2013 年 53 巻 10 号 p. 1696-1703
2013 年 53 巻 10 号 p. 1696-1703
The present paper investigated the influence of basicity and TiO2 content on the crystallization behavior of the Ti-bearing blast furnace slag (Ti–BF slag). Single Hot Thermocouple Technique (SHTT) was applied to construct the Time-Temperature-Transformation (TTT) diagrams. Scanning Electron Microscope equipped with Energy-Dispersive X-ray spectroscope (SEM-EDX) and X-Ray Diffraction (XRD) were employed to observe the morphology and determine the crystalline phase of the Ti-enriched crystals. It was found that rutile was formed as the Ti-enriched phase when the basicity of the sample was lower than 0.6, and perovskite appeared as the Ti-enriched phase with an increasing basicity to 1.0. It was also noticed that the addition of TiO2 could decrease the incubation time in the TTT diagram of the samples. The kinetics of the formation of Ti-enriched crystals, rutile and perovskite, was studied, and the mechanism of crystallization and growth was further discussed. The results indicated that the crystallization of rutile was one-dimensional interface-controlled growth, and the nucleation rate varied with the holding time at different TiO2 content. While the precipitation behavior of perovskite was three-dimensional diffusion-controlled growth, and its nucleation rate decreased with the holding time.
Titanium-bearing Blast furnace (Ti–BF) slag is a typical by-product of the iron and steel industry in China, which contains about 22 wt%–26 wt% TiO2 and is considered as a valuable secondary resource of titanium.1) Over the past decades, many researchers studied the properties of the Ti–BF slag,2,3,4) and lots of extraction methods, such as acid leaching,5,6) molten salt method7,8) and carbide-chloride method,9,10,11) were proposed in order to extract Ti element from Ti–BF slag effectively. However, the recycling of titanium from Ti–BF slag is still in the trouble of high cost and heavy pollution using the above-mentioned extraction methods.12) One reason is that the Ti–BF slag contains many impure elements, such as Ca, Si, Al and Mg etc, another reason is that Ti element in the Ti–BF slag is widely distributed in various titanium containing mineral phases. Therefore, a pre-treatment process called Selective Crystallization and Phase Separation (SCPS) method was proposed13) and applied to deal with the Ti–BF slag. This method has been successfully employed to recover boron and vanadium from the boron-bearing slags14) and vanadium-bearing steelmaking slags15,16) in recent years. The key point of the SCPS method is to enrich the dispersed Ti element into a certain phase (Ti-enriched phase) and to make the Ti-enriched crystals grow large enough by adjusting the composition of the Ti–BF slags and controlling the proper temperature. The conventional separation methods such as gravity separation and flotation separation are then applied to separate the Ti-enriched crystals from the glassy slags.17)
Since the enrichment of titanium is a vital part of the SCPS method, many researchers investigated the enrichment behavior of the Ti-enriched phases. Wang13) et al. studied the variation of the Ti-enriched phase with the addition of silica and titania into Ti–BF slag. However, the crystallization behavior and its kinetics of the formation of Ti-enriched phases were not discussed in Wang’s researches. Sui18,19,20) et al. proposed that perovskite (CaTiO3) could be acted as the Ti-enriched phase; however, it has been proved that perovskite is hard to be extracted from the slags due to its dentrite structure and the similar density with glassy phase. In earlier papers, the present author put forward that rutile is an appropriate Ti-enriched phase due to its rod-shaped structure and higher density.21) However, how the composition of the Ti–BF slag, especially the basicity and the TiO2 content, affect the crystallization behavior in the Ti–BF slag has not been investigated so far. Therefore, the present paper was motivated to discuss the influence of basicity and TiO2 on the crystallization behavior of the Ti–BF slag. And the objectives of this paper are to:
In situ observe the precipitation and the growth of the Ti-enriched phase and construct the time-temperature-transformation (TTT) diagram using the Single Thermocouple Hot Technique (SHTT).
Investigate the variation of Ti-enriched phase and discuss the impact of basicity and TiO2 content on the Ti-enriched phase.
Study the kinetics of crystallization and growth of the Ti-enriched crystal in order to find out its mechanism.
