2024 Volume 93 Issue 3 Pages 224-231
2024 Volume 93 Issue 3 Pages 224-231
When cut gerbera flowers absorb water after dry transport, some cultivars often exhibit petal curling, a phenomenon known as “Ben-sori” in Japanese. This study showed that the occurrence rate differed among gerbera cultivars, with ‘Aloha’, ‘Prime Time’ and ‘Kimsey’ being categorized as sensitive cultivars, while ‘Pinta’ and ‘Vivid’ were insensitive. In ‘Aloha’, petal curling caused the abaxial length and adaxial width of epidermal cells to be significantly shorter than in normal petals. ‘Aloha’ flowers harvested at later developmental stages were less affected by petal curling compared with those harvested at earlier stages. The petal length and width increased sharply at stage 4 (anthesis), and the development ray florets finished at stage 6 (flowers with anthers visible in three outermost rings in disk florets). Therefore, cut gerbera flowers that still have petal elongation potential may exhibit increased occurrence of petal curling symptoms. Lower absorption temperatures accelerated petal curling, and the most severe symptoms were observed at 5°C. Interestingly, when cut flowers absorbed water at 15°C, no symptoms were detected. We propose two ways to prevent the occurrence of petal curling in cut gerbera flowers: first, harvest flowers of sensitive cultivars at later developmental stages when anthers are visible in the two or three outermost rings of disk florets and second, allow water absorption at around 15°C after dry transport. These prevention strategies can resolve issues associated with petal curling for both flower retailers and customers and will improve the quality of cut gerberas.
Gerbera (Gerbera hybrida) is one of the most popular cut and pot flowers in Japan because of its numerous colors and varieties (Dole and Wilkins, 2005). A cut gerbera flower is normally composed of a terminal composite floral head (inflorescence) and a leafless stem (scape) (Huang et al., 2008; Fig. 1A).

Petal curling in cut gerberas. Normal inflorescence (A) and inflorescence with petal-curled ray florets (B) in ‘Aloha’ cut flowers. The bar corresponds to 2 cm. C, the procedure used to calculate the petal-curling index (= shortest length/longest length of a ray floret).
The vase life of cut flowers is a very important characteristic that determines their quality and the level of satisfaction of consumers who purchase them (Onozaki, 2018; Shibuya, 2018). Gerberas have been known to show low sensitivity to ethylene (Woltering and Van Doorn, 1988). Stem bending (scape bending), floret abscission, petal wilt, and other defects are also considered to lower the ornamental value of cut gerberas (Tonooka et al., 2019; van Meeteren, 1978a; Wernett et al., 1996). Stem bending in particular is a major factor that shortens vase life (Perik et al., 2012, 2014; Tonooka et al., 2023). The inhibition of bacterial growth in the vase water can reduce the incidence of stem bending, which suggests that bacteria are the main cause of this defect (de Witte et al., 2014). Pulse treatments using nanometer-sized silver (Ag+) particles were shown to extend vase life by more than double that of treatments based on water alone (Liu et al., 2008, 2021). In addition, the combination of sucrose with antimicrobial compounds, such as 8-hydroxyquinoline citrate and dichloroisocyanuric acid, was shown to prevent stem damage and significantly decrease the occurrence of stem bending (de Witte et al., 2014; Tonooka et al., 2021). On the other hand, it should be noted that bending is caused by net water loss from stems, particularly in an area with low mechanical strength in the upper part of stems that lacks a sclerenchyma cylinder (Perik et al., 2012; van Meeteren, 1978a). Previous studies have shown that calcium chloride treatments delayed stem bending, and the combination of sucrose and antibacterial compounds enhanced mechanical strength and extended the vase life of cut gerberas (Perik et al., 2014; Tonooka et al., 2023).
