2023 Volume 92 Issue 3 Pages 281-289
2023 Volume 92 Issue 3 Pages 281-289
In Japan, broccoli (Brassica oleracea L. var. Italica) is primarily harvested manually. Some Japanese broccoli cultivars have short stems, but these broccoli heads are more difficult to harvest, and complicate the introduction of mechanical harvesters. Therefore, research is focused on strategies to control stem length. The present study examined the effects of the plant hormone gibberellin (GA) on the morphological and agronomic traits of broccoli shoots. The broccoli cultivar ‘SK9-099’, widely distributed in Japan and characterized by a short stem, was studied. These experiments were conducted over three cropping seasons, i.e., spring (Exp. 1), summer (Exp. 2), and fall (Exp. 3), with four GA treatment plots at 0 ppm (Control), 20 ppm, 100 ppm, and 500 ppm. This study observed that, on average, GA elongated stem length from approximately 16.0 cm in the control plot to 24.7 cm in the 500 ppm plot for the three cropping seasons, without decreasing yield. In addition, GA treatment accelerated apical bud growth and shortened the overall growth period by 16 days in the 500 ppm plot compared to the control in the fall, equivalent to 20.5% of the growth period from transplantation to harvest in the latter.
This study highlights several practical benefits of GA application for growers, minimizing manual harvesting labor, avoid the difficult of designing mechanical harvesters for short-stemmed cultivars, and increase field usage efficiency by shortening the growth period.
In recent years, the types of broccoli cultivars distributed in Japan have exhibited increased flower buds (head) and a shortening of the stems (Takahashi et al., 2021; Takahashi and Sasaki, 2019). As harvesting is primarily by hand, shorter stems results in broccoli heads growing closer to the ground, which then requires workers to bend further down, increasing the physical demand of harvesting. While mechanical harvesters for broccoli are developed, their application on short-stemmed cultivars still faces challenges; for example, one harvester developed in Japan requires at least 23 cm of stem length (Yanmar Agribusiness Co., Ltd., 2021). Therefore, developing technologies controlling this trait in broccoli will reduce manual harvesting labor and facilitate harvesting machine introduction.
The plant hormone gibberellin (GA) significantly influences plant development (Hedden and Sponsel, 2015), specifically on stem elongation (Stowe and Yamaki, 1957), transition to the reproductive phase (Lang, 1957), root elongation (Tanimoto, 2012), and leaf shape (Gray, 1957).
In Arabidopsis, stem elongation arises through endogenous GA level changes (Thingnaes et al., 2003), which are determined by variations in temperature and day length. This results in epidermal and medullary cell expansion, with a limited increase in the number of cells in the stem (Thingnaes et al., 2003). Recent studies, in rice, exploring the relationship between stem elongation and GA, revealed that hormone-induced internode elongation initiation differs between floating rice and common rice (Nagai et al., 2020). This demonstrates that GA alone is insufficient to initiate elongation, with several other factors (e.g., ACE1 and DEC1) necessary to enhance the response to the hormone and modulate this process.
In agriculture, the mass-produced gibberellin GA3 is commonly used. In Japan, it is employed to accelerate germination, control plant height, and produce seedless fruits. For grape cultivation, GA3 is used to produce seedless fruit and, if applied twice, reduces yield loss. The GA3 biosynthesis inhibitor, paclobutrazol [(2RS,3RS)-1-(4-chlorophenyl) methyl-4,4-dimethyl-2-(1h-1,2,4-trizol-1-yl) penten-3-ol], is used to suppress lodging in rice (Asami and Kakimoto, 2016; Yim et al., 1997). In strawberries, this hormone improves fruit coloration and facilitates harvesting by elongating the fruit stalks (Asami and Kakimoto, 2016). Among vegetables, celery is treated with GA3 to promote growth (Ghosh and Halder, 2018) with 5 to 7 mL of GA3 solution per plant at a concentration of 50 to 70 ppm applied around 20 days before harvest.
In broccoli, GA controls reproductive transitions but has a limited effect on stem elongation (Duclos and Björkman, 2015). However, it may be possible that longer stems are induced by increasing either GA treatment spraying frequency or concentration. Controlling stem length will reduce harvesting labor requirements. In addition, a more rapid reproductive transition may shorten the growth period, thus contributing to field use efficiency and avoiding risks of disease and environmental disturbance.
The present study investigated the effects of GA treatment on the morphological and agronomic traits of broccoli, focusing on stem elongation and growth period.