Based on the chemical composition of Ti–BF slag in PANZHIHUA Iron and Steel (Group) Co. (shown in Table 1), the slag CaO-SiO2-12 wt%Al2O3-7 wt%MgO-TiO2 was selected to investigate the influence of basicity and TiO2 content on the crystallization of the Ti–BF slag. The designed compositions studied in the present paper were listed in Table 2. As can be seen, the TiO2 content varied from 15 wt% to 30 wt%, and the basicity varied from 0.5 to 0.9 at a certain TiO2 content.
| SiO2 | CaO | Al2O3 | MgO | TiO2 | MnO |
| 24.48 | 26.63 | 13.13 | 7.01 | 22.82 | 0.73 |
| Fe | V | Cr | Other | Total | Basicity |
| 1.47 | 0.25 | <0.1 | 4.48 | 100 | 1.1 |
| Slag No. | TiO2 | SiO2 | CaO | MgO | Al2O3 | Basicity | Total | |
|---|---|---|---|---|---|---|---|---|
| 1 | Designed | 15 | 44 | 22 | 7 | 12 | 0.5 | 100 |
| Measured | 14.65 | 41.70 | 24.72 | 7.23 | 11.70 | 0.59 | 100 | |
| 2 | Designed | 15 | 38.82 | 27.18 | 7 | 12 | 0.7 | 100 |
| Measured | 14.02 | 36.99 | 29.67 | 7.32 | 11.99 | 0.80 | 100 | |
| 3 | Designed | 15 | 34.74 | 31.26 | 7 | 12 | 0.9 | 100 |
| Measured | 13.83 | 33.45 | 33.66 | 7.37 | 11.69 | 1.01 | 100 | |
| 4 | Designed | 20 | 40.67 | 20.33 | 7 | 12 | 0.5 | 100 |
| Measured | 20.33 | 37.51 | 23.69 | 6.98 | 11.49 | 0.63 | 100 | |
| 5 | Designed | 20 | 35.88 | 25.12 | 7 | 12 | 0.7 | 100 |
| Measured | 18.82 | 34.69 | 27.46 | 7.13 | 11.90 | 0.79 | 100 | |
| 6 | Designed | 20 | 32.11 | 28.89 | 7 | 12 | 0.9 | 100 |
| Measured | 18.41 | 30.83 | 31.54 | 7.21 | 12.01 | 1.02 | 100 | |
| 7 | Designed | 25 | 37.33 | 18.67 | 7 | 12 | 0.5 | 100 |
| Measured | 22.98 | 36.61 | 21.05 | 7.36 | 12.00 | 0.57 | 100 | |
| 8 | Designed | 25 | 32.94 | 23.06 | 7 | 12 | 0.7 | 100 |
| Measured | 23.50 | 31.54 | 25.74 | 7.31 | 11.90 | 0.82 | 100 | |
| 9 | Designed | 25 | 29.47 | 26.53 | 7 | 12 | 0.9 | 100 |
| Measured | 23.00 | 28.60 | 29.31 | 7.07 | 12.01 | 1.02 | 100 | |
| 10 | Designed | 30 | 34 | 17 | 7 | 12 | 0.5 | 100 |
| Measured | 27.94 | 33.15 | 19.58 | 7.29 | 12.03 | 0.59 | 100 | |
| 11 | Designed | 30 | 30 | 21 | 7 | 12 | 0.7 | 100 |
| Measured | 27.97 | 29.09 | 23.70 | 7.24 | 12.00 | 0.81 | 100 | |
| 12 | Designed | 30 | 26.84 | 24.16 | 7 | 12 | 0.9 | 100 |
| Measured | 27.50 | 26.59 | 26.69 | 7.20 | 12.02 | 1.00 | 100 | |
Reagent grade CaO, SiO2, Al2O3, MgO and TiO2 was employed to synthesize the slags, which were carefully weighed, fully mixed and melted in a Pt crucible in air atmosphere using vertical MoSi2 furnace. The mixture was held at 1500°C for 30 min to completely homogenize the slag. The samples were then quenched into water, dried, crushed and grounded under 300 meshes for the further utilization. X-ray Powder Diffraction (XRD) with Cu Kα radiation was applied to determine the formed phase in the slags. The composition of the quenched slag may differ from those of the designed samples due to its evaporation losses. X-Ray fluoroscopy (XRF) was then applied to analyze the composition for a comparison, the results of which were also listed in Table 2. It can be seen that the measured values showed a small deviation compared with the designed composition.