In addition to the above-mentioned defects causing inconvenience to customers, there are other issues affecting cut gerberas that reduce their quality and market value for retailers. When absorbing water after dry transport, some gerbera cultivars often exhibit outer petal curling, a phenomenon known as “Ben-sori” in Japanese (Fig. 1B). Although it is not associated with senescence, this phenomenon greatly decreases the flowers’ ornamental value, and the affected cut flowers purchased from markets become unsellable. Therefore, numerous flower retailers have called for the development of postharvest technology to prevent petal curling in cut gerberas.
In the present study, we investigated variations in the rate of occurrence of petal curling among different gerbera cultivars. In addition, we compared biological and morphogenetic traits between cultivars considered to be sensitive and insensitive to petal curling under different water absorption temperatures. Based on the results obtained, we propose an optimal postharvest management strategy to prevent the occurrence of severe petal curling in cut gerberas.
Six gerbera cultivars, i.e., ‘Aloha’, ‘Pinta’, ‘Prime Time’, ‘Kimsey’, ‘Vivid’, and ‘Minou’, were grown in a greenhouse at Shizuoka University, Shizuoka, Japan. Flowers were harvested at the following six developmental stages: stage 1, ray floret corolla about as long as bracts; stage 2, emergence of the ray floret corolla from bracts with a length of 10 mm; stage 3, emergence of the ray floret corolla from bracts with a length of 20 mm; stage 4, fully opened inflorescence, anthers not visible in the disk florets; stage 5, anthers visible in one outermost ring in disk florets; stage 6, anthers visible in the three outermost rings in disk florets (Fig. S1). Stage 5 is usually the best for harvesting gerberas for commercial purposes; therefore, stage-5 flowers were also used for the experiments in the present study.
Evaluation of petal curling in cut gerberasGerbera flowers were harvested at the fifth developmental stage as mentioned above and immediately brought to the laboratory. Flower weight was measured (referred to as harvest FW), and the flowers were then dry stored in the dark at 25°C for 24 h, as postulated dry transport. The weight of the dry-stored flowers was measured (referred to as dry-storage FW), the flowers were cut to a scape length of 30 cm, and they were weighed again (cutback FW). The 30-cm cut flowers were inserted into a 50-mL tube containing 30 mL of distilled water, which was covered with parafilm. After storing the flowers in the dark at 4°C for 24 h, flower weight (absorption FW) and reduced water weight (reduced water) were measured. The natural evaporation was quantified as the reduced water amount in a test tube without a flower. Relative flower weight, water absorption, and transpiration were calculated using the following equations:
The ray florets were removed from the cut flowers, and both their shortest and longest distances from the top to the base were measured as shown in Fig. 1C. Petal curling was quantitatively evaluated by calculating the shortest distance to the longest distance ratio from the top to the base of a ray floret (petal-curling index). To evaluate the effect of water absorption temperature on petal curling, the ‘Aloha’ cut flowers were further cut to a 10-cm scape. The cut flowers were inserted into a 50-mL tube containing 30 mL of distilled water, and were stored in the dark at five different temperature (5, 10, 15, 20, and 25°C) for 24 h. All experiments were used at least 10 cut flowers for each treatment, and three to four replications were carried out. The Tukey-Kramer multiple comparisons test was used to assess significant differences among means.
Investigation of petal elongation and thickness in ray floretsPotted gerbera ‘Aloha’ with flowers at stage 1 were relocated to a plant incubator (CLE-303; Tomy, Tokyo, Japan) set at either 25/15°C or 35/25°C under a 16 h light condition. Every two days, three ray florets were collected, and their petal lengths were measured. This experiment was conducted with four replications.
To examine petal elongation during flower development, we measured the petal length and thickness of ray florets from three days before anthesis to 15 days after anthesis. Petal thickness was measured around the petal tip using a Digimatic indicator (ID-C112XBS; Mitsutoyo, Kanagawa, Japan). For this experiment, three ray florets were collected from intact flowers of potted gerbera ‘Aloha’ every three days. The experiment was conducted with three replications.