Three cultivation experiments, i.e., Experiment 1 (Exp. 1) planted in April as spring crop, Experiment 2 (Exp. 2) planted in May as summer crop, and Experiment 3 (Exp. 3) planted in August as fall crop, were conducted in 2021 in a field at the National Agriculture and Food Research Organization (NARO) (36.02 °N, 140.10 °E), Tsukuba City, Ibaraki Prefecture, Japan. The dates of sowing and transplanting were March 26 and April 13 for Exp. 1, April 13 and May 14 for Exp. 2, and August 2 and 31 for Exp. 3, respectively. Seeds were sown in cell trays (25 mL × 128 cells) filled with compost (NAPLA type S; YANMAR Co., Ltd., Osaka, Japan). Two weeks after sowing, the seedlings in each cell tray were fertilized with N:P2O5:K2O = 300:300:300 mg in the form of liquid fertilizer (OAT Agrio Co., Ltd., Tokyo, Japan). The centers of the plant beds were 150 cm apart, and each bed top was approximately 100 cm wide. Plants were transplanted into double rows placed 60 cm apart and spaced 40 cm from each other, resulting in a density of 3.3 plants·m−2. The field was then fertilized with N:P2O5:K2O = 30:30:30 g·m−2, half of which was slow-release fertilizer. The early-maturing cultivar ‘SK9-099’ (Sakata Seed Co., Ltd., Yokohama, Japan) was used in all three experiments, with each experiment and GA treatment independently replicated three to five times for each plot of different GA concentration. Environmental conditions such as temperature, accumulated daylight hours, and precipitation for each crop season are presented in Figure S1 and listed in Table S1.
GA treatmentAfter transplanting, 4 mL of GA3 solution (Sumitomo Chemical Co., Ltd., Tokyo, Japan, expressed as GA hereafter) was applied once or twice a week at concentrations of 0 ppm (Cont.), 20 ppm, 100 ppm, and 500 ppm on each plant. The hormone was sprayed at the center of the plant (including the apical bud and the main stem). The treatment continued until flower buds appeared; overall, GA was applied 10 times in Exp. 1, 11 times in Exp. 2, and 14 times in Exp. 3.
Examination of the stem and nodesAfter transplanting, plant heights (from the ground edge to the apical bud) were measured once or twice a week until flower bud initiation in Exps. 1 and 2.
After harvesting, the distance from the ground edge to the position just below the head was measured and recorded as stem length (Fig. 1). In Exps. 1 and 2, all plants were sampled at 63 days after transplanting (DAT), while those in Exp. 3 were individually sampled when their heads reached 15 cm in diameter.
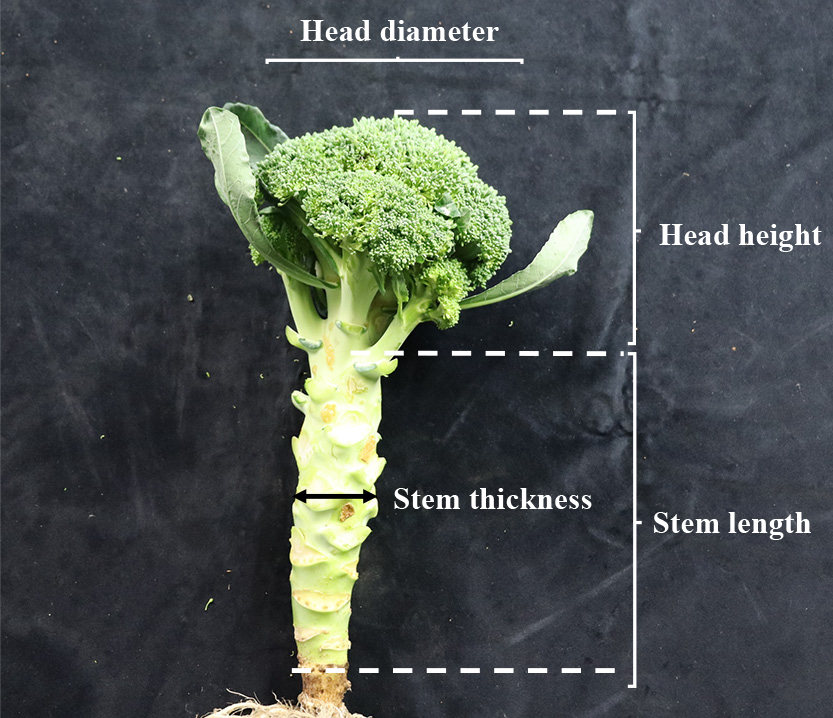
Anatomy of a broccoli plant in this study. Arrow distances indicate head diameter, head height, stem length, and stem diameter. The distance from the ground edge to just below the head was measured as stem length. The thickness at the center of the total stem length was used as stem diameter.
The number of nodes was counted in all three experiments. Each internode length was measured and compared in the control and 500 ppm GA treatment plots in Exp. 2.
Examination of the flower buds at harvestThe fresh weight (FW), diameter, and height of heads sampled in Exps. 1 and 3 were measured. Then, the heads were cut into floret segments of about 5 cm in length and weighed.
Examination of biomass allocation and variation of plant shoot morphology at harvestEach whole shoot in Exp. 3 was divided into four parts — namely, the apical head, leaves on the main shoot, side shoots (lateral branches), and the rest of the main stem—then the FW of each part was measured. The leaf area (LA), including leaf stalk, was also measured using ImageJ Fiji (Schindelin et al., 2012) to obtain the leaf weight per LA (g·cm−2). Stem length and thickness, defined as the diameter at the half height of the stem (Fig. 1, stem thickness), were also measured. The correlation between these two parameters was calculated for four plants in each GA treatment plot.
Observation of the apical bud and analysis of the growth periodPlants in each GA treatment were sampled at 30 DAT and the apical buds in Exp. 3 were observed under an optical microscope (Leica MZ6; Leica Camera AG, Wetzlar, Germany). The number of true leaves for five plants in each treatment was counted at 30 DAT in Exps. 1 and 3, and the number of days (after transplantation) required to reach a head size of 15 cm in diameter was defined as the growth period.
Statistical analysisStatistical analysis was performed in R (R Core Team, http://www.R-project.org., 2019).
No significant differences were observed in plant height among the four GA-treated plots until 17 DAT. By 23 DAT, significant differences were observed in the plots exposed to concentrations of 100 and 500 ppm compared to the control in Exp. 1 (Fig. 2). After this time point, the plant height in the 500 ppm plot was significantly higher than the control until 49 DAT, when flower buds appeared. Exp. 1 and Exp. 2 yielded the same trend, although the transplanting time of the latter was one month after that of the former (Fig. S2). In Exp. 2, significant differences began to appear between the control and GA treatments at 500 ppm at 18 DAT, and 100 ppm at 39 DAT.

