2024 Volume 93 Issue 1 Pages 58-67
2024 Volume 93 Issue 1 Pages 58-67
Strawberries can be categorized into June-bearing and ever-bearing depending on the environmental conditions that determine flower bud initiation. In Japan, the harvest yield and distribution of strawberry fruits during summer and autumn are low because of high temperatures and heavy fruit load. Therefore, cultivation of ever-bearing strawberries is limited to areas with cool summers such as Hokkaido and Tohoku. In this study, we investigated whether air treatment before liquefied carbon dioxide (CO2) application within the strawberry plant canopy could improve CO2 absorption efficiency and increase dry matter production. Four treatments were investigated: application of air within the plant canopy, application of CO2, application of CO2 after air application, and a control. We investigated the CO2 concentration, dry matter production, yield characteristics, individual leaf photosynthesis characteristics, projected leaf area, cumulative light interception, light use efficiency, and fruit quality. The results showed that the local application of only CO2 or Air and, application of CO2 after air application (Air/CO2) treatment within the plant canopy considerably increased the dry matter production. Total fruit yield of Air/CO2 was the highest among all treatments. This is probably because the application of CO2 and air expanded the leaf area, increased cumulative light interception, and improved light use efficiency. In addition, the photosynthetic rate of Air, CO2 and Air/CO2 treatments was higher than that of the control because of higher stomatal conductance. This suggests that local application of liquefied CO2 after air application can effectively increase fruit yield, and that air treatment will improve plant vigor, further increasing strawberry production in summer and autumn.
Strawberries (Fragaria × ananassa Duch.) prefer cool climates, and more than 90% of strawberries produced in Japan are grown in greenhouses (Yoshida, 2013). Depending on the environmental conditions that determine flower bud initiation, strawberries can be classified as June-bearing and ever-bearing. Specifically, June-bearing strawberries cannot develop flower buds under long-day conditions during summer and autumn, whereas ever-bearing strawberries exhibit flower bud differentiation even under long-day conditions (Okimura et al., 2011). There is demand for strawberry fruits not only during winter and spring, but also during summer and autumn. However, the harvest and distribution volumes in summer and autumn are small (Hamano et al., 2012). Strawberries have soft skin and are prone to vibration and shock; therefore, quality deterioration due to long-distance transportation is a problem (Hikawa-Endo, 2020). Consequently, it is necessary to expand the production area of ever-bearing strawberries.
Currently, the cultivation of ever-bearing strawberries in Japan is limited to areas with cooler summers, such as the Hokkaido and Tohoku regions (Ohta and Yasuba, 2019), and fruit production can be as low as 10,000 tons. Challenges include a decrease in photosynthetic capacity owing to high temperatures (Balasooriya et al., 2018) and fatigue owing to excessive fruit set (Nishiyama et al., 2020). Therefore, it is necessary to suppress the deterioration of photosynthetic capacity and prevent the decline in plant vigor during the cultivation period for stable cultivation in warmer areas such as the Kanto to Kyushu area. Most strawberries are cultivated using protected horticulture in plastic houses (Inaba, 2008), and carbon dioxide (CO2) is used as a photosynthesis-promoting technique in protected horticulture. It is difficult to apply CO2 during summer because it is commonly applied through combustion in plastic houses using a CO2 gas generator. However, precise control was developed using an application controller with a liquefied CO2 gas cylinder, which made it possible to apply CO2 locally within the plant canopy. When this application method was applied to the cultivation of ever-bearing strawberries, we succeeded in maintaining plant vigor during the cultivation period and increasing the yield by 30% (Mochizuki et al., 2022). However, CO2 application promoted the expansion of leaf area, but self-shading of the leaves did not improve light use efficiency. In such a plant canopy with strong vigor, there is a possibility that the applied CO2 will be retained and the application efficiency may be low.