Observation of epidermal cells in ray floretsThe petal tops in the petal curling-sensitive ‘Aloha’, ‘Prime Time’ and ‘Kimsey’, and -insensitive ‘Pinta’ and ‘Vivid’ ray florets were observed using a scanning electron microscope in low vacuum pressure mode (JSM6510; JEOL, Tokyo, Japan). Epidermal cell size was measured using Image J version 1.5. The Student’s t-test was used to compare means.
The petal-curling index values for the harvested flowers ranged between 0.94 and 0.98, which meant that petal curling symptoms were not observed, and results were not significantly different among the five gerbera cultivars examined (Fig. 2A). Upon water absorption at 4°C after the dry-storage period at 25°C, the petal curling index values in ‘Pinta’, ‘Vivid’, ‘Kimsey’ ‘Aloha’ and ‘Prime Time’ were significantly reduced to 0.90, 0.84, 0.77, 0.74, and 0.70, respectively (Fig. 2A). ‘Kimsey’, ‘Aloha’ and ‘Prime Time’ exhibited more severe petal-curling symptoms than ‘Pinta’ and ‘Vivid’ (Fig. 2E–I).

Differences in the petal-curling index, relative fresh weight, water absorption amount, and transpiration amount among the five gerbera cultivars. The harvested flowers of each cultivar were dry stored for 24 h, and allowed to absorb water at 4°C for 24 h. This experiments were used at least 10 cut flowers for each cultivar. Values are expressed as the mean ± standard error. A, petal-curling index for ray florets after harvest and water absorption. B, relative fresh weight of flowers after dry storage and after water absorption compared with harvest FW. Water absorption amount (C) and transpiration amount (D) in cut flowers during absorption over 24 h. Values with different letters denote significant differences (P < 0.05) based on the Tukey-Kramer multiple comparisons test. (E)–(I) Images of typical inflorescences in ‘Aloha’ (E), ‘Pinta’ (F), ‘Vivid’ (G), ‘Prime Time’ (H), and ‘Kimsey’ (I) after water absorption at 4°C. The bar corresponds to 2 cm.
The relative flower weights of ‘Prime Time’, ‘Pinta’, ‘Aloha’, ‘Kimsey’, and ‘Vivid’ cut flowers after dry storage fell to 90.0%, 89.3%, 88.4%, 86.3%, and 83.9%, respectively, and those of ‘Vivid’ decreased significantly compared with ‘Pinta’ and ‘Prime time’ (Fig. 2B). The relative flower weights of ‘Prime Time’ and ‘Pinta’ flowers upon water absorption after dry storage increased to 110.2% and 109.7%, respectively, and increased significantly compared with those of ‘Aloha’ and ‘Vivid’ flowers. The amount of water absorption in ‘Aloha’ (0.20 mL·g−1FW) was significantly lower than that in ‘Kimsey’, but was not significantly different from the amounts in the other cultivars (Fig. 2C). The transpiration amount in ‘Vivid’ (0.03 mL·g−1FW) was significantly higher than in ‘Prime Time’, ‘Aloha’, and ‘Pinta’ (Fig. 2D). Therefore, based on the above results, significant differences in the occurrence of petal curling were detected among cultivars.
Effect of growth temperatures on ray floret developmentIn the aforementioned experiment, it was observed that petal curling symptoms, typically seen in sensitive cultivars, were hardly noticeable in cut flowers harvested in the summer. A comparison was made between the growth rates of ray florets in intact potted gerbera incubated under two different temperature conditions (25/15°C and 35/25°C). ‘Aloha’ flowers grown at 35/25°C developed earlier than those at 25/15°C (Fig. 3A). The ray petals of flowers at 35/25°C rapidly elongated up to six days after incubation, after which petal elongation ceased. Conversely, the ray petal length of flowers up to 16 days after incubation at 25/15°C continued to increase. The optimal harvests (stage 5) for the flowers grown at 35/25°C and 25/15°C were achieved at 6.8 and 11.6 days, respectively. The subsequent experiments used cut flowers harvested in spring and autumn.

