traits. Stems with leaves at harvesting stage are picked selectively, and young shoots regrow from the remaining nodes (Odawara et al., 1995; Yamamoto et al., 1993). In the case of garland chrysanthemums, there are no reports of specific horizontal cutting harvesting methods except for an early description by Ogawa (1978). Ogawa reported a method in which lateral branches are horizontally cut after the main stem is plucked. In conventional ratooning, the main stems of garland chrysanthemum are picked leaving behind four nodes, with the regrown lateral branches subsequently picked, leaving behind two nodes on the branches. Kawamura et al. (2008) reported that shoots at the lower position of the stems grew before the main stem was pinched, while shoots at the higher position grew after the main stem was pinched. We considered that these results indicate that the lateral buds under the cutting surface may begin to grow after horizontal harvest. Considering the branching traits of garland chrysanthemum, and by implementing a similar machine harvest technique to that used for spinach, it appears possible to use machine harvesting in ratooning.
Tea cultivation in Japan involves tea shoot harvesting using machinery at a uniform tree height, after which new shoots grow from under the harvested surface. Nakano (1998) studied the effect of fall trimming on the first flush of the following spring in tea plants, and reported that the trimming height affected the branch structure and growth. By trimming at the high position of the tree, the harvest date was delayed, number of apical buds increased, and the number of lateral buds just below the cut surface were reduced. In rice ratooning research, the highest total yield was achieved when the first crop was harvested at a normal harvest time with a high cutting height (Nakano et al., 2020). In addition, Nakano et al. (2020) reported that the second crop ripening required more time than the first due to low temperature conditions post first harvest. The above observations indicate that cultivation conditions as well as changes in the environment may affect regrowth and harvest of plants in ratooning.
In this study, we tried to establish a ratooning cultivation method for garland chrysanthemum with future machine harvesting in mind. We believe that harvest timing and cutting height parameters influence the volume of stems, leaves, and branching structures, which in turn affect regrowth and yield. This study aimed to investigate the effects of plant height at harvesting and cutting height on regrowth for ratooned garland chrysanthemum harvested at a uniform cutting height. We evaluated the effects of three harvesting plant height levels and three cutting height levels on ratooning in spring and fall seasons.
Four trials were performed at NARO (36°01′N, 140°06′E), Tsukuba, Japan, during spring (April 27 to July 3, 2021; May 21 to July 10, 2021) and fall (September 17 to December 10, 2021; October 4 to December 16, 2021). The garland chrysanthemum of medium leaf type cultivar ‘Oedo’ (The Musashino Seed Co., Ltd., Tokyo, Japan) was used in these trials. Four seeds per cell were sown in 128-cell seedling trays filled with growth media (Yosaku N-8; JCAM AGRI. CO., LTD., Tokyo, Japan) containing 80N-1500P-150K (mg·L−1) which were then thinned to one plant per cell after seedling emergence. At 2 or 3 weeks after germination, the cell trays were soaked in liquid fertilizer (OK-F-9; OAT Agrio Co., Ltd., Tokyo, Japan) with 150N-150P-150K (mg·L−1) once a week. Seedlings were raised under a rain-protected plastic film shelter. The seedlings were transplanted to an unheated plastic film green house, 17.0 m long × 4.5 m wide × 3.0 m high. The soil, an andosol, was fertilized with 15N-15P-15K (kg/10a) before transplantation. Plants were grown in 2 rows with staggered planting on a 60 cm wide bed. Each row was separated from the other by 30 cm and plants within rows were separated by 30 cm. For April, May, September, and October planting, sowing and transplanting dates were April 1 and April 27, April 28 and May 21, August 31 and September 17, and September 13 and October 4, respectively. In spring trials, 45% shading rate cloth (SlimWhite45; Nihon Widecloth Co., Ltd., Osaka, Japan) was spread 1.7 m above the ground in the greenhouse to protect from heat.
Experimental designA schematic diagram of ratoon cropping is shown in Figure 1. Target plant height at harvest was set to three levels (20 cm, 30 cm, and 40 cm). Harvests were carried out when the average test plant height exceeded the target height. Cutting height was set to three levels (5 cm, 10 cm, and 15 cm). Ratooning was evaluated in six different combinations of plant and cutting height, as shown (Fig. 1). The widths of harvesting were varied from 10 cm to 30 cm. Of the six treatments, 20-5 (plant height-cutting height) and 20-10 were harvested at the low plant height (20 cm). At the mid-plant height of 30 cm, cutting heights were set to three levels (30-5, 30-10, and 30-15). Furthermore, 40-10 was harvested at the higher plant height of 40 cm. Each plot consisted of four plants, and each treatment comprised three plots.
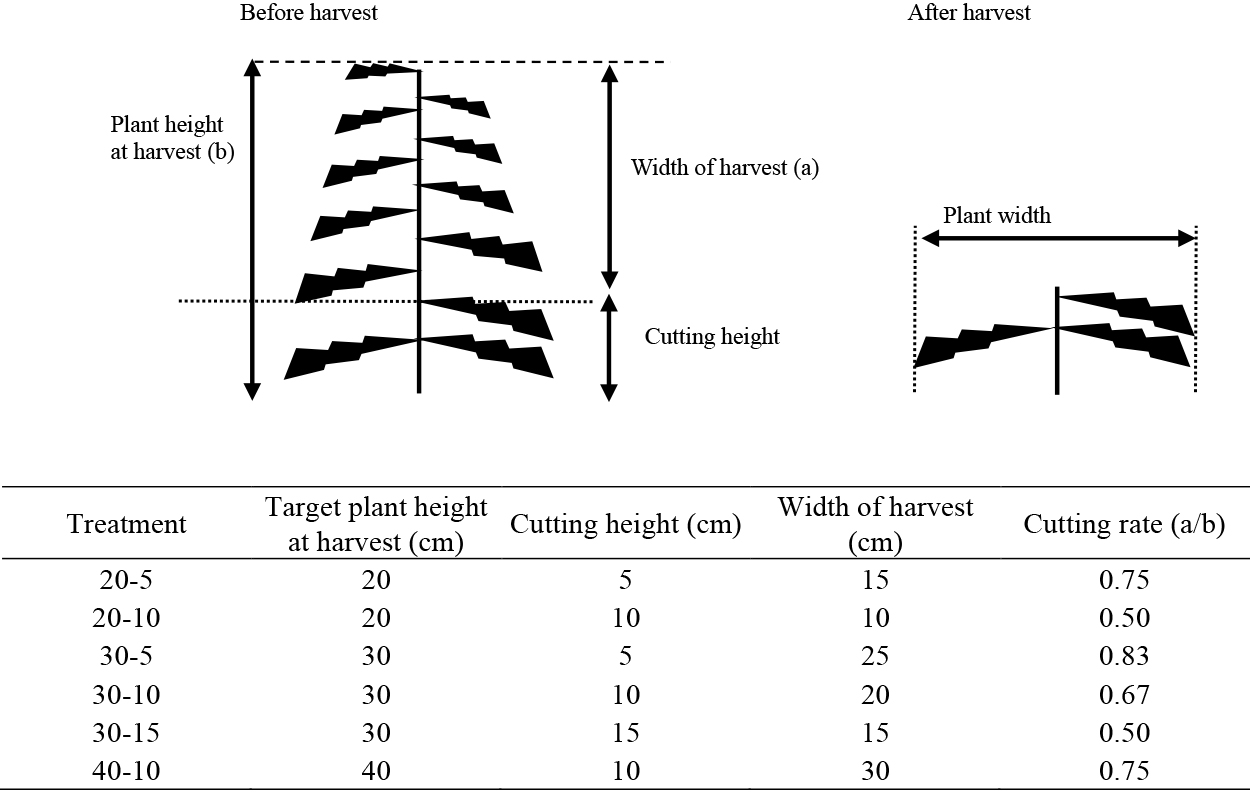
Schematic diagram of garland chrysanthemum ratooning.