Changes in height after planting caused by different GA concentrations in Exp. 1. Samples in the control and GA treatments at 20 ppm, 100 ppm, and 500 ppm are represented by circles, squares, triangles, and crosses, respectively. Vertical bars indicate the SD (n = 4) and different letters listed above the bars represent significant differences based on Tukey’s test (P < 0.05).
Concentration-dependent stem growth was observed in all three experiments with stem length in the 500 ppm plot increased significantly over that of the control (Figs. 3 and 4). For Exp. 2, significant stem elongation differences were observed, even in the 20 and 100 ppm plots, compared to the control. On average, stem length increased from approximately 16.0 cm in the control to 24.7 cm in the 500 ppm plot over the three cropping seasons, corresponding to an overall stem length increase of 54.4%. In Exp. 1 and Exp. 2, each plant was harvested and measured at about 63 DAT to standardize growth period. However, for Exp. 3, the growth stage was standardized using head size, with harvesting performed upon reaching 15 cm in diameter, but this had little influence on the results.

Stem length at harvest in Exp. 1 (a), Exp. 2 (b), and Exp. 3 (c). Vertical bars indicate the SD (Exp. 1 n = 4, Exp. 2 n = 3, Exp. 3 n = 5). The different letters listed above the bars represent significant differences based on Tukey’s test (P < 0.05).

Whole broccoli plants at harvest in Exp. 3. As photographs were taken, the leaves at the front were removed to facilitate recognition of the stem and head shape.
The number of nodes in Exp. 2 (almost 25) was higher than that in Exps. 1 and 3 (approximately 21) (Table 1). Although, in Exp. 3, this parameter significantly increased for the 500 ppm plots, no clear or common effect of GA on internode number was observed over the three experiments. All internode lengths for the 500 ppm plots were longer than those of the control after the third node (Fig. 5). The difference in internode length was the greatest at node # 12 (P < 0.001), but the elongation effect did not seem biased to any specific node.