Plant leaves absorb CO2 through the stomata during photosynthesis. Air stagnation is defined as a boundary layer around the leaves. If air circulation is insufficient, the leaf boundary layer resistance increases, restricting gas exchange and reducing CO2 absorption efficiency (Kim, 1996; Kitaya et al., 1998). A circulation fan is introduced to create airflow in plastic houses, but in the canopies of crops with small plant heights, such as strawberries, there is a possibility that air circulation is insufficient. It has been reported that turbulent air circulation with a wind speed of 0.5 m·s−1 or more is effective in increasing material production at production sites, such as greenhouses, where the wind speed is low (Harazono, 1982). Furthermore, it has been reported that the leaves are affected by wind and swaying, which was effective in increasing the total dry weight of cucumbers (Umeda et al., 2016). The porous tube which was used our previous study (Mochizuki et al., 2022) can supply not only CO2, but also air using the same tube. By increasing the amount of CO2 applied, a further increase in income can be expected, but the investment and increase in income cannot be balanced economically. Therefore, we considered air application, which can be implemented with almost no increase in capital investment, to assess whether it is possible to increase dry matter production by performing air treatment before applying CO2 to the strawberry plant canopy. In addition, the effects of air treatment on strawberry plants were investigated from the perspective of destructive measurements and photosynthetic characteristics.
Frigo bareroot plants were grown from May to November 2021, dug up from a greenhouse, and kept in plastic bags at −2°C until transplanting. Fungicide treatment was done before storage. We cultivated the ever-bearing strawberry ‘Suzuakane’ (Hokusan Co., Ltd., Hokkaido, Japan). On 14 April 2022, six frigo bare root plants (crown size was approximately 1.0 cm, Fig. 1) were planted in a polystyrene container (35.5 cm × 75.0 cm × 14.5 cm deep; 24 L) filled with black peat moss (BVB soil substrate; Toyotane, Aichi, Japan). Frigo bare root plants were planted 30 cm apart, with 10 cm between rows. Plant density was 7.0 plants·m−2. There were 84 plants (14 containers) for each treatment, and they were assigned randomly. Guard plants were placed between those with and without CO2 treatment to avoid the effects of CO2 application. Plants were grown on a high bench in a plastic house at the College of Agriculture, Ibaraki University, until 31 October 2022. The size of plastic house was 5.5 m frontage, 27.5 m depth, and 3.5 m height. The bench height was 1.0 m. A circulation fan was placed in a plastic house and side windows were fully open for ventilation. Plants were irrigated via an automatic drip-irrigation system (Aqua-touch; Sunhope-Aqua, Chiba, Japan) with ~30 mL per plant at 06:00 every morning and again after every 2.0 MJ·m−2 of cumulative solar radiation. A solar-radiation sensor was placed in the plastic house. Fertigation was prepared from OAT-A nutrient solution (OAT Agrio, Tokyo, Japan) with electrical conductivity (EC) adjusted to 0.3–0.5 dS·m−2 and pH to 6.5–7.0. The nutrient solution contained 2.2 to 3.7 mM NO3−, 1.0 to 1.7 mM K+, 1.0 to 1.7 mM Ca2+, 0.4 to 0.7 mM Mg2+, 0.6 to 1.0 mM H2PO4−, 0.3 to 0.5 mg·L−1 Fe, 0.2 to 0.3 mg·L−1 Mn, 0.2 to 0.3 mg·L−1 B, 0.01 to 0.02 mg·L−1 Zn, 0.003 to 0.005 mg·L−1 Cu, and 0.003 to 0.005 mg·L−1 Mo. The buds were managed so that there were less than four. The senescent leaves were removed weekly from July to October 2022. Artificial pollination was used to set up fruits. The temperature inside the greenhouse was measured at 10 min intervals (Fig. 2) using a TR-76Ui CO2 Recorder (T&D, Tokyo, Japan). Air was supplied by a compressor and CO2 was supplied from a gas cylinder to the plants through the same porous tubes (WTR-100; Aomidori, Tokyo, Japan) laid within the canopy under the control of a controller (Omnia Concerto, Tokyo, Japan; Fig. 3). Four treatments were used in this study: air only (Air), CO2 only (CO2), CO2 supplied after air supplied (Air/CO2), and a control (C). Treatments were started on 11 July. CO2 and air were supplied daily from sunrise to 6 h before sunset at a pressure of 0.2 MPa at a flow rate of 15 L·min−1. Application of air and CO2 was performed at intervals of 1 minute on and 5 minutes off using a controller to maintain the CO2 concentration at around 1,000 μmol·mol−1 during application, as in our previous study. In the Air/CO2 treatment, air was supplied for 1 minute at first, and then CO2 was supplied immediately for 1 minute. After 4 minutes, the air treatment was repeatedly performed again. This cycle was repeated for Air/CO2. To measure the diurnal changes in CO2 concentration, we installed a TR-76Ui CO2 Recorder within the canopy of each treatment on 1 Sep., which recorded measurements at 10 s intervals. The average CO2 concentration was calculated every 15 min. The average CO2 concentrations every 15 min during CO2 application in the plant canopy of C, Air, CO2, and Air/CO2 were 411, 414, 729, and 736 μmol·mol−1, respectively (Fig. 4).