Stems with leaves were harvested a maximum of three times. The date of each harvest, actual plant height at each harvest and regrowth rates are listed in Table 1. Planting month, plant height at harvest and cutting height varied with harvest dates after planting. The third harvests of 30-5, 30-10, and 40-10 in May planting were canceled, due to disease occurrence with rising temperature. Regrowth of fall trials required more days than spring trials due to slow growth in low temperatures. Therefore, the third harvests of 30-5 and 40-10 of the September plantings, third harvests of 20-5, 30-5, and 30-10 of the October plantings and second and third harvests of 40-10 of the October plantings were canceled. There were plants that were not regrowing in 20-5, 30-5 and 40-10 at spring plantings. All harvested plants reached the target height. Harvest plant heights on May 18, June 18, and June 20 were 6 cm higher than the target heights. However, this difference was recorded in one of two or three harvests, and these data were used for analysis.
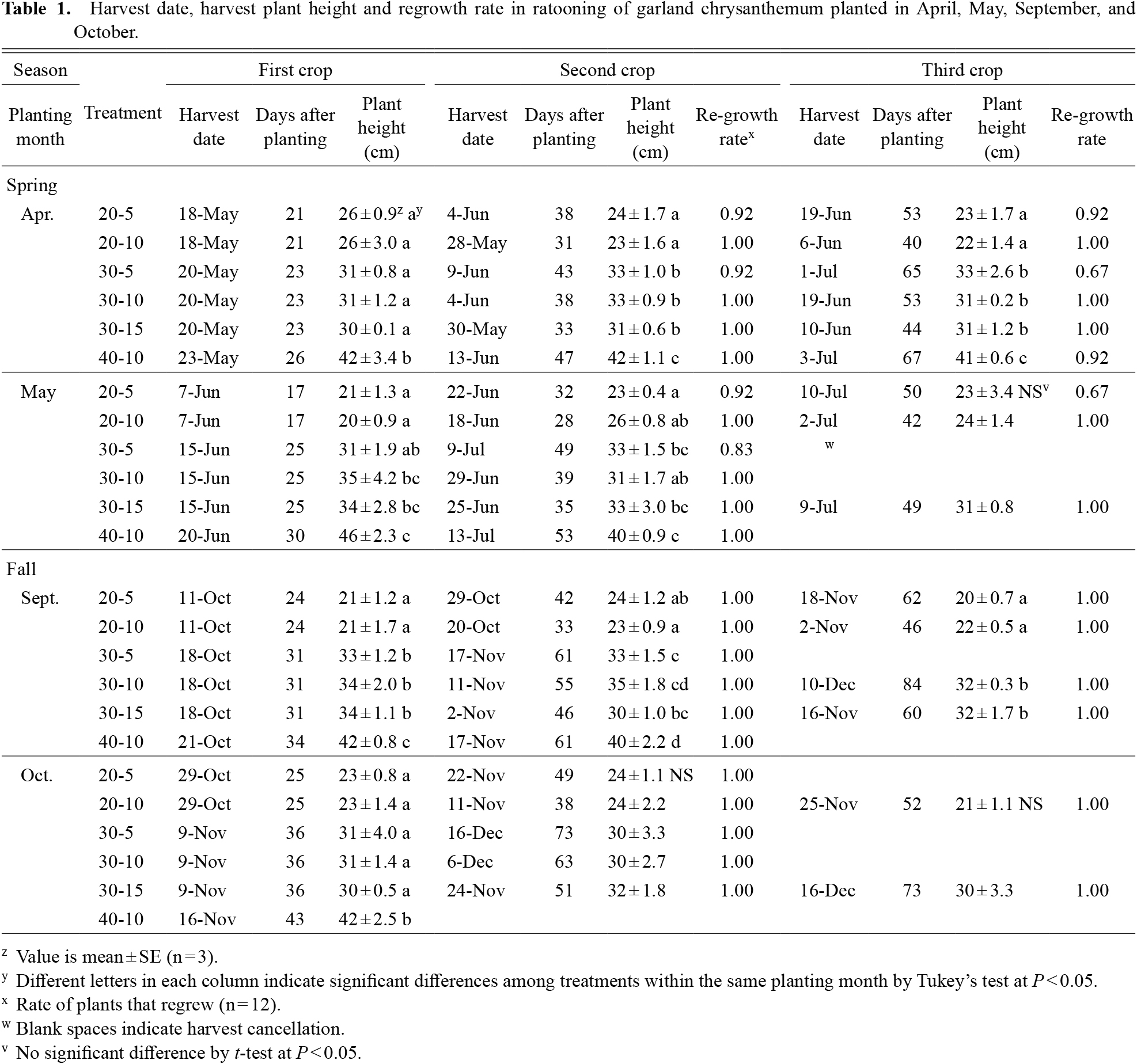
Harvest date, harvest plant height and regrowth rate in ratooning of garland chrysanthemum planted in April, May, September, and October.
At harvest, stems with leaves above the cutting surface were cut and collected from each plant. Collected stems and leaves were classified as shown in Figure 2. Stems with leaves longer than 15 cm were separated into harvest crops and their weights and numbers were measured as yields (Fig. 2). Of harvest crops, stems with leaves longer than 25 cm were cut to 25 cm from the top of the leaves. Extremely thin stems with leaves (shorter than 20 cm and of which base is thinner than 4 mm) were excluded from harvest crops.

Classification of harvests based on the length of stems with leaves in garland chrysanthemum.
Test plant characteristics were evaluated before or after harvest. Plant heights were measured before harvest and plant widths were measured after harvest (Fig. 2). Leaf spreading was measured as plant width at two orthogonal locations per plant and averaged. Main stem leaf number and length were measured before first harvest. Internode length was calculated by dividing stem length by the number of leaves (Fig. 3). The number of first lateral branches under the cutting surface was counted after the first harvest. The number of first lateral branches whose apical buds were pinched and the number of nodes on the lateral branches whose apical buds were pinched were measured after the harvests (Fig. 3). The number of growing points that are potentially harvestable (nodes number) summed the number of the first lateral branches whose apical buds were not pinched and the number of nodes on the lateral branches whose apical buds were pinched.