Effects of GA treatment on the number of nodes at harvest.

Internode length in plots exposed to GA concentrations of 0 ppm (Cont.) and 500 ppm in Exp. 2. Values are shown as means ± SD (n = 4). Symbols are same as Fig. 2. Asterisks indicate significant difference between Cont. and 500 ppm GA treatment at each node number by t-test (***: P < 0.001, **: P < 0.01, *: P < 0.05).
No significant differences were detected in head weight, floret weight, and head diameter among GA treatments (Fig. 6a–c, e–g). However, GA concentration-dependent increases in head height were observed for Exp. 1 (Fig. 6d) and Exp. 3 (Fig. 6h). Flower bud images from each GA treatment plot are shown in Fig. 6i. No significant differences in yield were observed among GA treatments; however, in terms of quality, it was observed that treatment resulted in roughness of the flower bud surface (Fig. 6i).

Agronomic traits of flower buds at different GA concentrations. (a)–(d) Exp. 1; (e)–(h) Exp. 3; (i) Shape of flower buds in Exp. 3. Vertical bars indicate the SD (Exp. 1 n = 4, Exp. 3 n = 5). The different letters listed above the bars represent significant differences based on Tukey’s test (P < 0.05).
No significant variations were observed in the total FW of above-ground plant shoots between GA-treated plots and the control (Fig. 7). Variations in the FW of plant shoots was primarily caused by increases or decreases in leaf weight, while the head and stem weights changed only slightly (Fig. 7).

Fresh weight of broccoli parts at harvest for each GA concentration in Exp. 3. Vertical bars indicate the SD (n = 5). The different letters listed above the bars represent significant differences based on Tukey’s test (P < 0.05).
LA and leaf weight showed a similar trend (Fig. 8a, b). The slight increase with the 20 ppm plot was not significant. Then a marked decreasing trend was observed for the 100 ppm and 500 ppm plots. Leaf weight decreased more sharply than LA, which resulted in a GA concentration-dependent decrease in leaf weight per LA (Fig. 8c).

Variations in leaf morphology at harvest in Exp. 3. Vertical bars indicate the SD (n = 5). The different letters listed above the bars represent significant differences (P < 0.05).
Stem weight did not vary among GA treatments, but the stem itself became thinner and longer under increasing GA concentrations (Fig. 9). The correlation between stem length and thickness was relatively high (R2 = 0.62).

Correlation between stem length and thickness at harvest. Symbols are same as Fig. 2. The dashed line shows an approximate curve.
Apical buds were larger in the 500 ppm plot than in the control at 30 DAT, which corresponds approximately to the flower development stage (Fig. 10).

Microscopic images of apical buds at 30 DAT in Exp. 3.
Furthermore, the number of true leaves tended to increase with GA concentration at this stage (Table 2). In Exp. 3, the average number of true leaves for the control plot was 13.3 whereas for the 500 ppm plot it was 15.5. Therefore, approximately two additional leaves per plant were produced in the 500 ppm GA-treated plot.

Number of leaves at 30 DAT in flower development stage.
The growth period from transplantation to harvest was clearly shortened with increasing GA concentration in Exp. 3 (Table 3). The growth period was shortened by 16 days in 500 ppm plot compared to the control plot, corresponding to a 20.5% decrease in the overall growth period.