2.2. Experimental ProcessIn order to construct the TTT diagram, the isothermal experiments was performed using SHTT in this paper. The principle of the SHTT22) could be briefly illustrated as the followings. A Pt-Rh thermocouple (B Type) was used to heat and measure the temperature simultaneously. The heating and cooling processes were controlled using a computer program. The images of the samples were observed and recorded through a video camera. Pure CaF2 was used to calibrate the temperature. During the experiments, about 10 mg samples were mounted on the tip of the thermocouple. It was heated to 1500°C at a heating rate of 15°C/s, held for 120 s to eliminate the bubbles and then rapidly cooled down to the pre-setting temperatures holding for different times to in situ observe the crystallization behavior of the samples. Then according to the analysis of sample images, the TTT diagram was constructed.
2.3. Measurement and AnalysisIn order to identify the structure of the crystals, the samples were quenched from different temperatures and examined by X-Ray Powder Diffraction (XRD, Rigaku, Dmax/2400) analysis. Intensities were collected by 2θ scanning. The generator voltage and tube current were 40 kV and 40 mA, respectively. The scanning range from 10°–80° was measured at a step of 0.02°.
Scanning Electron Microscope (SEM, HITACHI S4800) equipped with Energy-Dispersive X-ray spectroscope (EDX, BRUKER) was carried out in order to observe the microstructure of the samples and identify the chemical compositions of the crystals. The accelerating voltage of electron beam was 10 kV. And the Back Scattered Electron (BSE) images were obtained in this work.
The SHTT was applied to in situ observe the crystallization of the samples. Among all the samples, slag No. 1 was held at the temperature range of 1000°C to 1300°C for 30 min, and no crystallization was observed. Thus the slag No. 1 would not be discussed in the following sections. Figure 1 showed the in-situ observed images of the slags at 1200°C. It can be seen that two types of Ti-enriched crystals, rod-structured crystal and hill-like crystal, were observed in the slag melts. And it was noticed that the type of the crystals was mainly determined by the basicity of the samples. When the basicity was 0.6, the rod-structured crystal was formed in the slag melt. With the basicity increasing to 0.8, the hill-like crystal and the rod-structured crystal appeared simultaneously. And when further increasing the basicity to 1.0, the hill-like crystal became the only crystals in the slag. Thus it can be concluded that lower basicity was favorable for the formation of rod-structured crystals.
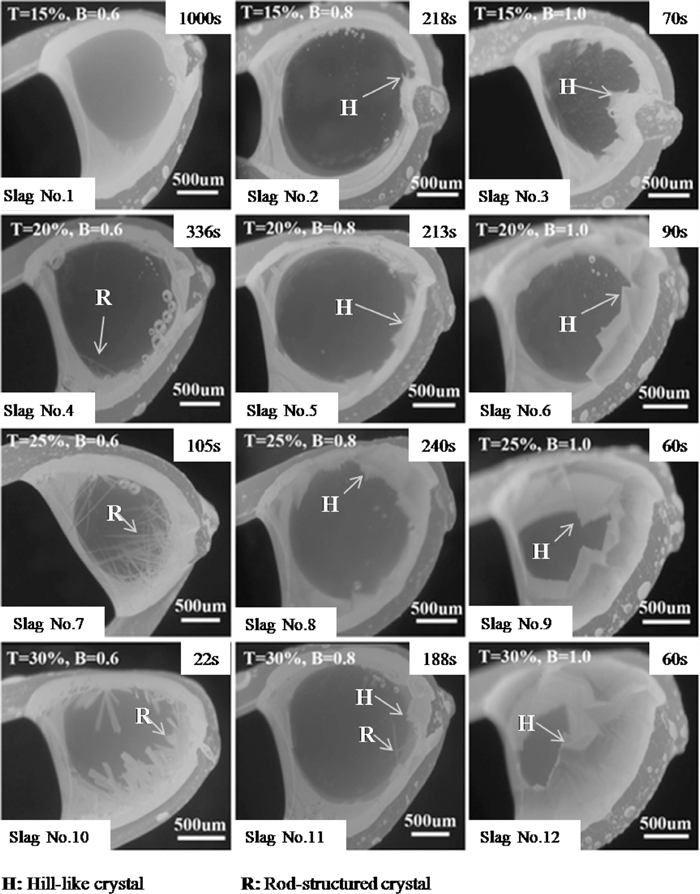
The in situ observed images of the samples at 1200°C, where T means TiO2 content and B means the basicity of the slag.