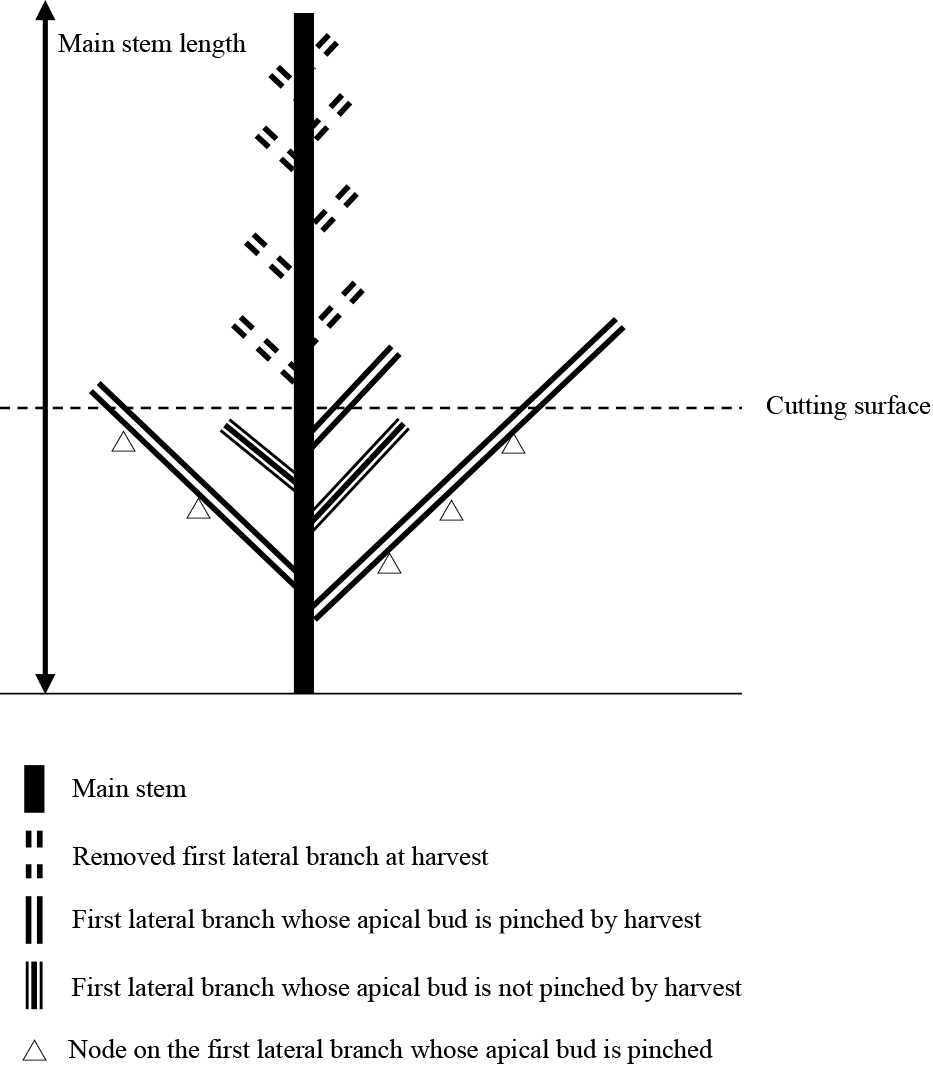
Branch structure at first harvest in ratooning of garland chrysanthemum.
The air temperatures in the green house were collected at a height of 0.5 m, and daily sunshine duration at the nearest weather station (Tsukuba) was obtained from the weather data of the Japan Meteorological Agency (https://www.data.jma.go.jp/obd/stats/etrn/). The daily air temperature at the green house and daily sunshine duration data are summarized in Figure 4. During spring trials (April 27 to July 13), the temperature gradually increased from April to July. The optimum temperature for the growth of garland chrysanthemum is between 15°C and 20°C (Suzuki, 2004); however, the daily average temperature consistently exceeded 20°C after June. During the first harvest period (May 18 to May 23) of the April planting, the days with short duration of sunshine continued. During the first harvest period (June 7 to June 20) of the May planting, temperatures remained high. During Fall trials (September 17 to December 16), after mid-October, the minimum temperature was occasionally lower than 10°C. At the end of November, the maximum temperature remained near 20°C due to the green house set up, but the minimum temperature dropped below 0°C.
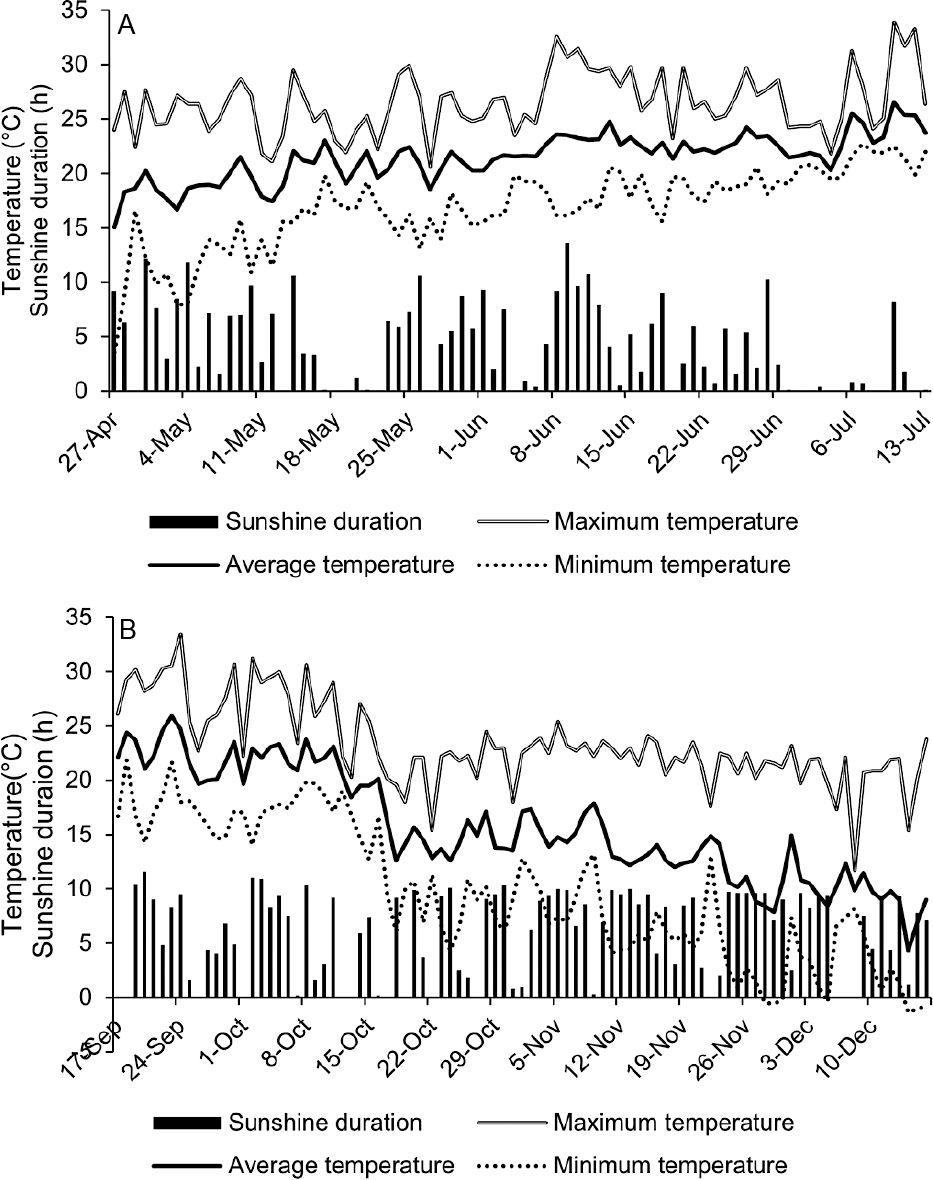
Daily air temperature inside the green house and daily duration of sunshine at the nearest weather station (Tsukuba) during spring trials (A) and Fall trials (B).