A frigo bare root plant of ‘Suzuakane’. Bar indicates 5 cm.

Average, maximum, and minimum temperatures in the plastic house.

Schematic diagram of the local air and CO2 application system. It consisted of a porous tube connected to a controller. Treatment was conducted from sunrise to six hours before sunset.
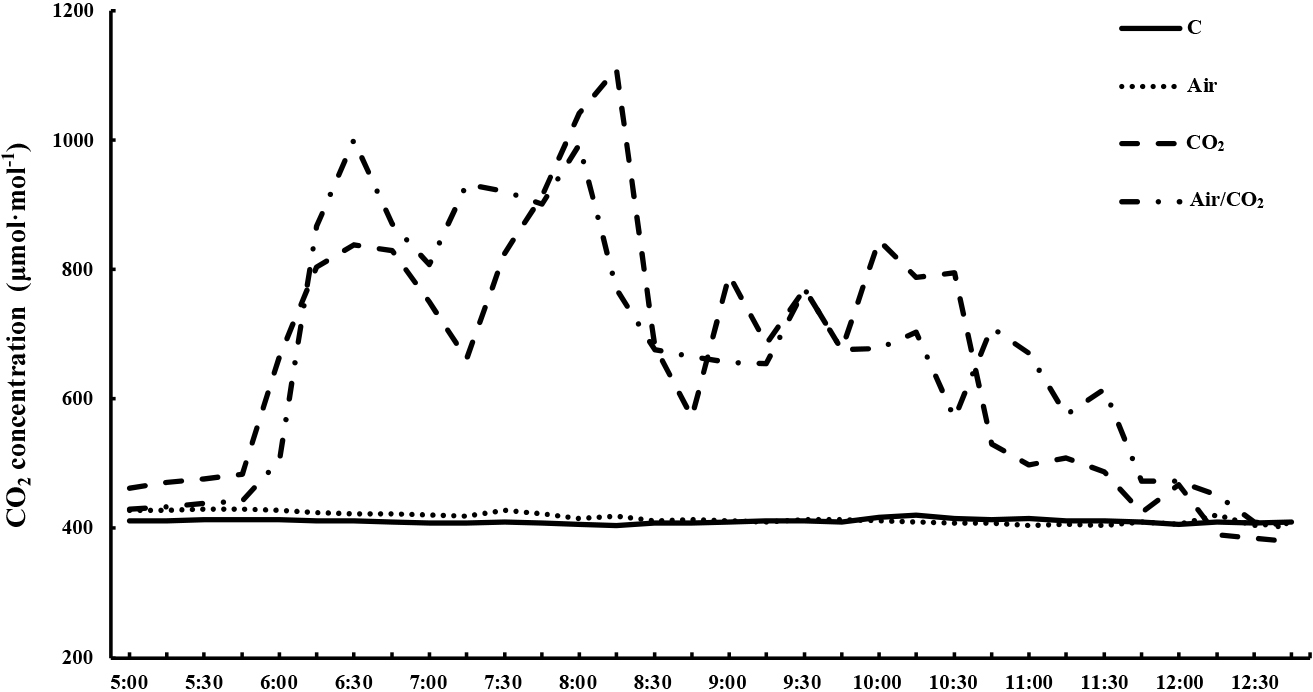
Diurnal changes in CO2 concentration on 1 Sep. The average CO2 concentration was calculated every 15 min. CO2 was supplied from sunrise (5:10) to six hours before sunset (12:09).