To further observe the morphologies of the Ti-enriched crystals, SEM was applied, as shown in Fig. 2, which displayed the typical morphologies of the Ti-enriched crystals. As can be seen, the rod-structured crystal in the SHTT images actually showed rod structure, whereas the hill-like crystal was composed of many dendrite-structured crystals.
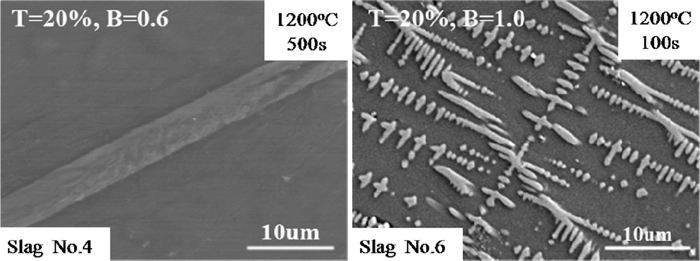
The typical morphology of the Ti-enriched crystals observed in the samples, where T means TiO2 content and B means basicity of the slag.
Then, in order to indentify the structure of the crystals in the slag melt, a Pt pan containing the glassy slag was put into the heating zone of a vertical MoSi2 furnace. The furnace was heated to 1500°C to homogenize the slag, and then followed by a rapid cooling to 1200°C holding for 4 h. The slag samples were then quenched for XRD measurement and EDX analyses. Table 3 showed the EDX analysis results. It can be seen that the rod-structured crystal mainly contained Ti and O elements, whereas the dendrite-structure crystal consisted of Ti, Ca and O elements. Figure 3 displayed the corresponding XRD results. As can be seen, the rod-structure crystal was rutile, and the dendrite-structure crystal was perovskite, which was in agreement with the EDX results.
| Basicity | Morphorlogy | Phase | Si | Ca | Al | Mg | Ti | O |
|---|---|---|---|---|---|---|---|---|
| 0.6 | Rod-structure | Rutile | 0.58 | 0.99 | 0.23 | – | 61.88 | 34.10 |
| Other | Amorphous | 17.74 | 13.51 | 5.34 | 3.81 | 12.50 | 44.58 | |
| 0.8 | Rod-structure | MgTi2O5 | – | 2.00 | 1.64 | 8.11 | 33.14 | 52.36 |
| Dendrite structure | Perovskite | – | 29.76 | – | – | 37.65 | 29.74 | |
| Other | Amorphous | 17.97 | 13.49 | 5.81 | 3.78 | 9.43 | 47.27 | |
| 1.0 | Dendrite structure | Perovskite | 1.38 | 31.66 | 0.59 | 0.18 | 35.21 | 28.61 |
| Other | Amorphous | 18.49 | 16.44 | 6.30 | 4.15 | 4.16 | 48.23 |
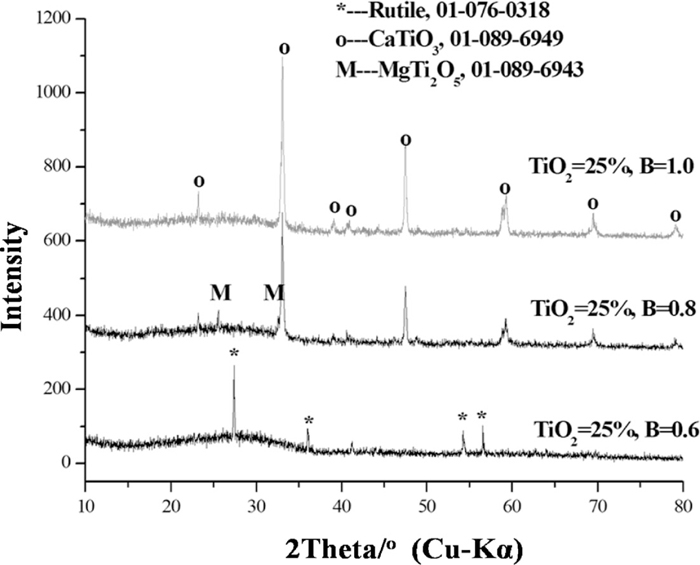
The XRD results of the samples at 1200°C for 4 h.
