2013 年 53 巻 10 号 p. 1779-1785
2013 年 53 巻 10 号 p. 1779-1785
The formation of titanium carbonitride has been used as a conventional method to protect the refractory wear in the hearth. Because titanium carbonitride is formed only in the molten iron, the area that can form a protective layer in the hearth is limited. There is another possibility to protect the refractory wear by introducing the compounds with high melting point in the slags. If the compounds with high melting point are simultaneously formed in the molten iron and slag by adding TiO2, it might be more effective to form the protective layer and to prevent the refractory wear of hearth. However, the change of slag compositions and the formation of slag compounds can affect the slag viscosity and critical temperature, which might cause serious problems with blast furnace operation.
In this study, to find the slag compositions for the effective compounds formation with maintaining the slag fluidity, the viscosity measurements, in-situ observation of compound formation by a confocal laser scanning microscopy and thermodynamical evaluation by FactSage has been carried out. Based on these results, the suitable slag compositions were suggested to form a protective layer in the hearth.
The campaign life extension of a blast furnace is of a great concern in the steelmaking industry and premature hearth erosion is one of the most important factors limiting campaign life. There are several measures to reduce the erosion of the blast furnace hearth including the addition of the titania bearing materials into the blast furnace.1,2,3) This is believed to promote the formation of a protection layer, so-called ‘‘titanium-bear,’’ on the refractory brick.
Besides titanium carbonitride formation as a method of making a protective layer in the hearth region, there is another possibility to form a protective layer by using oxide compounds such as magnesium aluminate spinel (MgAl2O4), magnesium titanate spinel (Mg2TiO4) or perovskite (CaTiO3) with high melting point higher than about 2073 K. The Al2O3 content in the slag is now going to increase due to the depletion of high grade iron ore resources and slag compounds such as spinel or perovskite can be formed more easily as Al2O3 content in the slag is increased. If those slag compounds such as spinels or perovskite are formed along with titanium carbonitride by the addition of TiO2, it will be more effective to form a protective layer in the BF hearth. However, high TiO2 content may result in significant issues such as variations in the liquidus temperature of the slag,4,5) reduced refining capacity of the slag,6,7) and slag viscosity deviations from optimum processing conditions.8,9,10,11)
Du Hegui12) showed the equilibrium distribution ratios of sulphur (Ls=(S)/[S]) between molten iron and slag were affected by the basicity and titanium oxides contents. The desulphurization capacity of slag was lowered as titanium oxides increased. Handfield and Charette13) showed small TiO2 additions decreased the slag viscosity, but large TiO2 additions had the opposite effect. Ohno and Ross14) described TiO2 additions to increase slag viscosity in the CaO–SiO2–Al2O3–TiO2 slags under reducing conditions. The work done by Shankar et al.4) showed the effect of TiO2 in the CaO–SiO2–MgO–Al2O3 slag system under Ar, where TiO2 up to 2 mass% decreased the viscosity at the various extended Vee ratio [(CaO + MgO)/(SiO2 + Al2O3)] between 0.46 and 0.83. Saito et al.11) also studied the effect of TiO2 in the CaO–SiO2–MgO–Al2O3 slag system at 10 and 20 mass% TiO2 content. TiO2 lowered the viscosity of the slag and the corresponding activation energy of the viscous flow decreased with TiO2 content. Thus, slag viscosity can be affected by the amount of TiO2 additions. Recently, Park et al.15) found that TiO2 decreased the slag viscosity up to 10 mass% in the CaO-SiO2-17 mass%Al2O3-10 mass%MgO-TiO2 slag system at various basicity ranges. Therefore, it is not clear whether TiO2 addition increase or decrease the slag viscosity.
The most important factor in the BF slag is the liquidus temperature and the critical temperature below which the normal operation becomes unavailable due to the sharp increase of the viscosity. Handfield et al.13) added titanium oxides to CaO–MgO–Al2O3–SiO2 slag and found that TiO and Ti2O3 increased the liquidus temperature substantially after a slight decrease at low concentrations of the oxides. Gruzdev et al.16) actually linked the changes in liquidus temperature accompanied changes in primary phase, but their results appear to prove the opposite of those of Handfield et al.13) with respect to the relative effects of TiO2 and Ti2O3 upon the viscosity. In both cases, the ratio of Ti4+ to Ti3+ is the crucial factor to affect liquidus temperature. However, it is not clear the effect of the relative ratio of TiO2 and Ti2O3 on the liquidus temperature. Also, it was found that the ratio of Ti4+ to Ti3+ was affected by the slag basicity according to several investigations. Morizane et al.7) found that the ratio of Ti4+ to Ti3+ was increased with increasing the basicity in the CaO–SiO2–MgO–Al2O3–TiO2 slag equilibrated with carbon saturated iron. According to Ito and Sano,17) the ratio of Ti4+ to Ti3+ was increased in the basicity range over 1.0 with increasing slag basicity, while the result was opposite in the basicity range below 1.0. Thus, it is also not clear whether slag basicity can increase or decrease the ratio of Ti4+ to Ti3+.