Fruits with ≥ 80% of the skin color were harvested, and those > 4 g were counted as marketable fruits. The harvest period was from 1 July to 31 October. The Brix content and acidity of fruit were measured with a pocket sugar acidity meter (PAL-BX|Acid F5; Atago, Tokyo, Japan). The Brix content was measured in the juice of the crushed, fully colored fruit. Acidity was measured in 1:50 diluted juices. The cumulative dry weights (DW) of fruits were calculated as follows:
| Eqn. 1 |
where FW1, FW2, and r are the cumulative fresh weight of fruits harvested by the day of the destructive measurement date, the fresh weight of fruits that had set on the day of destructive measurement, and pre-measured dry matter ratio (0.1), respectively.
Dry matter production and growth analysisDestructive measurements were performed on 11 July, 31 August, and 31 October. Nine to 22 plants were harvested from each treatment and separated into roots, crowns, petioles, leaves, fruits, and peduncles, which were dried at 80°C for 72 h in a circulation drier, cooled, and weighed.
To measure the total leaf area, we used leaves from destructively measured plants. The leaves were photographed, and the leaf area was calculated from the photos using lia32 leaf area analysis software (https://www.flatworld.jp/soft/142711.html). The cultivation period was divided into two periods from July to October. The values of relative growth rate (RGR), net assimilation rate (NAR), and leaf area ratio (LAR) during each period were calculated as:
| Eqn. 2 |
| Eqn. 3 |
| Eqn. 4 |
where TDW1 and TDW2 are the total dry weights (g) at t1 and t2, respectively, and A1 and A2 are the leaf areas (m2) at t1 and t2, respectively.
Projected leaf area (PLA), cumulative light interception, and light use efficiency (LUE)From 23 July to 29 October, the upper part of the plant canopy was photographed 14 times (23 July, 2, 14, 23, 26 August, 5, 10, 17, 24 September, and 2, 8, 15, 22, 29 October) with a smartphone (iPhone 11; Apple, California, USA). After the leaves were extracted from the photographs using Microsoft PowerPoint software, the light-receiving leaf area was calculated using lia32 software. This value was used to calculate the projected leaf area. Daily PLA was estimated using linear interpolation. Daily light reception (MJ·m−2·day−1) was calculated as (Monsi and Saeki, 2005):
| Eqn. 5 |
where DSRtotal, CPAR, LTgreenhouse, PLA, and PD were total daily solar radiation (data of Tsuba, Ibaraki were used from website of Japan Meteorological Agency), photosynthetically active radiation coefficient (0.5), our plastic house light transmittance (0.8), daily PLA and planting density (7.0 plants·m−2), respectively. Cumulative light interception was evaluated by accumulating daily light reception. LUE was the slope of the regression lines between cumulative light interception and total DW.
Photosynthetic characteristicsOn 27 July, 24 August, and 28 September, the photosynthetic rate (Pn), stomatal conductance (gs), leaf intercellular CO2 concentration (Ci), and transpiration rate (Tr) were measured using a portable photosynthesis system (LI-6800XT; LI-COR, Lincoln, NE, USA). Measurements were conducted between 09:00 and 14:00. The third fully expanded leaves of five to seven plants in each treatment were used for the measurements. The photosynthetic characteristics were measured under base conditions of humidity 65–75%, leaf temperature of 25°C, light intensity of 0, 350, 750, and 1,500 μmol·m−2·s−1, CO2 concentration 400 μmol·mol−1, and air flow 500 μmol·s−1.