Furthermore, TTT diagrams were constructed based on the Isothermal experiments. Figure 4 displayed the TTT diagrams of the samples. It was found that the incubation time decreased with the addition of TiO2 at the same basicity, and the higher basicity of the samples was, the smaller influence of the TiO2 addition on the crystallization would be, which indicated that the addition of TiO2 was more beneficial to the crystallization of the slags at lower basicity. It was also observed that the incubation time initially increased and then decreased with increasing the basicity at the same TiO2 content, which may suggest that the crystallization was firstly inhibited and then promoted with the basicity increasing at the same TiO2 content.
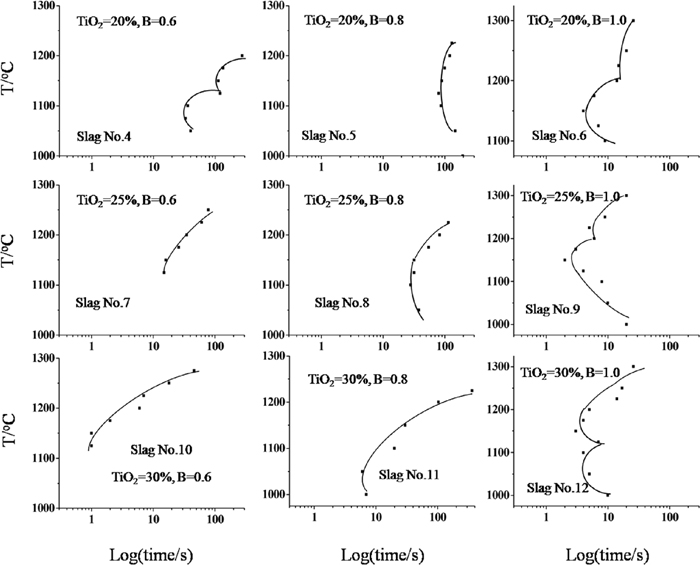
The TTT diagram of the slags.
Moreover, it was seen that the TTT diagram of slag No. 4, No. 6, No. 9 and No. 12 showed double-C shape, which may imply the different crystals formed or the change of the crystallization mechanism at different temperatures in the samples. Combined with the in situ images observed by SHTT, as shown in Fig. 5, the different nucleation mechanism may be the reason since the morphology of the Ti-enriched crystals did not apparently change, i.e., the crystal showed heterogeneous nucleation at higher temperatures, and it transformed to homogeneous nucleation with decreasing the temperature.
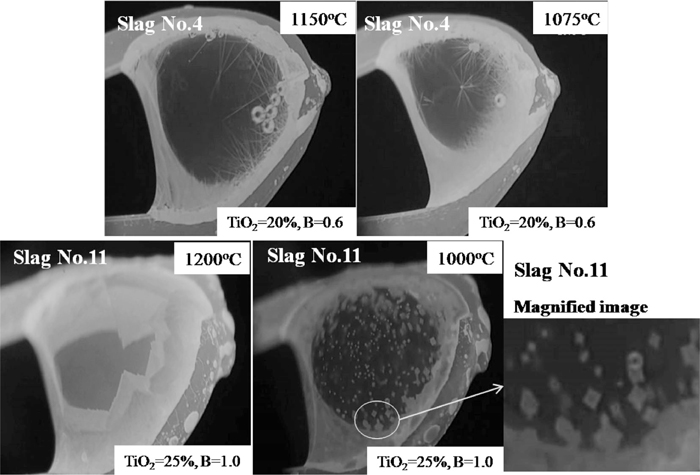
The SHTT images of the sample at different temperatures.
In a word, the influence of basicity and TiO2 content on the crystallization of the Ti-bearing blast furnace slag was studied based on the analyses of Figs. 1,2,3,4,5. And it can be seen that the formation of the Ti-enriched crystals was mainly determined by the basicity of the samples, which transformed from rutile to perovskite with the increase of the basicity (Figs. 1,2,3). The incubation time of the samples varied when the Ti-enriched crystals was different, and the variation of the incubation time in different samples showed similar pattern, i.e., the more TiO2 content in the sample was, the easier occurrence of crystallization in the sample would be (Fig. 4). And the related mechanism was further investigated in the followings.