Due to the uncertainties of the effect of TiO2 addition on the slag properties, in the present study, the experimental investigations were undertaken to understand the effects of TiO2 addition into the BF slag on the viscosity and critical temperature as well as the formation of compounds with high melting point.
The slag samples were prepared by using special grade chemicals of Al2O3, MgO, SiO2 and TiO2. CaO was prepared by calcining CaCO3 of 99.5 mass% purity at 1000°C in a box furnace for 5 hrs. To measure slag viscosity using a viscometer, a powder mixture of 140 g was pre-melted. Each chemical was weighted for the target compositions and mixed with zirconia balls in a cylindrical ball mill for 24 hrs, and then the mixture was pre-melted at 1823 K in Ar atmosphere of 800 mL/min in a Pt crucible (54 mm ID × 65 mm Height) by employing a SiC vertical tube furnace. The moisture and oxygen in Ar gas were removed by passing it through columns of CaSO4, silica gel and magnesium turnings heated at 673 K. The pO2 after deoxidation treatment was measured by using O2 meter and was found to be around 1×10–16 atm. All of experiments were carried out under these deoxidized Ar atmospheres. The pO2 under the carbon saturated condition at 1873 K is about 1.6×10–16 atm. Namely, the present experimental pO2 atmosphere is almost the same to that at the hearth zone of BF. The temperature was controlled using a Pt-Pt/10 mass%Rh thermocouple and a temperature controller. The fully melted slag was taken out from the furnace and then quenched in a flushing Ar on the water cooled copper plate. The slag was analyzed using XRF spectroscopy. Table 1 shows the composition of pre-melted slag samples used in the viscosity measurement.
| Sample | Content (mass%) | |||||
|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | TiO2 | CaO/SiO2 | |
| Slag_T0_B1.35 | 38.9 | 28.8 | 17.3 | 15.0 | 0 | 1.35 |
| Slag_T1.0_B1.35 | 38.3 | 28.4 | 17.3 | 15.0 | 1.0 | 1.35 |
| Slag _T1.0_B1.25 | 37.2 | 27.5 | 17.3 | 15.0 | 1.0 | 1.25 |
| Slag _T3.0_B1.35 | 37.2 | 27.5 | 17.3 | 15.0 | 3.0 | 1.35 |
| Slag _T5.0_B1.35 | 36.0 | 26.7 | 17.3 | 15.0 | 5.0 | 1.35 |
To investigate the formation of slag compounds with high melting point in slag, the disks prepared by powder mixture were used for in-situ observation with a confocal laser scanning microscopy (CLSM) equipped with an image furnace. Uniformly mixed powder by the same method as pre-melted slag was pressed into the disks of 5 mm diameter with hydraulic press of 5 Mpa for 2.5 min. As shown in Table 2, Slag A and B have the same composition except for MgO and Slag B and C have almost the same composition with different slag basicity (CaO/SiO2).
| Sample | Content (mass%) | |||||
|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | TiO2 | CaO/SiO2 | |
| Slag A | 41.2 | 30.5 | 17.3 | 10.0 | 1.0 | 1.35 |
| Slag B | 38.3 | 28.4 | 17.3 | 15.0 | 1.0 | 1.35 |
| Slag C | 37.1 | 29.7 | 17.3 | 15.0 | 1.0 | 1.25 |
The viscosities of slags were measured by using the general rotating cylindrical method. A digital viscometer with the viscosity range of 1–40000 poise was used in the present study. The viscometer head was connected to the working spindle by two Pt-Pt/10 mass%Rh hooks. The crucible, suspending wire and spindle used in the experiments were made of Pt-10 mass%Rh to prevent the slag contamination. The viscometer was calibrated by using the standard oils with 1, 5, 10 and 50 poises at room temperature. The experimentally measured viscosity values were corrected considering the effect of thermal expansion on the viscosity increase. Three different rotating speeds (30, 60 and 100 rpm) were employed at 1873 K to ensure that the viscosity values were independent of rotation speed and that the homogeneous melt shows a Newtonian behavior at 1723 K. The repeatability of the viscosity values (100 × standard deviation/average value) was evaluated to be less than 1% at 1723 K. The uniform heating zone of the furnace was preliminarily decided by measuring the temperature profile along the vertical position of the furnace. Alumina tube and isolite brick were applied to place the Pt crucible containing slag samples in the uniform heating zone.