Growth period from transplantation to harvest in Exp. 3.
The GA treatment increased plant height in a concentration-dependent manner (Fig. 2). Although no significant differences in plant height were observed within the first week after treatment, stem elongation became visible after approximately 20 days. This study clearly confirmed the effect of GA on stem elongation, despite Duclos and Björkman (2015) previously suggesting that neither GA3 nor GA4+7 significantly affect this trait. This discrepancy may arise from the GA concentration used in the present research. In the above-mentioned study, broccoli plants were treated three times with 2 μL of GA solution at a concentration of 2 μg·μL−1. This is equivalent to about 0.012 mg of GA and is sufficient to induce GA responses in ‘Alaska’ peas (Pisum sativum L.) (Moore, 1967; Mandel et al., 1992). In contrast, broccoli in the present study were sprayed with 4 mL of GA solution at concentrations of 20 ppm (= 0.04 μg·μL−1), 100 ppm (= 0.2 μg·μL−1), and 500 ppm (= 1 μg·μL−1) at least 10 times. This resulted in more than 1.6 mg, 8 mg, and 40 mg of GA treatment in total, quantities 100 to 3,000 times higher than that used by Duclos and Björkman (2015). It is possible that exogenous GA treatment effects are observed in broccoli only at high concentrations and doses. This is likely due to the larger plant body than that of Pisum sativum L. and the hard epidermal tissue of broccoli, which impedes GA penetration to internal tissues. Alternatively, broccoli stem tissue may have a higher threshold for GA response than that of Pisum sativum L.. Supporting this is the observation of the limited response at a concentration of 20 ppm, while yielding an obvious response at 500 ppm. Thus, the GA concentration selections in the present study are sufficient to demonstrate the effect of GA on stem elongation in broccoli. This effect was most obvious in Exp. 2, where even the plots treated at 20 ppm and 100 ppm showed significant differences from the control (Fig. S2). Previous studies of Arabidopsis stem elongation observed increased rates in higher daytime temperatures, ranging from 12°C to 27°C, and a relationship between the amount of inactive endogenous GA29 and temperature (Thingnaes et al., 2003). Similarly, temperature-induced changes in endogenous GA concentrations are reported in peas (Grindal et al., 1998), carrots (Hiller et al., 1979), and lettuce (Fukuda et al., 2009). Thus, broccoli endogenous GA levels or sensitivity may also change with temperature, producing the greater stem elongation effect observed in Exp. 2 (summer crop), in which the average temperature was higher than for both Exp. 1 (spring crop) and Exp. 3 (fall crop) (Fig. S1).
Total FW slightly increased in plants subjected to a GA concentration of 20 ppm, while it slightly decreased in those exposed to 100 ppm and 500 ppm compared to the control (Fig. 7). This variation is primarily due to leaf weight fluctuations. However, as node numbers changed only slightly, there may be a defoliation effect contribution (Table 1; Fig. 8). In terms of the GA effects on leaves, previous studies have shown that exogenous GA reduces leaf shape complexity and the number of unfolding leaves in tomato (Fleishon et al., 2011). In cauliflower, GA causes early defoliation and a reduction in the number of remaining leaves (Booij, 1990), which is consistent with our results. However, in the present study, leaf weight per LA was significantly reduced by the high GA concentration (Fig. 8). Therefore, it is inferred that leaf thickness was decreased by the hormone treatment. This variation in leaf thickness may be explained by decreased cell numbers or cell-shape modification, but confirmation requires further investigation.
As for the changes in stem shape, a negative correlation was identified between stem length and thickness (Fig. 9). Thingnaes et al. (2003) reported that, in Arabidopsis plants grown at 12°C during the day and 22°C at night, endogenous GA29 levels significantly increased, and stem width diminished compared to those in plants grown at 22°C during the day. Cell cross-section analysis detected significant changes in cell longitudinal length and width, but not in number. This suggests that the GA-induced stem elongation observed in the present study may be also due to cell-size changes rather than cell number. GA application to broccoli cultivations may require measures to avoid the lodging risk, such as increasing the ridging frequency in view of the longer and thinner stems expected.
As for the number of nodes, Duclos and Björkman (2015) reported that GA treatment in broccoli had no significant influence on this trait, confirmed in the present study with no common trend on this trait detected over the three cropping seasons (Table 1). This suggests that environmental conditions such as temperature and day length, or cultivar characteristics, may have a greater influence on node number than exogeneous GA.