Statistical analysis was performed using R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria).
Total yields from ratooning of garland chrysanthemum are indicated in Figure 5. Plant height at harvest, cutting height, and planting month affected total yield. The highest crop yields were produced in 40-10 for the April planting (228 g per plant), 40-10 for the May planting (131 g per plant), 30-10 for the September planting (309 g per plant), and 30-5 for the October planting (197 g per plant) (Fig. 5A, C, E, G). The second and third harvest yields tended to be higher than the first.
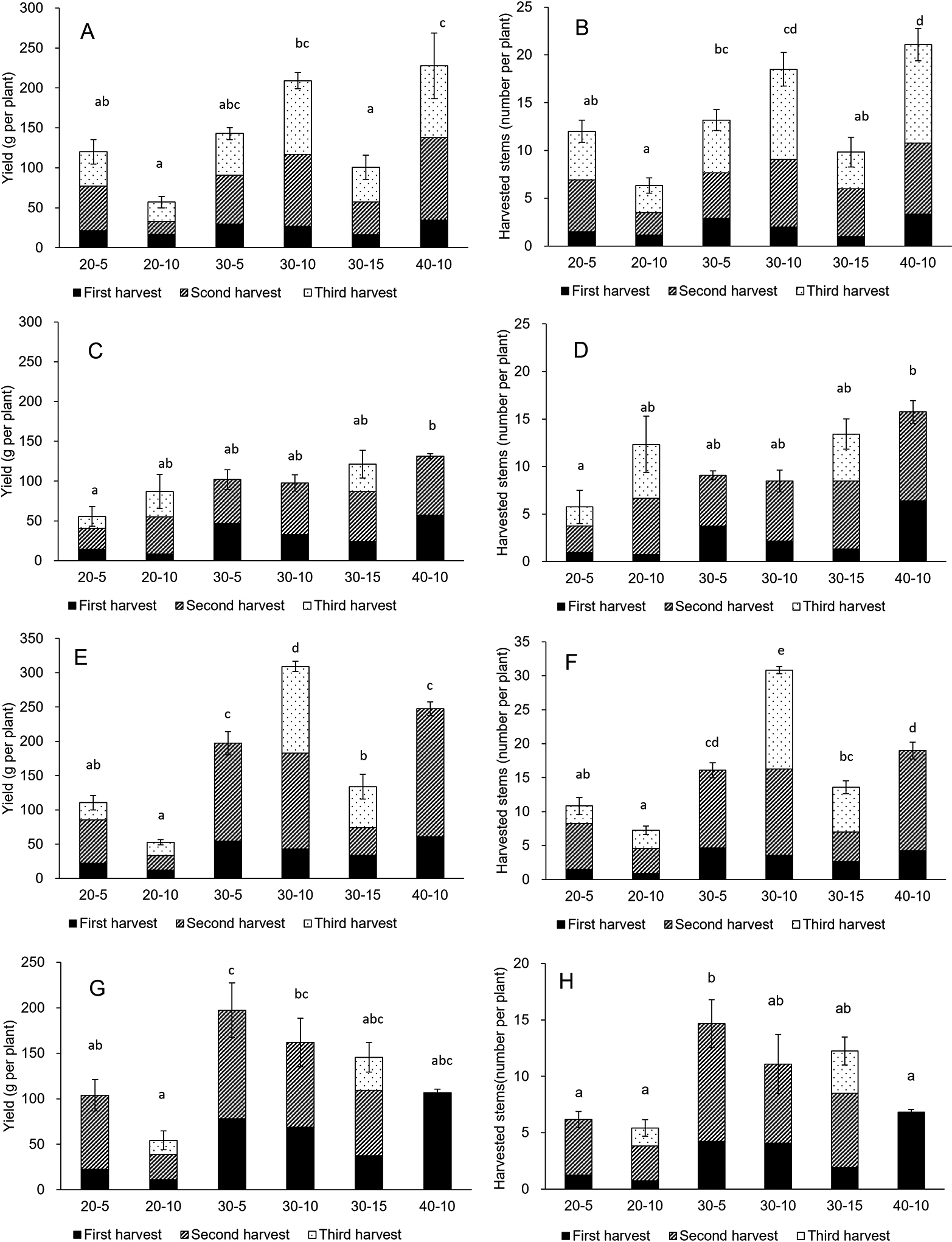
Total yield of fresh weight and number of harvested stems per plant in garland chrysanthemum planted in April (A, B), May (C, D), September (E, F), and October (G, H). Bars are SE (n = 3). Different letters in each column indicate significant differences in total yields among treatments within the same planting month by Tukey’s test at P < 0.05.
The effect of plant height at harvest on total yield was evaluated by comparison at the same cutting height. In the case of a 10 cm cutting height (20-10, 30-10, and 40-10), the lowest plant height at harvest (20-10) produced a significantly lower total yield than 30-10 except in the May planting, and 40-10 except in May and October (P < 0.05) (Fig. 5A, C, E, G). For the 5 cm cutting height (20-5 and 30-5), the lower plant height at harvest (20-5) produced significantly lower total yields than 30-5 in September and October.
Cutting height affected total yields (Fig. 5A, C, E, G). For a 30 cm plant height at harvest (30-5, 30-10, and 30-15), the highest cutting height (30-15) produced the lowest total yield, while 30-10 produced the highest total yield for both April and September plantings. There were no significant differences among treatments in the May and October plantings. For the 20 cm plant height at harvest (20-5 and 20-10), there were no significant differences among treatments. The cutting rate and width of harvest also affected total yield (Fig. 5A, C, E, G). Plants of 20-10 and 30-15 treatments were harvested with the lowest cutting rate i.e., higher cutting height relative to plant height, and 20-10 was also harvested with the lowest width of harvest (Fig. 1). These treatments tended to result in lower total yield than other treatments in April and September.
The effect of plant height at harvest and cutting height on the number of harvested stems could be correlated with yield results (Fig. 5). The 30-10 and 40-10 treatments of the April planting and 30-10 of the September planting produced a large number of harvested stems (Fig. 5B, D, F, H).
Effects of plant height and cutting height on cumulative yieldHigher total yields were produced from the April planting in spring and September planting in fall, while there were many canceled harvests in May and October (Table 1; Fig. 5). As April and September were considered desirable for planting, the changes in cumulative yields were compared. Figure 6 shows the change in cumulative yields for April and September plantings. Cumulative yields linearly increased with days of regrowth. The steeper lines in Figure 6 represent higher yields per regrowth days. The number of days for regrowth for each harvest tended to be longer in September plantings than April plantings.