The pre-melted slag sample of about 120 g, which corresponds to approximately 29 mm height from the bottom of the Pt crucible, was held in the Pt crucible (ID 54 mm × Height 60 mm) and the Pt crucible was then placed inside the furnace. Then a stainless steel pipe (ID 30 mm) connected with a cylindrical isolite brick was installed into the top of the alumina reaction tube to fill the gap between the viscometer and the reaction tube and place the spindle above the center of the Pt crucible. After the viscometer was installed using suspension wires, the bob was lowered and kept at 50 mm above the slag layer inside the crucible. The viscometer and bob were properly aligned along with the axis of the crucible. Prior to the initiation of the experiments, the reaction chamber was flushed with Ar gas for 30 min and a constant gas flow rate of 800 mL/min was maintained during the viscosity measurement. The Ar gas was purified according to the similar procedure adopted in the process of pre-melting. The furnace was heated up to 1723 K and the bob was immersed into the slag melt while the furnace temperature was held at 1723 K for 2 hrs. The bob was adjusted to be placed 2 to 3 mm above the bottom of crucible and rotated with 100 rpm during the viscosity measurement. The viscosity was continuously measured during the cooling process from 1723 to 1623 K. The temperature interval was 10 K until it reached 1693 K and then below 1623 K, the interval was adjusted to be 5 K for the purpose of obtaining more accurate critical temperature. The temperature was maintained for 30 min at each step to attain equilibrium state of the slag melt. To verify that 30 min is enough to reach equilibrium state, the slag viscosity with the composition (39.8 mass% CaO, 37.6 mass% SiO2, 16.5 mass% Al2O3, 5.6 mass% MgO and 0.48 mass% TiO2) at 1708 K was continuously measured for 18 hrs. The viscosity was found to be reached its equilibrium value within 5 min. The difference between the maximum and minimum value was only 0.7% during the viscosity measurement. Therefore, the holding time of 30 min is enough to reach an equilibrium state for the viscosity measurement at each temperature. After the viscosity was measured until 1623 K, the temperature was increased up to 1723 K and then held for 30 min at the temperature. Thereafter, the Pt crucible was taken out from the furnace and the slag melt was quenched as the same manner with pre-melted slag sample manufacture. The temperature, where the viscosity of the slag was abruptly increased, was defined to be a critical temperature.
2.3. Experimental System and Procedure for in-situ ObservationA compacted disk of powder mixture prepared by the procedure shown in 2.1 was placed in a Pt crucible (ID 6 mm × Height 5 mm) and then the formation of precipitated compounds in slag was observed in the process of heating and cooling by CLSM in an Ar atmosphere (200 mL/min). Moisture and oxygen in Ar gas were removed as the same manner as that for the preparation of pre-melted slag previously. After completing the in-situ observation for each disk by CLSM, a Field-emission Scanning Electron Microscope (FE-SEM) equipped with a Energy Dispersive X-ray (EDS) was employed to observe the microstructures and identify the phases in each sample.
Figure 1 shows the in-situ observation results with CSLM for Slag A (41.2 mass% CaO, 30.5 mass% SiO2, 17.3 mass% Al2O3, 10.0 mass% MgO, 1.0 mass% TiO2, CaO/SiO2 of 1.35) in the process of heating and cooling. In the process of heating up to 1823 K, the disk of powder mixture started to melt at 1668 K and then fully melted at the temperatures of higher than 1763 K. The circles observed in the pictures are all bubbles. Therefore, there were no solid compounds left over 1763 K. In the process of cooling, any solid compounds did not be appeared and the melt was solidified as glassy phase below 1587 K under the relatively fast cooling rate.

In-situ observation for Slag A during heating and cooling process.