The internode lengths measured in Exp. 2 demonstrated that the effect of GA on stem elongation was not biased toward any specific node after the third one, which was beginning to grow at the start of the treatment (Fig. 5). Although the first and second nodes were also exposed to the treatment, no clear effect was observed. In general, GA strongly affects immature cells during the early growth stages (Hedden and Sponsel, 2015). Therefore, it is inferred that the hormone had a limited effect on the first and second nodes, whose cells were almost fully differentiated when GA treatment began, and that the elongation effect appeared after the third node was formed. The reason for the different elongation effect according to the number of the node, for example, the #12 internode length between the control and 500 ppm was significantly different while that of #11 showed almost no difference (Fig. 5), may be due to GA treatment frequency. Treatment was performed once or twice a week. Therefore, uneven intervals of GA treatment may have missed the optimal timing for certain nodes. Another possible factor is the weather condition post GA treatment. Good weather for plant growth may additively promote internode elongation, or unexpected rainfall immediately after GA treatment may limit the GA effect. Therefore, it is unlikely that GA affects differently in response to node number.
Effects of GA treatment on apical buds, reproductive phase transition, and growth periodNo significant differences were observed among GA treatments across seasons for head weight and floret weight (Fig. 6a, b, e, f). As all the heads were harvested at the same time in Exp. 1 (while those in Exp. 3 were individually harvested when they reached 15 cm in diameter), significant differences in head diameter were possible in this experiment; however, this was not observed for any GA-treated plots (Fig. 6c). On the other hand, head height increased with the 500 ppm treatment regardless of harvesting condition (Fig. 6d, h). This increase was caused by the elongation of the peduncle (the stalk part of the floral shoot) (Fig. 6i), which is previously reported (Duclos and Björkman, 2015). These results indicate that GA does not promote broccoli head expansion or increase in floret weight but does influence peduncle elongation. Longer peduncle may assist cutting heads into florets, particularly in processing. A slight roughness of the head surface was noted after GA treatment (Fig. 6i). These changes in head shape and appearance influence head quality, especially for fresh market broccoli appeal. Therefore, to minimize these negative effects, it is necessary to optimize concentration, frequency, and treatment timing, for example, avoiding GA application immediately prior flower initiation or decreasing applied hormone concentration at this developmental stage.
In Exp. 3, the growth period was shortened in a GA concentration-dependent manner (Table 3), a behavior similarly observed in cauliflower (Booij, 1990). In fact, based on the leaf number observed at 30 DAT, vegetative growth was promoted by GA only in the fall crop (Table 2). This suggests that GA treatment may be an effective method to shorten growth periods, although a seasonal dependency of the hormone’s effect appears present. If GA shortened the growth period independently of season, head diameters would differ with treatment strength in Exp. 1, given the simultaneous harvesting (Fig. 6c). Alternatively, this shortened growth period may be obvious only in the fall crop (Exp. 3) as the growth period in this season is longer due to the cooler temperatures than in spring (Exp. 1). In any case, further studies are necessary to establish a practical method to shorten the growth period in all seasons.
Generally, GA activates the transcription of LFY and SOC1 in Arabidopsis, a member of the Brassicaceae family, to induce the reproductive growth phase (Blazquez et al., 1998; Bonhomme et al., 2000; Gocal et al., 2001; Moon et al., 2003). However, there are conflicting reports for the genus Brassica. One maintains a GA association with the transition from vegetative to reproductive growth (Wittwer and Bukovac, 1957), while others do not support this theory (Duclos and Björkman, 2015; Fontes and Ozbun, 1970). In cauliflower, the additive reactivity between GA and vernalization is reported (Booij, 1989, 1990). These studies suggest variations in the mechanism and sensitivity of Brassica spp. responsiveness to GA. In the present study, larger flower buds were observed in the 500 ppm plot than in the control at 30 DAT (Fig. 10). This result demonstrated apical bud developmental stage advancement by GA treatment, and it is inferred that the floral bud differentiation timing was increasingly anticipated under the influence of GA concentrations (500 ppm > 100 ppm > 20 ppm ≃ Cont.) resulting in the apical bud size difference. In addition, an increase in leaf number for the 500 ppm GA-treated plots at 30 DAT (Table 2) was observed. Overall, based on the results of this study, both the vegetative growth and transition to the reproductive phase are accelerated by exogenous GA treatment, and the growth period was shortened in the fall crop.
ConclusionsIn this study, GA was revealed to elongate the stem and accelerate apical bud growth in broccoli without diminishing yield. Also, for the fall crop, GA treatment shortened the growing period without reducing yields. These results assist effective GA use in agriculture. With these optimizations, GA treatment reduces the labor burden, facilitates harvest mechanization, and reduces pest control and fertilizer costs due to shortened growth periods. Practical application of these findings may require measures to avoid the risk of lodging associated with longer and thinner stems. Also, GA treatment field-optimization must consider environmental factors such as temperature and day length to ensure stable and effective treatment effects.
We thank Ms. A. Nakagawa for providing technical assistance in cultivation. We wish to thank Dr. M. Fukuda for the advice on researching vegetable cultivation in open fields.