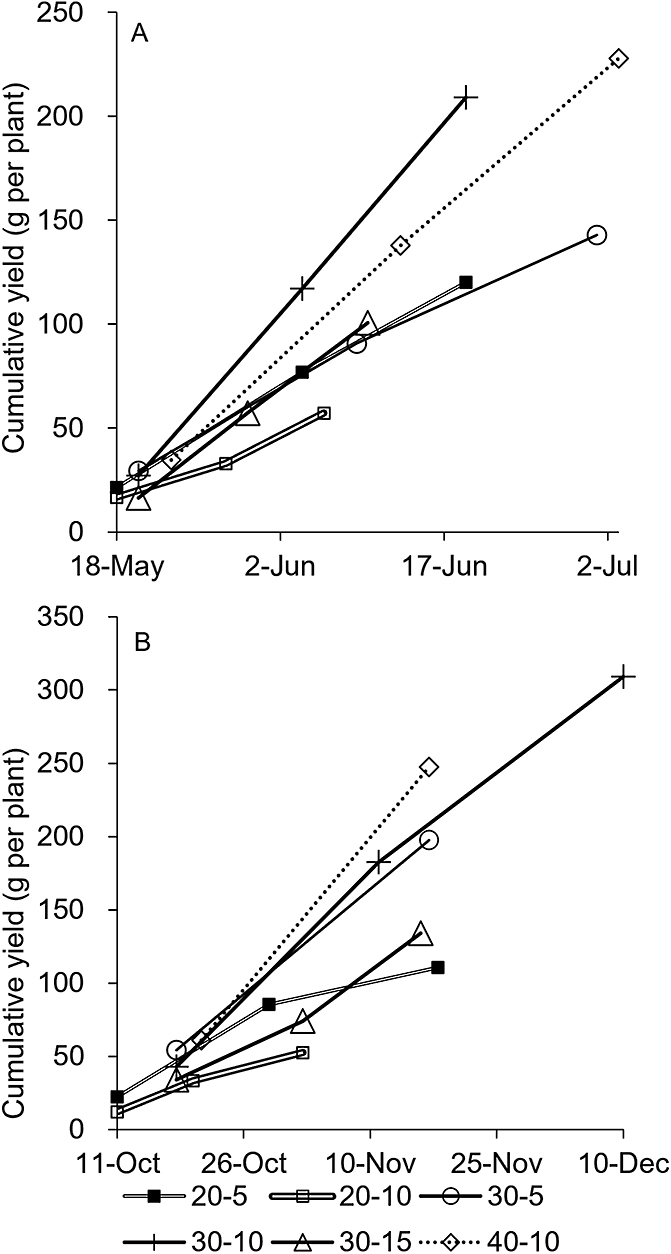
Changes in cumulative yields per plant in ratooning of garland chrysanthemum planted in April (A) and September (B).
Higher plant heights at harvest required more days to regrow (Fig. 6). For the 10 cm cutting height, the higher plant heights at harvest required more days to regrow for both planting months. Similar results were observed for the 5 cm cutting height. Lower cutting heights also took more days to regrow (Fig. 6). For the 30 cm plant height at harvest, the lower cutting height took more days to regrow for April and September plantings. Similar results were observed for the 20 cm plant height at harvest.
The lowest plant height at harvest (20 cm) tended to produce lower yields per regrowth days (Fig. 6). For the 10 cm cutting height, the lowest plant height at harvest (20-10) resulted in lower yields per regrowth days than 30-10 and 40-10 in each planting month. For the 5 cm cutting height, there were no considerable differences in the April planting; however, the yield per regrowth days for 20-5 was less than that of 30-5 for the September planting. For 30 cm plant height at harvest, the highest yield per regrowth days was produced by 30-10, while a lower yield was produced in 30-5 and 30-15 for the April planting. In the September planting, 30-5 and 30-10 produced higher yields per regrowth days than 30-15 (Fig. 6). The effects of cutting height on yield per regrowth days was different between April and September for 30-5. In the case of 20 cm plant height at harvest, there was no large difference between 20-5 and 20-10 as observed in the case for a 30 cm plant height.
Morphological characteristics of plants at first harvestTable 2 presents the plant morphological characteristics at first harvest. In all trials, higher plant height at harvest resulted in an increased number of leaves and main stem length. The internode lengths of plants for the April planting were longer than those in other plantings. At the first harvest, an apical bud of the main stem is cut, allowing the growth of remaining main stem lateral shoots (Fig. 3). The number of first lateral branches on the main stem was measured after the first harvest (Fig. 7). For treatments with the same cutting height, there were no considerable differences among plant heights. At the same plant height harvest, the higher cutting heights tended to leave a large number of first lateral branches on the main stem in all four trials. The number of first lateral branches during the spring trials tended to be less than that during the fall trials. The number of first lateral branches for 30-10 was 6.3, 8.3, 10.8, and 10.0 per plant for the April, May, September, and October plantings, respectively.
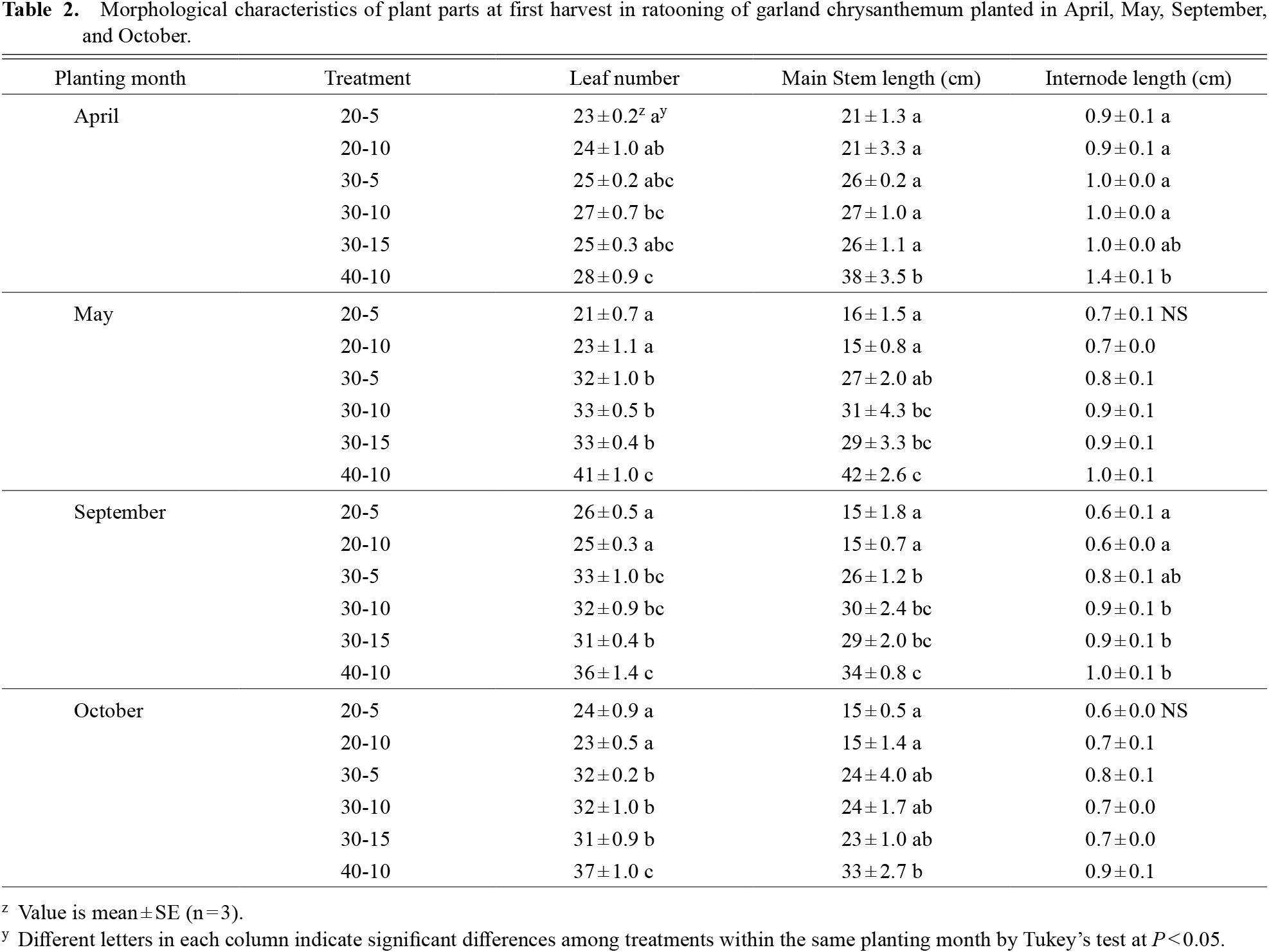
Morphological characteristics of plant parts at first harvest in ratooning of garland chrysanthemum planted in April, May, September, and October.