Temporal development pattern in ‘Aloha’ray petals. A, Potted gerbera ‘Aloha’ with flowers at stage 1 were incubated at either 25/15°C (○) or 35/25°C (●). The petal lengths of ray florets were measured every two days, and flower development was evaluated by observation of anthers visible in disk florets. Open and closed down-pointing triangles indicate days when the optimal stage for harvest was reached (stage 5) for the flowers grown at 25/15°C and 35/25°C, respectively. B, the change in petal length and thickness of ray florets in gerbera grown in April. Broken gray line indicates the optimal stage for harvest. The flowers in stage 3, 4, 5, and 6 corresponded with those at −3, 0, 3, and 6 days after anthesis, respectively.
In April 2018, a study was conducted on the temporal changes in petal length and thickness of ray florets using ‘Aloha’. The petal length experienced a sharp increase from three days before anthesis (stage 3) to the day of anthesis (stage 4) and continued to elongate for up to 15 days post-anthesis (Fig. 3B). The thickness of the petals increased up to six days after anthesis (stage 6), after which it gradually decreased. These findings suggest that the optimal harvest stage (stage 5), characterized by the visibility of anthers in one outermost ring of the disk florets, does not mark the completion of ray floret development
Effect of harvesting flowers at different developmental stages on petal-curling occurrenceBased on the above experiments, ‘Aloha’ and ‘Pinta’ were defined as sensitive and insensitive to petal curling, respectively. Gerberas belonging to these two cultivars were harvested at different developmental stages (stage 4 to 6), dry stored, and subjected to water absorption for 24 h. Then, their level of petal curling was evaluated. The ray florets of ‘Aloha’ flowers harvested at stage 4 were the most severely affected by petal curling, with an index value of 0.80, while those harvested at stages 5 and 6 showed only moderate symptoms, with values of 0.86 and 0.88, respectively (Fig. 4A). On the other hand, petal curling was not observed in all ‘Pinta’ flowers harvested at stages 4 to 6. The relative fresh weights of ‘Aloha’ flowers after water absorption did not significantly differ among these three developmental stages (data not shown). However, the water absorption and transpiration amounts were higher in the late flower development stage (Fig. 4B, C).

Differences in the petal-curling index, water absorption amount, and transpiration amount among flowers harvested at different developmental stages. The curling-sensitive ‘Aloha’ and the insensitive ‘Pinta’ were used in this experiment. Flowers harvested at stage 4 (earlier harvest), 5 (general harvest), and 6 (later harvest), were dry stored for 24 h, and then allowed to absorb water at 4°C for 24 h. All experiments used at least 10 cut flowers for each harvested stage. Values are expressed as the mean ± standard error. A, petal-curling index of ray florets after water absorption. B, water absorption amounts during absorption for 24 h. C, transpiration amounts during absorption for 24 h. Values with different letters denote significant differences (P < 0.05) based on the Tukey-Kramer multiple comparisons test.
Observation of petals in ray florets revealed an increase in cell size and the presence of cuticle elevations in the adaxial epidermal cells along with flower development in both ‘Aloha’ and ‘Pinta’ (Fig. 5A–H). The cuticle elevations in ‘Pinta’ were deeper and longer than those in ‘Aloha’. However, when the ray florets at stage 5 in other cultivars were also observed, the degree of cuticle elevations in the adaxial epidermal cells was not correlated with the occurrence of petal curling symptoms (Fig. 5I–K).