Based on the aforementioned experimental results, the Ti-enriched crystal was transformed from rutile to perovskite with the increase of the basicity, while the TiO2 content had little impact on the variation of the Ti-enriched phase. Thus, it could be concluded that the variation of the Ti-enriched crystals was mainly determined by the basicity of the slags, and it could be explained as the followings. According to the thermodynamic analysis calculated by HSC 5.0 software,23) as shown in Fig. 6, the standard Gibbs free energy changes of the chemical reactions (5) and (7) are much smaller than that of reaction (1), i.e., CaO preferred to react with SiO2 in the presence of Al2O3 and MgO, rather than TiO2 in the slag melt. Therefore, based on the molecular theory, it can be predicted that the activity of CaO in this Ti-bearing slag melt got smaller with decreasing the basicity of the slag. Thus, it could be concluded that the decreasing basicity inhibited the formation of perovskite and is beneficial to the formation of rutile. To further support this viewpoint, the phase diagram of CaO–SiO2–Al2O3–MgO–TiO2 was calculated by FactSage 6.1,24) and the slag No. 1–No. 12 were labeled in the diagram, as shown in Fig. 7. Compared with the actual phase diagram of CaO-SiO2-TiO2,25) it can be seen that CaAl2Si2O8 was easier to be produced and the formation of CaTiSiO5 was inhibited in this slag melt, which was in agreement with the analysis in Fig. 6. It was also observed from Fig. 7 that the primary phase transformed from perovskite (CaTiO3) to rutile (TiO2) with the decrease of basicity, and the variation of primary phase was mainly determined by the basicity. It is in accordance with the experimental results. However, some deviations were also noticed by comparing the experimental results with the calculated phase diagram, which was listed in Table 4. And it could be explained that the calculated phase diagram showed the equilibrated phases in the slag, whereas the experiments were carried out under a super-cooling conditions.
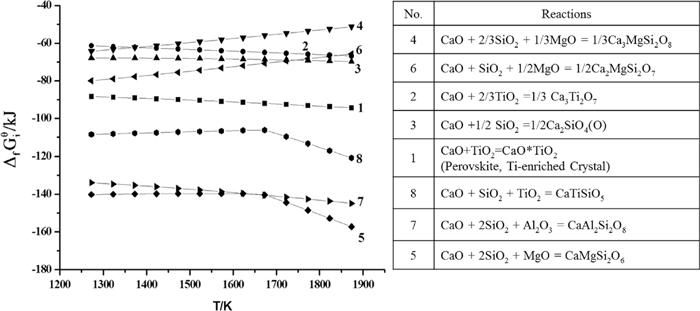
The comparison of the detla Gibbs free energy of the reactions in the Ti–BF slag.
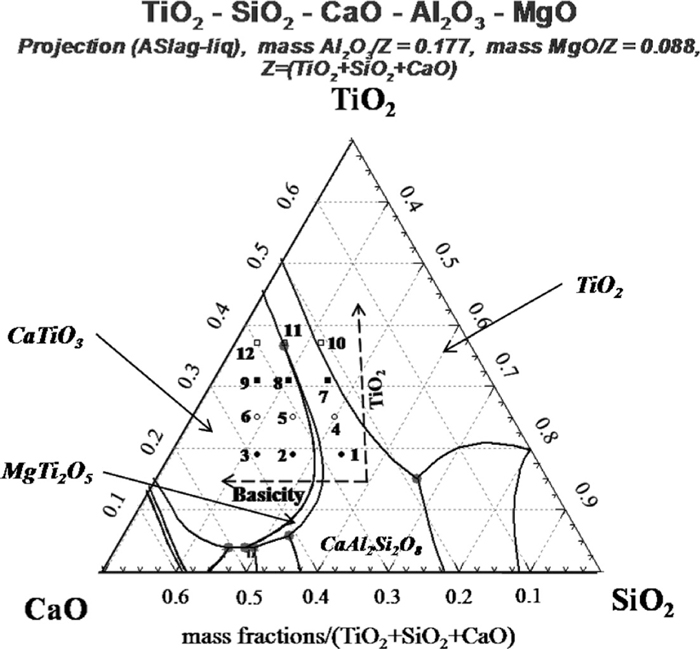
The calculated phase diagram of the Ti–BF slag.