Statistical analysisAll data were tested using the one-way ANOVA followed by Tukey-Kramer test. LUEs were compared among treatments using 95% confidence intervals (Higashide et al., 2015). All statistical analyses were performed using Excel (Social Survey Research Information, Tokyo, Japan).
The fruit yield of Air/CO2 was higher than that of C treatment in July and September (Table 1). The total fruit yield of Air/CO2 was highest among treatments, followed by CO2, Air, and C, and there was a considerable difference in fruit yield in September. The total number of fruits in CO2 and Air/CO2 treatments was higher than that in C (Table 2). The average weight of the fruit in Air/CO2 was also the highest among the treatments in July, August, as well as overall (Table 3). Brix was 7.0–9.2% and acidity was 0.52–0.70%; however, there was no difference in treatments during the entire growing period (data not shown).

Effect of Air and CO2 application within a strawberry plant canopy on fruit yield.
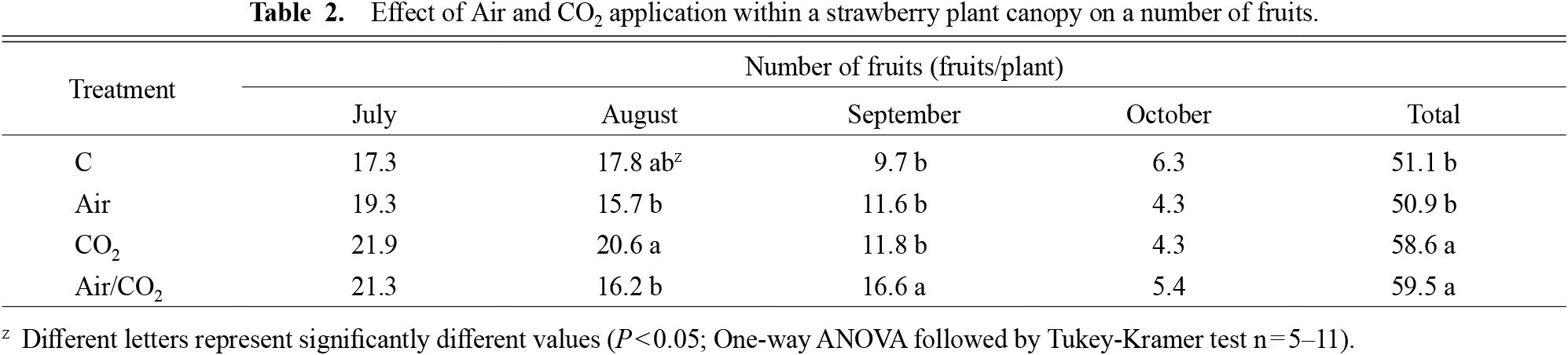
Effect of Air and CO2 application within a strawberry plant canopy on a number of fruits.

Effect of Air and CO2 application within a plant canopy on the average weight of fruit.
On 31 August, the leaf DW of Air/CO2 was higher than that of the other treatments, root DW of Air was higher than that of C, total DW of Air/CO2 was higher than that of C, as well as Air (Table 4). On 31 October, the leaf DW of CO2 was higher than that of C and Air plants. Peduncles DW of CO2 and Air/CO2 were higher than that of C. Fruit DW of Air/CO2 was the highest, followed by CO2, Air, and C. Total DW of CO2 and Air/CO2 was higher than those of Air and C, and the total DW of Air was higher than that of C.

Changes in total dry weight and in dry weights of each plant part.
The growth period was divided into two periods (11 July to 31 August; First period, 31. August to 31 October; Second period. 11 July to 31 October; Entire period) for growth analysis. RGR of CO2 and Air/CO2 were higher than that of C in the first and entire periods (Table 5). NAR of Air, CO2 and Air/CO2 were higher than that of C in the first period; however, NAR of C and Air were higher than those of CO2 and Air/CO2 in the second period. LAR of CO2 and Air/CO2 were higher than those of C and Air in the second period.

Comparison of growth analysis among treatments.