Observation of adaxial epidermal cells in the curling-sensitive ‘Aloha’ and curling-insensitive ‘Pinta’. The adaxial epidermal cells of ray florets in flowers harvested at different developmental stages were observed by scanning electron microscopy. The figure includes images of the flower development stages in ‘Aloha’ (A)–(D) and ‘Pinta’ (E)–(H). (A) and (E) depict ray florets at stage 1, (B) and (F) at stage 3, (C) and (G) at stage 5, and (D) and (H) at stage 6. The adaxial epidermal cells of ‘Prime Time’(I), ‘Kimsey’(J), and ‘Vivid’(K) at stage 5 were observed. The bars corresponds to 50 μm.
Next, we observed the epidermal cells of normal ray petals and those with petal curling symptoms. After water absorption, the adaxial epidermal cell lengths in normal and curling-affected ‘Aloha’ ray florets were 95.2 and 93.5 μm, respectively (Fig. 6A). However, the abaxial epidermal length was significantly shorter in the latter (113.1 μm) than in the former (128.3 μm). In addition, the adaxial cell width in curled petals was also significantly shorter than in normal petals in ‘Aloha’ (Fig. 6B).
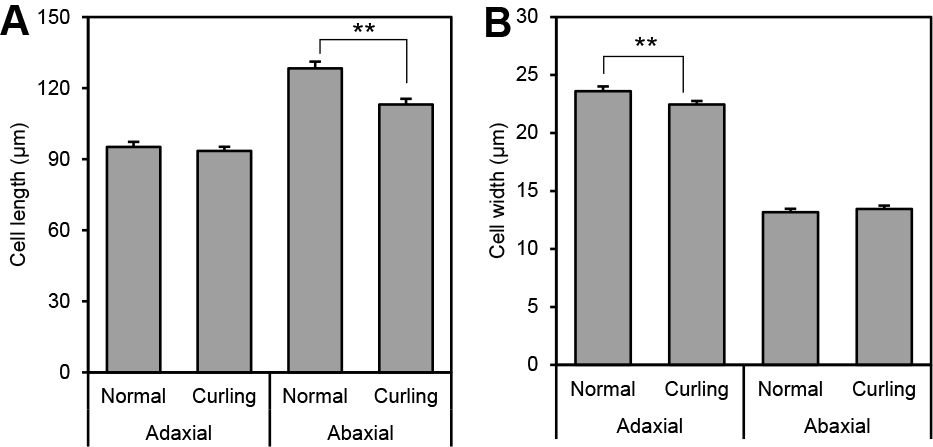
Comparison of epidermal cell size in normal and curling-affected ‘Aloha’ ray florets. Average cell length (A) and cell width (B) of adaxial and abaxial epidermal cells in normal and curling-affected ray florets. Values are expressed as the mean ± standard error (n = 80). ** indicates significant differences at P < 0.01 based on the Student’s t-test.
In the above experiments, water absorption treatments after dry storage were conducted at 4°C for 24 h. This experiment was repeated with water absorption temperatures of 5, 10, 15, 20, and 25°C. The most severe petal-curling symptoms were observed at 5°C, resulting in a petal-curling value of 0.69 (Fig. 7A). Higher water absorption temperatures suppressed petal curling in ray florets, with the best results being achieved at 15°C. Inversely, temperatures above 20°C had the opposite effect and accelerated petal curling of ‘Aloha’ ray florets. Water absorption amounts increased as absorption temperatures rose, with treatments at 5, 10, 15, 20, and 25°C resulting in absorption amounts of 0.27, 0.28, 0.34, 0.44, and 0.49 mL·g−1FW, respectively (Fig. 7B). Similarly, transpiration amounts also increased with increasing absorption temperatures (data not shown).

Effect of water absorption temperature on petal-curling occurrence in ‘Aloha’. The harvested flowers of each cultivar were dry stored for 24 h and allowed to absorb water at 5, 10, 15, 20, and 25°C for 24 h. This experiment used at least 10 cut flowers for each temperature. Values are expressed as the mean ± standard error. A, petal-curling values of ray florets after water absorption at different absorption temperatures. B, water absorption amounts over a 24-h period. Different letters denote significant differences (P < 0.05) based on the Tukey-Kramer multiple comparisons test.