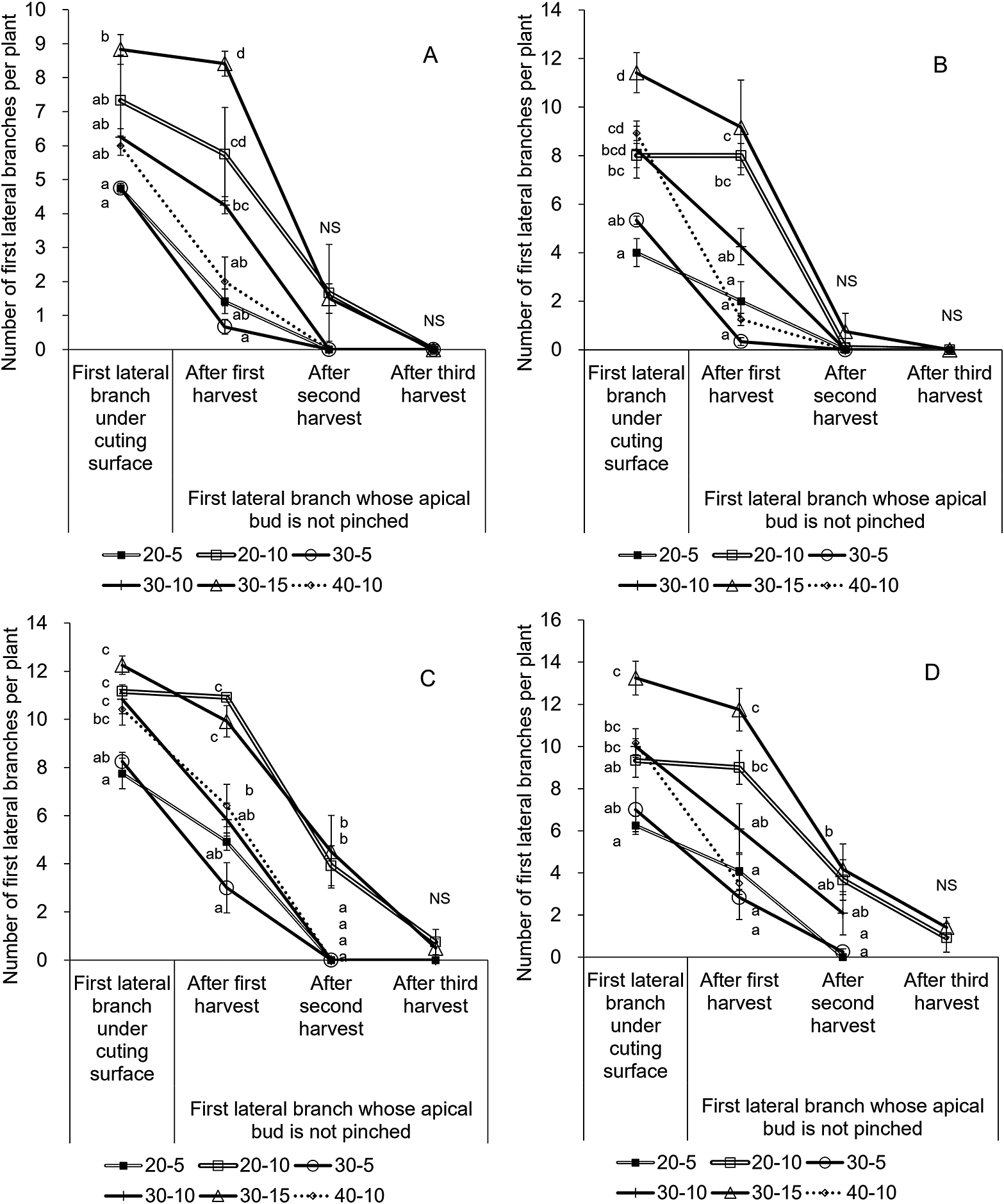
Number of first lateral branches and changes in the number of first lateral branches whose apical buds are not pinched while ratooning, planted in April (A), May (B), September (C), and October (D). Bars are SE (n = 3). Different letters in each harvest indicate significant differences among treatments within the same planting month by Tukey’s test at P < 0.05.
Apical buds of first lateral branches situated higher than the cutting surface are pinched at harvesting, allowing the growth of second lateral branches (Fig. 3). In contrast, apical buds of first lateral branches situated lower than the cutting surface are not pinched at harvesting and are allowed to grow (Fig. 3). The number of first lateral branches whose apical buds were not pinched was counted after harvest to evaluate the regrowth process (Fig. 7).
For a 10 cm cutting height, lower plant heights at harvest tended to retain more first lateral branches whose apical buds were not pinched in all harvests, except in the first harvest of 30-10 and 40-10 in the September plantings (Fig. 7). Not all branches were pinched at second harvest for the 20-10, but all lateral branches were pinched at the second harvest for 30-10 and 40-10, for the April, May, and September plantings. The 5 cm cutting height showed no significant differences. For the 30 cm plant height at harvest, the higher cutting height tended to retain more lateral branches that were not pinched for all harvests (Fig. 7). Not all branches were pinched in the second harvest for 30-15, but all lateral branches were pinched by the second harvest for 30-5 and 30-10 except in the October planting. Similar results were observed for the 20 cm plant height at harvest. The higher cutting height considerably tended to remain more lateral branches not pinched. For 20-10 and 30-15 at the second harvest, several first lateral branches did not reach the cutting surface, indicating that branches are not fully grown, which may result in premature harvesting of lateral branches with shorter lengths (Fig. 7). These results demonstrate why excessively higher cutting height relative to plant height (20-10 and 30-15) resulted in lower total yields (Fig. 5).
Effects of plant height and cutting height on node numbersThe node number (the sum of the number of first lateral branches whose apical buds were not pinched and number of nodes on the lateral branches whose apical buds were pinched) indicates the number of growing points that are potentially harvestable (Fig. 3).