Except for 26 August and 5 September, there was no difference in PLA during the growing period (Fig. 5A). Cumulative light interception of CO2 was the highest (117.3 MJ·m−2) followed by Air/CO2 (113.7 MJ·m−2), C (108.5 MJ·m−2), and Air (106.0 MJ·m−2) (Fig. 5B) on 31 Oct. There was almost the same cumulative light interception among treatments until the beginning of September. LUE is represented by the slopes in Figure 6. The LUEs of C, Air, CO2 and Air/CO2 were 2.87, 3.42, 4.03, and 4.14, respectively. The treatments with CO2 for LUE were 1.2–1.4 times higher than those without CO2 treatments based on 95% confidence intervals.

Changes in projected leaf area (A) and cumulative light interception (B) in the four treatments. z: Different letters represent significantly different values (P < 0.05; Tukey-Kramer test; NS; not significant; n = 4–5).

Comparison of light use efficiency in the four treatments. The slopes of the regression lines, that is, light use efficiency (95% confidence intervals) of C, Air, CO2, and Air/CO2 were 2.87 (2.40–3.34), 3.42 (3.06–3.78), 4.03 (3.79–4.27), and 4.14 (3.87–4.41), respectively. z: Increase in dry weight after starting treatment.
The photosynthetic rate of Air/CO2 in July was the highest among treatments followed by CO2, Air, and C, at 300 μmol·m−2·s−1 and over (Fig. 7A). The photosynthetic rate of Air/CO2 in August was the highest among treatments followed by CO2, Air, and C, at 750 μmol·m−2·s−1 and over (Fig. 7B). The photosynthetic rate of Air in September was higher than that of C at 300 μmol·m−2·s−1 and over (Fig. 7C); however, there was no difference in the respiration rate (0 μmol·m−2·s−1) each month. Stomatal conductance was the same as the photosynthetic rate; however, in July, there were significant differences at 300 and 1,500 μmol·m−2·s−1 and in August, there was only significance at 1,500 μmol·m−2·s−1 (Fig. 8A, B). The stomatal conductance of air in September was higher than that of C at all light intensities (Fig. 8C). There were no substantial differences in leaf intercellular CO2 concentration or transpiration rate among treatments at any light intensity or month (data not shown).

Photosynthetic rate at different light intensities in July (A), August (B), and September (C) in the four treatments. z: Different letters represent significantly different values (P < 0.05; Tukey-Kramer test; NS; not significant; n = 5–7).

Stomatal conductance at the different light intensities in July (A), August (B), and September (C) in 4 treatments. z: Different letters represent significantly different values (P < 0.05; Tukey-Kramer test; NS; not significant; n = 5–7).
The total fruit yield of the Air/CO2 was higher than that of the other treatments because it produced more fruits from July to September (Table 1). The total fruit yield of Air/CO2 and CO2 were 1.44 and 1.3 times higher than that of C. The number of fruits (Table 2) and average fruit weight (Table 3) showed the same tendency as fruit yield. Tagawa et al. (2022) reported that CO2 application increases the fruit weight of June-bearing strawberries. Kumakura and Shishido (1994) reported that the higher the temperature, the shorter the fruit ripening period; however, Air/CO2 treatment could increase fruit weight during the hot season because of the large amount of photoassimilates. Umeda et al. (2016) reported that air treatment of cucumber leaves increased the total DW. Higashide and Heuvelink (2009) and Mochizuki et al. (2013, 2014) reported that fruit yield is affected by the total DW of tomatoes and strawberries. Therefore, Air/CO2 produced more fruit yield than the other treatments. However, there was no difference in the fruit yield in October. Nishiyama et al. (2009) reported a critical photoperiod for flower bud initiation in ever-bearing strawberries; they preferred a photoperiod of more than 15 h. Hamano and Kimura (2018) reported that when long-day treatment (24 h, 2 weeks) was given to ever-bearing strawberry in July, fruit yield in October increased. In addition, Tagawa et al. (2022) reported that when CO2 was applied to June-bearing strawberries, the sugar content increased. However, the fruit quality did not change in this study (data not shown). It has been reported that the sugar content of strawberries decreases during high-temperature periods because the fruits are harvested in a state when sufficient sugar has not accumulated since the high temperature accelerates fruit coloration (Kawanobu et al., 2011). In this study, it is thought that the sugar content did not improve even with CO2 and Air application owing to the same factors.