In rose, carnation, and other flowers, petal in-rolling and wilting phenomena occur due to senescence induced by ethylene after cutting (Onozaki, 2018; Pun and Ichimura, 2003). However, the curling of outer petals in cut gerberas differs from the petal in-rolling observed in other species (Fig. 1B). The vase life of cut gerberas has been extensively studied (van Meeteren, 1978a, b, 1979, 1980; van Meeteren and van Gelder, 1980), but no research has been reported on petal curling, which is an issue of concern for flower retailers. To evaluate the degree of petal curling in cut gerberas, we introduced a petal-curling index, with values calculated as the shortest distance to longest distance ratio from the top to the base of a ray floret, as shown in Figure 1C. Values below 0.8 denoted severe petal curling, signifying the loss of ornamental value. The occurrence of petal-curling symptoms differed among the five cultivars investigated in this study. The petal-curling index values in ‘Kimsey’, ‘Aloha’ and ‘Prime Time’ were reduced significantly to 0.77, 0.74, and 0.70, respectively, and indicated more severe symptoms (Fig. 2A). Relative flower weight, water absorption amount, and transpiration amount after absorption following dry storage were only slightly correlated with petal curling (Fig. 2B–D). Therefore, these results revealed that the severity of petal-curling symptoms depends on the gerbera cultivar. During dry transport to market and retailers in Japan, cut gerbera flowers are usually covered by plastic caps to protect flowers from injury. We found that plastic caps were reduced the cut flower weight during dry storage. However, the petal curling occurrence was not different with or without a plastic cap in ‘Aloha’ (data not shown). Therefore, the reduced degree of relative fresh weight during dry transport did not affect the occurrence of petal curling symptoms.
Xylem blockage by bacteria is known to reduce water absorption, although these organisms are responsible for stem breakage in cut flowers (Perik et al., 2012; Tonooka et al., 2023; van Meeteren, 1978a). Cut gerberas with broken stems were usually shown to exhibit wilted petals, but not petal curling. In the present study, the sensitive ‘Aloha’ flowers harvested at earlier developmental stages exhibited severe petal-curling symptoms (Fig. 4A). Specifically, cut flowers at the beginning of anthesis (stage 4) were the most sensitive in this cultivar. Cut flowers harvested at stage 4 exhibited a significant increase in both the petal length and thickness of their ray florets (Fig. 3B). Conversely, those harvested at stage 6 had completed development of their ray petals. Gerbera producers often report that the petal curling symptom in cut gerbera flowers occurs frequently in the summer, which contradicts our observations. Under high-temperature conditions, flower development progresses faster compared to low temperatures (Fig. 3A). The growth from stage 1 to stage 5 was achieved in 6.8 days. We hypothesize that many cut flowers may be harvested earlier than stage 5 during summer harvesting. In addition, the petals of ray florets developed under high temperatures were shorter than those developed under low temperatures, suggesting that petal elongation may be inhibited by high temperatures (Fig. 3A). Therefore, cut gerbera flowers harvested in summer may resume petal elongation when exposed to a cold chain after harvest. Cut flowers that retain the potential for petal elongation may exhibit increased occurrence of petal curling symptoms. We propose this hypothesis regarding the mechanism of the petal-curling symptom, but we were unable to investigate the development pattern of ray petals in cultivars other than ‘Aloha’. Further studies are needed to understand the petal curling symptom in cut gerbera flowers.
The adaxial epidermal cells in ‘Pinta’ gerberas were covered with cutin arranged in small transverse ridges (Fig. 5). On the other hand, the adaxial epidermal cells in ‘Aloha’ gerberas at stage 3, which is the optimal stage for harvest, did not exhibit this cutin layer. Thus, the presence or absence of cutin on the epidermal cells between sensitive and insensitive cultivars may affect cell strength and the extension of ray florets.