| Slag No. | Primary phase (Measured) | Primary phase (Calculated) |
|---|---|---|
| 1 | – | CaAl2Si2O8 |
| 2 | CaTiO3 | CaTiO3 |
| 3 | CaTiO3 | CaTiO3 |
| 4 | TiO2 | CaAl2Si2O8 |
| 5 | CaTiO3 | CaTiO3 |
| 6 | CaTiO3 | CaTiO3 |
| 7 | TiO2 | CaAl2Si2O8 |
| 8 | CaTiO3, MgTi2O5 | CaTiO3 |
| 9 | CaTiO3 | CaTiO3 |
| 10 | TiO2 | TiO2 |
| 11 | CaTiO3, MgTi2O5 | CaTiO3, MgTi2O5 |
| 12 | CaTiO3 | CaTiO3 |
Johnson-Mehl-Avrami-Kolmogorov (JMAK) equation,26,27,28,29) as shown in Eq. (1), was applied to study the kinetics of the Ti-enriched crystals in the slag.
| (1) |
| (2) |
Herein Ac is the area of Ti-enriched crystals and AT is the total area of the sample. Based on the value of n, the nucleation and growth mechanism could be investigated.30,31,32,33)
Equation (1) was rearranged to obtain Eq. (3), and then the value of n could be obtained and the result was shown in Fig. 8.
| (3) |
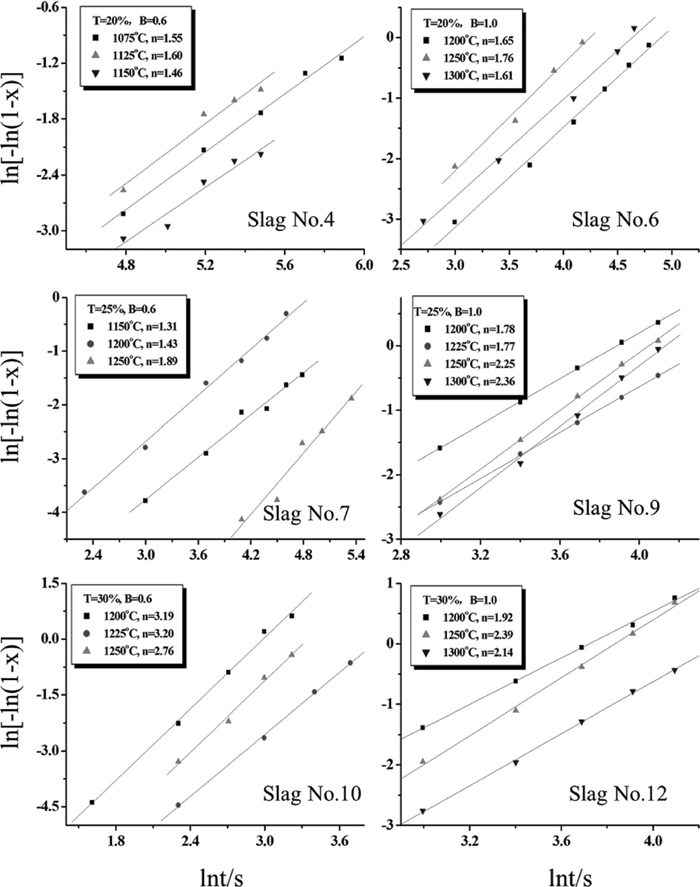
ln[–ln(1–x)] as functions of lnt of the samples at different temperatures.
Based on the experimental results, two types of Ti-enriched crystals, rutile and perovskite, were found in the samples. And it was also known that rutile could be formed when the basicity of the samples was 0.6; perovskite was precipitated when the basicity increased to 1.0. Thus, Table 5 displayed the variation of value n of the samples to better illustrate the crystallization and growth mechanism of the Ti-enriched crystals.
| Basicity | TiO2 content | Temperature/°C | n | lnk | Mean Value |
|---|---|---|---|---|---|
| 0.6 | 20% | 1075 | 1.55 | –10.2 | n=1.537 |
| 1125 | 1.60 | –10.16 | |||
| 1150 | 1.46 | –10.122 | |||
| 25% | 1150 | 1.31 | –7.68 | n=1.543 | |
| 1200 | 1.43 | –6.97 | |||
| 1250 | 1.89 | –12.02 | |||
| 30% | 1200 | 3.19 | –9.54 | n=3.05 | |
| 1225 | 3.2 | –10.72 | |||
| 1250 | 2.76 | –10.86 | |||
| 1.0 | 20% | 1200 | 1.65 | –8.08 | n=1.673 |
| 1250 | 1.76 | –7.49 | |||
| 1300 | 1.61 | –7.46 | |||
| 25% | 1200 | 1.78 | –6.92 | n=2.04 | |
| 1225 | 1.77 | –7.73 | |||
| 1250 | 2.25 | –9.12 | |||
| 1300 | 2.36 | –9.76 | |||
| 30% | 1200 | 1.92 | –7.14 | n=2.15 | |
| 1250 | 2.39 | –9.17 | |||
| 1300 | 2.14 | –9.21 |
When the basicity of the sample was 0.6, rutile was formed. And based on Fig. 8 and Table 5, the value of n was about 1.5 when the TiO2 content was 20% and 25%, whereas the value of n increased to 3.0 when the TiO2 content was 30%.