For a 10 cm cutting height, the lowest plant height at harvest (20-10) tended to display the smallest node number among the three plant heights, except in the April planting. In the case of a 5 cm cutting height, there was no significant difference between 20-5 and 30-5. The lowest cutting height (5 cm) tended to yield a smaller number of nodes than other cutting heights for the 30 cm plant height at harvest except in October (Fig. 8). Also, in the 20 cm plant height at harvest, the lower cutting height (5 cm) tended to display fewer nodes in May. The node number in the spring trials was less than that in the fall trials (Fig. 8). The node number of 30-10 increased from 10 to 32 over harvests of the April planting and from 23 to 92 for the September planting. The node number of 30-5 in April remained much smaller than that for September.
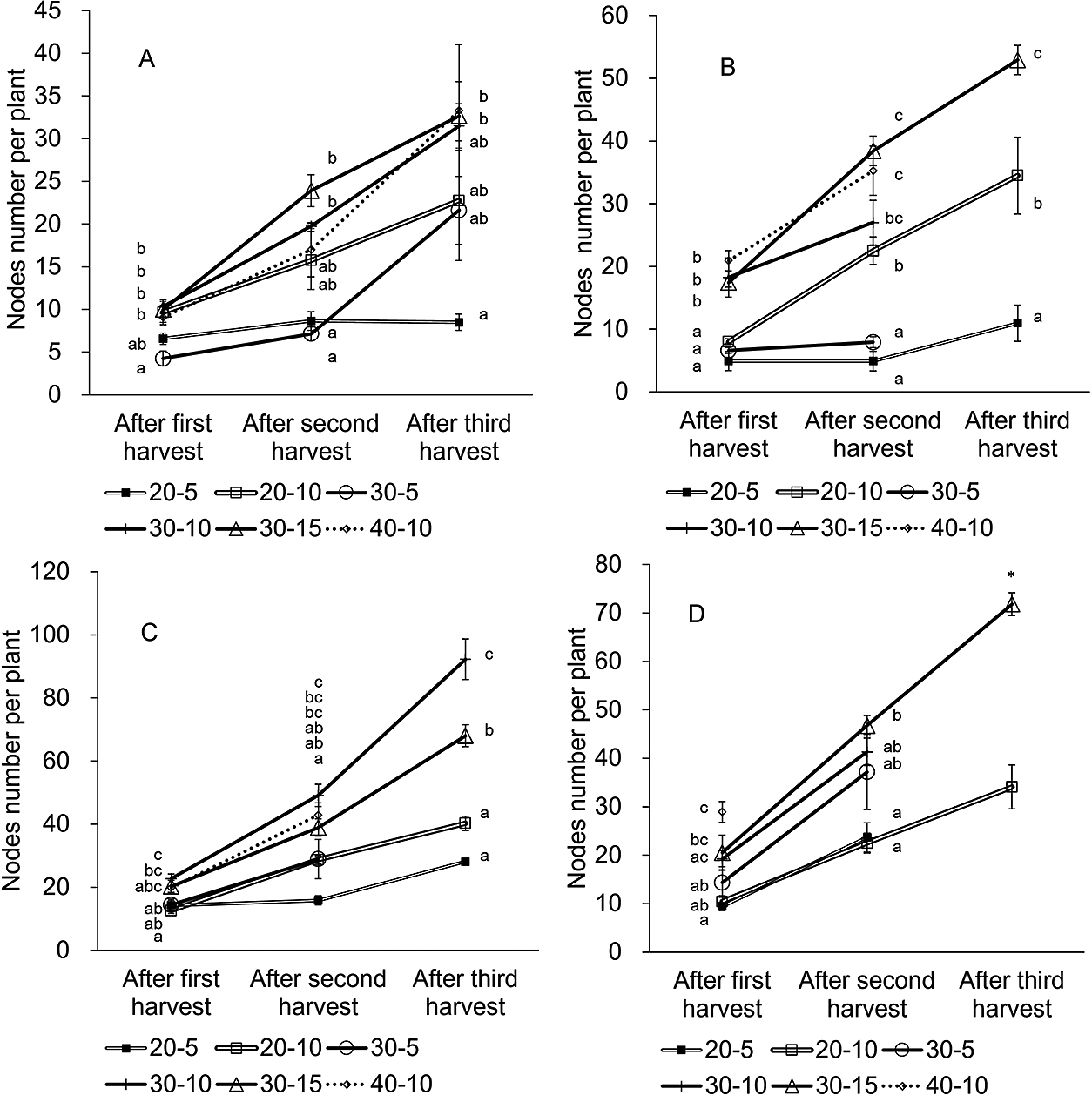
Changes in the number of nodes in plants planted in April (A), May (B), September (C), and October (D). The number on the ‘y-axis’ refers to the total number of first lateral branches whose apical buds were not pinched and number of nodes on the lateral branches whose apical buds were pinched. Bars are SE (n = 3). Plants that did not regrow were excluded from observation. Different letters in each harvest indicate significant differences among treatments within the same planting month by Tukey’s test at P < 0.05. Asterisk (*) indicates significant differences among treatments within the same planting month by t-test at P < 0.05.
At the same cutting height, the plant width of 40-10 was significantly wider than that of 20-10 after first harvest in October (P < 0.05), but there was no clear tendency for other harvests. At the same plant harvest height, the lowest cutting height (5 cm) tended to yield narrower plant widths than other cutting heights except in October (Fig. 9). The plants in the fall trials were wider than those in the spring trials.
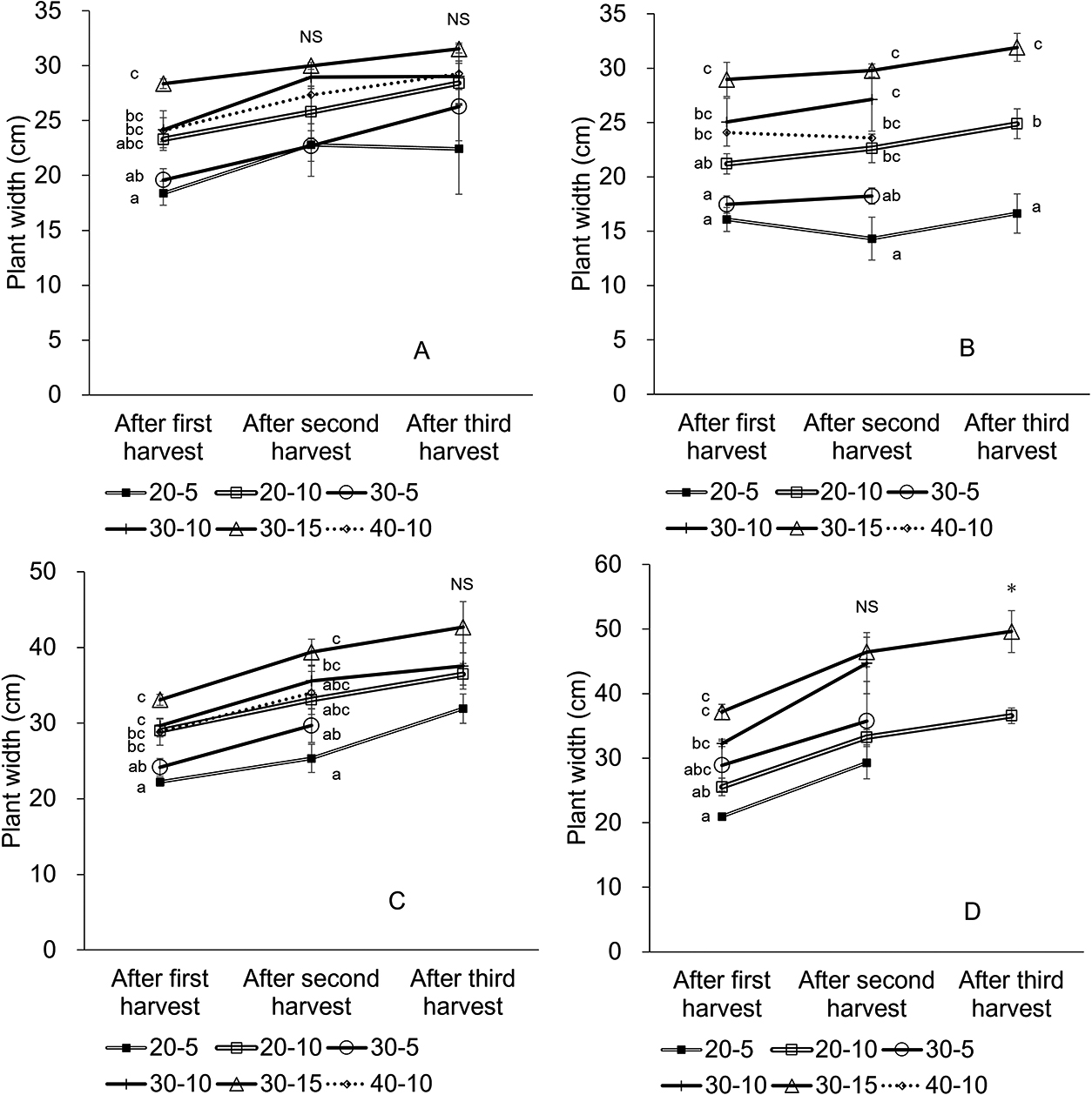
Changes in widths of plants planted in April (A), May (B), September (C), and October (D). Bars are SE (n = 3). Plants that did not regrow were excluded from observation. Different letters in each harvest indicate significant differences among treatments within the same planting month by Tukey’s test at P < 0.05. Asterisk (*) indicate significant differences among treatments within the same planting month by t-test at P < 0.05.