The physical and chemical properties of the petal surface of cutin in Arabidopsis mutants have been found to play a crucial role in the generation of specialized plant surfaces with important biological functions, such as facilitating the gliding of organs during rapid growth (Mazurek et al., 2017). However, deeper and longer cuticle elevations were observed in the petal-curling-sensitive cultivars ‘Prime Time’ and ‘Kimsey’ (Fig. 5I, J). Therefore, it appears that the degree of cuticle elevation in the adaxial epidermal cells of ray florets may not be involved in the occurrence of the petal-curling symptom.
Ethylene is widely known to induce epinasty, which is a characteristic morphological phenomenon occurring in leaves (Sandalio et al., 2016). In addition, auxin and abscisic acid have also been shown to regulate cell division and elongation in this phenomenon. Gerberas are known to have a low sensitivity to ethylene (Woltering and Van Doorn, 1988) and in the present study petal-curling symptoms were not detected when the cut flowers were treated with ethephon (2-chioroethanephosphonic acid, data not shown). Upon observation of the epidermal cells, both abaxial cell length and adaxial cell width in curling-affected ray florets were reduced compared with normal ray florets (Fig. 6). Light and gibberellins are important regulators of plant organ growth. In gerbera ray floret petals, gibberellin (GA) has been shown to exert a synergistic positive effect on cell length, but an antagonistic effect on cell width depending on the light signal (Zhang et al., 2012). On the other hand, abscisic acid (ABA) treatments were shown to significantly reduce the size of ray floret petals (Li et al., 2015). Petal elongation is associated with cell elongation and is regulated by the antagonistic effects of GA and ABA. These endogenous phytohormones may be involved in the occurrence of petal curling in cut gerberas.
A previous study of the effect of temperature during dry storage revealed that storage at 2°C was the most suitable to maintain the postharvest quality and ornamental value of cut gerberas (Durigan and Mattiuz, 2009). However, the effect of water absorption temperature after dry storage on the postharvest quality of cut gerberas has not yet been investigated. In general, after transportation to flower shops from the market, gerberas absorb water under cool conditions. Interestingly, in this study, the most severe petal-curling symptoms were observed when ‘Aloha’ flowers absorbed water at a temperature of 5°C (Fig. 7). On the other hand, when water absorption occurred at 15°C, no symptoms were observed. Our study revealed that the temperature of water absorption significantly influences the rate of petal curling in ‘Aloha’, which is sensitive to this factor. Contrary to the common belief among many gerbera producers that low-temperature absorption reduces the occurrence of petal curling in cut gerbera flowers, our findings suggest the opposite. An increase in water temperature leads to a decrease in kinematic viscosity, thereby reducing water conductance in the xylem vessels (Doi and Tsuruga, 2009). Similarly, environmental temperature was found to affect the degree of petal elongation in the ray flowers of ‘Aloha’ (Fig. 3A). A majority of gerbera cut flowers are shipped by dry transport, and wet transport is hardly used. Of the few farmers that ship cut gerbera flowers by wet transport, it was reported that severe petal curling resulting in unsaleable flowers did not occur. We hypothesize that a disturbance in the balance between the amount of water and the development potential of ray florets in cut gerbera flowers may accelerate the occurrence of petal curling symptoms.
The present study revealed that the sensitivity to petal curling varied among gerbera cultivars and the occurrence of petal curling was potentially associated with the growth timing of ray florets. Two strategies are proposed to avoid petal curling: one is to harvest flowers at later developmental stages, preferably when anthers are visible in the three outermost rings in disk florets; the other is to allow cut flowers to absorb water at a temperature of around 15°C after dry storage and transport. This procedure has been established using the petal-curling sensitive ‘Aloha’ cultivar and could improve postharvest management if applied to other gerbera cultivar cut flowers.