It has been known that the value of n was related to the nucleation and growth process,34) as shown in Eq. (4):
| (4) |
Where n is the Avrami constant; a is the constant related with the nucleation rate of the crystals, b is the constant associated with the growth dimension of the crystals, and c is the constant related with the growth rate of the crystals. The meaning of their value was displayed in Table 6 in detail.34)
| Value | Meaning | |
|---|---|---|
| a | 0 | Nucleation rate is constant |
| 0<a<1 | Nucleation rate decreased with holding time | |
| a>1 | Nucleation rate increased with holding time | |
| b | 1 | 1-dimension growth |
| 2 | 2-dimension growth | |
| 3 | 3-dimension growth | |
| c | 1 | Interface-controlled growth |
| 0.5 | Diffusion-controlled growth |
According to the meaning of b and c, their value could be calculated based on the experimental data. As seen from SHTT images, rutile was rod structure and grew along the thermocouple. So it can be concluded that rutile was one-dimensional growth, and b was assumed to equal to 1. Moreover, based on Jackson’s studies,35) when the length of rutile increased linearly with the holding time, as shown in Fig. 9, the controlling step of rutile growth was the interfacial reaction and c equals to 1. Therefore, it could be calculated the value of a, which was about 0.5 when the TiO2 content was 20% and 25%. This may suggest that the nucleation rate decreased with holding time. The value of a was about 2 when the TiO2 content increased to 30%, which showed that the nucleation rate increased with holding time.

The length of rutile as function of holding time at 1100°C when the basicity was 0.6 and the TiO2 content was 20%.
Therefore, it could be concluded that the crystallization and growth mechanism of rutile was one-dimensional interface-controlled growth. Its nucleation rate varied with the TiO2 content. When the TiO2 content was 20% and 25%, the nucleation rate decreased with the holding time, whereas it increased with the holding time when the TiO2 content was 30%.
Similarly, the crystallization and growth mechanism of perovskite was studied. According to the SHTT and SEM image, the growth of perovskite showed 3-dimension phenomena, i.e., b equals to 3. Based on Lou’s experimental results,36) the controlling step of perovskite crystal was diffusion, and c equals to 0.5. And then it could be calculated that the value of number a was less than 1, and it implied that the nucleation rate of perovskite crystal decreased with holding time.
The crystallization and growth of the Ti-enriched crystals have a vital effect on the extraction of Ti from Ti–BF slags based on Selective Crystallization and Phase Separation (SCPS) method. In view of the importance of TiO2 content and the basicity on the crystallization behavior, the influence of basicity and TiO2 content on the precipitation behavior in the Ti–BF slag was therefore investigated in this paper.
It was found that rutile showed the rod-structure and was formed in the Ti–BF slag in the lower basicity region. With increasing the basicity, the Ti-enriched crystals transformed from rutile to perovskite. It was also noticed that the incubation time of the samples was mainly influenced by the TiO2 content, and the incubation time decreased with increasing TiO2 content of the samples, i.e., the addition of TiO2 is favorable for the formation of the Ti-enrichment crystals.
The kinetics of the two types of Ti-enrichment crystals, rutile and perovskite, was also studied in this paper. It was concluded that the crystallization of rutile was one-dimensional interface-controlled growth, and the nucleation rate of rutile decreased with the holding time when the TiO2 content was 20% and 25%, whereas it increased with the holding time when the TiO2 content was 30%. Meanwhile, the precipitation of perovskite showed three-dimensional diffusion-controlled growth, and the nucleation rate of perovskite decreased with the holding time.
Financial supports from National Natural Science Foundation of China (51172003, 51074009), Key Projects in the National Science & Technology Pillar Program (2011BAB03B02, 2011BAB02B05), and the National High Technology Research and Development Program of China (863 Program, 2012AA06A114) are gratefully acknowledged.