We studied the ratooning methods of garland chrysanthemums with a view to develop and implement mechanical harvesting methods. In this study, crops were repeatedly harvested by horizontal cutting which differs from the conventional method of selective hand harvesting. The effects of plant height at harvest and cutting height on yields and regrowth were investigated.
Plant height at harvest, cutting height, and planting month affected the total yield of garland chrysanthemum (Fig. 5). While the April and September plantings witnessed the highest total yields for the spring and fall plantings, respectively, there were many canceled harvests for the May and October plantings (Table 1; Fig. 5). Therefore, April and September are considered optimal planting months.
Among the treatments in the April and September plantings, 30-10 and 40-10 produced higher total yields and yields per regrowth days (Fig. 5). The lowest plant height at harvest (20 cm) generally resulted in lower total yield as well as yield per regrowth days (Figs. 5 and 6). The decrease in total yield for the lowest plant height may be due to the narrower width of harvest area (Fig. 1) and smaller node numbers (Fig. 8). The excessively high cutting height relative to plant height (e.g., 20-10 and 30-15) tended to result in lower total yields (Fig. 5). The cases of 20-10 and 30-15 may result in harvesting lateral branches prematurely. The lower cutting height treatments took more days to regrow (Fig. 6), and these treatments decreased the number of first lateral branches and nodes (Figs. 7 and 8). In addition, the lower cutting height narrowed the plant widths; in other words, it reduced the volume of leaves and stems left on the plants after harvest (Fig. 9). In the case of 30-5, the node numbers in the April planting were much smaller than those in the September planting (Fig. 8). These results indicate that the low cutting height treatments during the spring trials caused non-regeneration in ratooning plants. The results also indicate the reason behind the higher yield per regrowth days obtained for fall trials than for spring trials (Table 1; Fig. 6). The higher plant height at harvest resulted in longer regrowth time. The 40-10 treatment produced high yields, but regrowth took a long time (Table 1; Figs. 5 and 6). Therefore, this treatment may be unsuitable for ratooning. We determined that harvesting at a 30 cm plant height with a 10 cm cutting height was suitable for ratooning, and resulted in high yields. This combination resulted in a sufficient number of first lateral branches and nodes on plants, enabling the harvest of fully grown leaves.
Yamamoto et al. (1993) evaluated the number of first lateral branches that should be left at the first harvest for hand picking. In the August planting of cultivars ‘Kiwamechuba’ and ‘Fusanoharu’, they suggested that the optimal first branch number was 5–6 for ‘Kiwamechuba’ and four for ‘Fusanoharu’, and leaving three branches caused low productivity owing to the small number of leaves. In our trials, more than four first lateral branches were left in all treatments (Fig. 7). However, horizontal cutting removed part of the stems and leaves of the first lateral branches in lower position of the main stem. Therefore, insufficient nodes, stems, and leaves were left behind. This indicates that more nodes need to be left behind for horizontal cutting than with hand-picking.
Motizuki and Hiraoka (1976) studied internode elongation of cultivars and strains of garland chrysanthemums, reporting that medium leaf type cultivars have high internode elongation in May and low internode elongation in September. The authors also reported that high planting density promoted internode elongation. Our results also indicated a seasonal change in internode elongation; node length in spring trials was longer than that in fall trials. Plant internode length for the April planting was the longest among the four trials. The seasonal response of internode elongation may explain why planting month affected the total yield of garland chrysanthemum.
In ratooning, the sowing and planting times are very important. Garland chrysanthemum growth was inhibited due to disease emergence in high temperatures, and delayed growth in low temperatures (Fig. 4; Table 1). Our results suggest that April and September are suitable planting months (Figs. 4 and 5; Table 1). Iwasaki (2009) studied ratooning of komatsuna (Brassica rapa var. perviridis) and reported that sowing from late February to early April and from late August to early September enabled for three of more harvests; the plants escaped disease under high temperature and also escaped low temperature effects on flower-bud differentiation and flower stalk development.
The cutting height affected the regrowth days. Therefore, it may control the harvest schedule if sufficient node numbers remain. In Welsh onion ratooning, Sato (2019) reported that mowing before harvest could control harvest schedule.
Our study provides a new framework for garland chrysanthemum ratooning. Based on the results, we propose a plant height of 30 cm with a cutting height of 10 cm as the optimum parameters for high yields. This cutting height would leave enough nodes and first lateral branches that were not pinched at the first harvest and would enable the harvest of mature stems at the second harvest. This ratooning method is novel in the use of horizontal harvesting, which is different from conventional selective hand-picking ratooning. Through these experiments, we open the possibility of machine harvest ratooning as reported for spinach (Suzuki et al., 2018).
Although many garland chrysanthemum cultivars are released by seed companies, our results were obtained using a single cultivar and therefore need validation for other cultivars. The optimum harvest plant height and cutting height may leave enough nodes and first lateral branches for photosynthesis. However, we did not evaluate the light-intercepting characteristics of ratooning. In addition, light intensity affects plant morphology. Further studies are needed to evaluate the relationship between light and ratooning. We attempted three harvests in our trials, but there is a possibility of increasing the number of harvests to four with April and September planting, considering regrowth dates and seasonal temperature changes. Future studies must also focus on increasing harvest number as well as evaluate the regrowth processes including rate of regeneration from nodes and plant branching patterns.