In this study, Air, CO2 and Air/CO2 treatments increased total DW (Table 4). According to Matsugaki et al. (1998), the DW of the leaves increases because photosynthesis increases due to the application of CO2, creating a surplus of assimilated products supplied to the vegetative organs. During the fruit maturation stage, most of the assimilated products produced in strawberries are distributed to the flowers and fruits, and the rate of distribution to the roots is greatly reduced (Nishizawa and Hori, 1988). However, since environmental measurements such as the temperature on the underside of the leaf and the wind speed in the plant canopy were not measured, further investigation and consideration are needed. Furthermore, the RGR of CO2 and Air/CO2 were higher than that of C in the first and entire periods (Table 5). This was mainly due to the large NAR of Air, CO2, and Air/CO2 in the first period, and the large LAR of CO2 and Air/CO2 in the second period. The PLA of CO2 treatment tended to be larger than that of C (Fig. 5A). Therefore, Air/CO2 and CO2 exhibited greater cumulative light interception (Fig. 5B) and higher LUE (Fig. 6). Higashide et al. (2015) reported that elevated CO2 with fogging treatment increased LUE 1.5 times more than that of ambient CO2 without fogging in tomatoes. Harazono (1982) reported that if the air near the leaves does not circulate, the concentration of CO2 and the photosynthetic rate will decrease. The LUE of Air/CO2 was higher than that of C because the air treatment may have removed the boundary layer and brought a higher concentration of CO2 into the stomata. In addition, LUE is influenced by the photosynthetic rate (Higashide and Heuvelink, 2009). Photosynthetic reduction involves long-term acclimation to elevated CO2 levels (Keutgen et al., 1997). However, an increase in the photosynthetic rate due to CO2 application was observed in this study (Fig. 7). In addition, Li et al. (2013) reported that elevated CO2 increased the dark respiration of tomato leaves during the night, but there was no difference in the photosynthetic rate under 0 μmol·m−2·s−1 light intensity. Moreover, stomatal conductance decreases because of long-term elevated CO2 (Xu et al., 1994). However, stomatal conductance of Air, CO2, and Air/CO2 were higher than that of C (Fig. 8). In contrast, Itani et al. (1999) reported that photosynthesis in crops was promoted under high CO2 concentrations in a short time, resulting in increased yield. Thus, we did not see the effects observed in some reports indicating adverse effects of CO2 elevation in this study because the supplemental CO2 time was only from sunrise to six hours before sunset.
In the present study, the local application of CO2 and air to strawberry plants increased dry matter production and total yield. Larger PLA, cumulative light interception, and high LUE helped produce a large amount of total DW and high fruit yield. The photosynthetic rate of the CO2 and Air/CO2 treatments was also high because of the high stomatal conductance. In addition, Air/CO2 plants could produce more fruits than other treatments, including the CO2 treatment alone. Assuming the costs of a CO2 cylinder (30 kg) and price of strawberry fruits (1 kg) were 1,000 and 2,000 yen, respectively, the cost for CO2 treatment under this application condition was 220 yen per strawberry plant. The yield increase needed to balance this cost is 110 g per plant. In this study, the yield of the Air/CO2 treatment increased by 115.8 g compared to C, so the investment cost and income from the increase in yield were balanced. However, CO2 was applied even on cloudy and rainy days because the sunlight sensor and the CO2 application device were not linked so there is a possibility that the amount of CO2 applied could be reduced. Therefore, repeated application of air for 1 minute, and then CO2 for 1 minute, and 4 minutes off within the plant canopy may be an effective method for increasing the production of ever-bearing strawberries in summer and autumn culture.
We thank Mr. Keita Fujiwara and Mr. Akemitsu Sugita (Omnia Concerto, Tokyo, Japan) for supporting this research, especially for the air and CO2 treatments.