In the present study, added titanium oxides in slags can be existed as Ti4+, Ti3+ and/or Ti2+ ions (TiO2, Ti2O3 and/or TiO in the oxide form) depended on the basicity, temperature and pO2. However, it is hard to determine the amounts of each titanium ions in slag. Thus, in the present study, the various Ti contained slag system was represented by using the initially added TiO2 content such as CaO–SiO2–Al2O3–MgO–x%TiO2 system instead of CaO–SiO2–Al2O3–MgO–TiOn system. For example, “CaO–SiO2–Al2O3–MgO–2%TiO2 slag system” simply means that the slag was prepared by adding 2 mass% of TiO2 to the CaO–SiO2–Al2O3–MgO slag system. Namely, the description “slag with x%TiO2” in the present study does not means that x% of “TiO2” is existed in slag but it is the slag with initially n% of TiO2 was added to the master slag. The amounts of Ti4+, Ti3+ and Ti2+ ions in slag can be constant once temperature, basicity and pO2 are fixed.
Figure 2 shows the projection of iso-basicity (CaO/SiO2 of 1.35) line for Slag A with pO2 of 1×10–16 atm, which is simulated from FactSage. According to the FactSage calculation, the slag exists as a fully liquid melt at the temperatures higher than 1743 K. However, spinel can be possibly formed even at 1723 K under the slow cooling rate. This calculation result is in good agreement with the experimental result for Slag A. (cooling rate is fast)
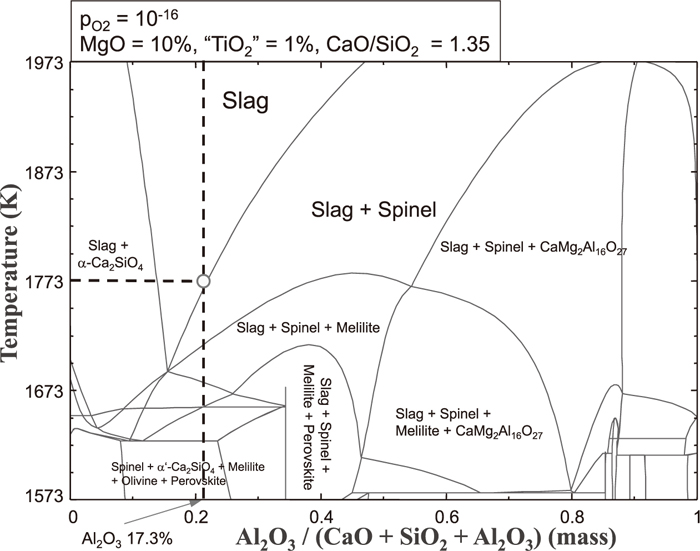
Projection of 1.35 iso-basicity line for Slag A (PO2 = 10–16 atm).
Unlike Slag A, in the case of Slag B (38.3 mass% CaO, 28.4 mass% SiO2, 17.3 mass% Al2O3, 15.0 mass% MgO, 1.0 mass% TiO2 and CaO/SiO2 of 1.35), slag compounds were observed in the slag even at high temperature of 1823 K. Until the temperature reached to 1723 K, the slag was not melted. The small solid crystals were came appeared as soon as the slag was partially melted at 1723 K and did not melt even though the temperature was increased up to 1823 K. Figure 3 shows the projection of iso-basicity (CaO/SiO2 of 1.35) line for Slag B with pO2 of 1×10–16. Spinel can appear in the temperature range below 1823 K and the region, where crystal compound can be coexisted, is expanded to higher temperature zone with increasing Al2O3 content. The quenched Slag B was analyzed by using FE-SEM equipped with EDS. From FE-SEM images, they were consisted of two bright and dark phases. The resuls of EDS analysis is shown in Table 3. The point A and C (drak phase) will be corresponded to the spinel phase, and point B (bright phase) will be the bulk slag phase.
Projection of iso-basicity (CaO/SiO2 of 1.35) line for Slag B (PO2=10–16 atm).
| Atomic% | O-K | Mg-K | Al-K | Si-K | Ca-K | Ti-K |
|---|---|---|---|---|---|---|
| Point A | 50.7 | 15.0 | 34.3 | |||
| Point B | 35.0 | 7.3 | 3.3 | 20.9 | 32.8 | 0.7 |
| Point C | 51.3 | 14.5 | 31.4 | 1.8 | 1.0 |
The melting behavior of Slag C (37.1 mass% CaO, 29.7 mass% SiO2, 17.3 mass% Al2O3, 15.0 mass% MgO, 1.0 mass% TiO2 and CaO/SiO2 of 1.25) was almost the same with Slag B. Figure 4 shows the projection of iso-basicity (CaO/SiO2 of 1.25) line for Slag B with pO2 of 1×10–16. The phase diagram shown in Fig. 4 is almost similar to that of Fig. 2 except for the kinds of existing compounds. While monoxide and spinel exist as compounds in the temperature range over 1723 K for Slag B, only spinel exists as a compound in the case of Slag C.

Projection of iso-basicity (CaO/SiO2 of 1.25) line for Slag C (PO2=10–16 atm).
Figure 5 and Table 4 show the result of viscosity measurement for the slag samples (CaO-SiO2-15 mass%MgO-17.3 mass%Al2O3-xTiO2) with C/S 1.35. The compositions are shown in Table 1. All slag viscosities and critical temperatures were decreased with increasing TiO2 content. In the case of Slag_T0_B1.35 (TiO2 0 mass%, C/S 1.35), the viscosity at 1723 K was 4.94 poise and critical temperature was 1703 K. On the other hand, Slag_T5.0_B1.35 (TiO2 5 mass%, C/S 1.35) showed the viscosity of 2.22 poise at 1723 K and the critical temperature of 1658 K. Compared with the slag without TiO2, the viscosity of slag containing 5 mass% TiO2 at 1723 K decreased in half and the critical temperature appeared to be below 1673 K.

Effect of TiO2 content on slag viscosities (0–5 mass%).
| Sample | Viscosity at 1723 K (poise) | Critical Temperature (K) | TiO2 content (mass%) |
|---|---|---|---|
| Slag_T0_B1.35 | 4.94 | 1703 | 0 |
| Slag_T1.0_B1.35 | 3.43 | 1693 | 1.0 |
| Slag _T3.0_B1.35 | 2.90 | 1688 | 3.0 |
| Slag _T5.0_B1.35 | 2.22 | 1658 | 5.0 |
Kondratiev et al.18) said that the most important factors to be considered in estimating the viscosities of slags comprising solid and liquid phases are the volume fraction of solid and the coexisting liquid composition. Figure 6 shows the calculation results by the Factsage forth e volume percentage of coexisting phases in each slag sample. It indicates that the liquid portion rapidly falls down at relatively lower temperature with increasing TiO2 content. The temperature where the liquid fraction is rapidly decreased is in good accordance with the experimentally measured critical temperature of each slag. The critical temperature for each slag was noticed when the volume fraction of liquid phase reaches below 40–45%. From another view point, it is likely that the critical temperature is closely related with the appearance of merwinite phase. In case the liquid portion is sharply decreased, most of the reduced liquids transform to merwinite. The critical temperature of each slag seems to correspond to the temperature that the fraction of merwinite reaches more than 35%, and the appearance of merwinite is delayed with increasing TiO2 content.

Volume fractions of slag constituents depending on TiO2 content under the pO2 of 1×10–16 atm (calculated from FactSage).
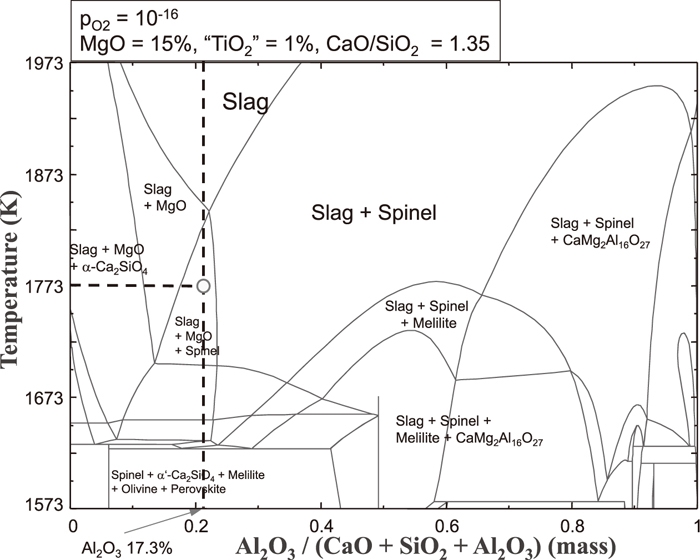
According to the FactSage calculation shown in Fig. 7, spinel is not likely to be formed within the current operational condition in CaO–SiO2–Al2O3–MgO–TiO2 slag system (5 mass% MgO, 0.5 mass% TiO2, less than 17 mass% Al2O3, and 1.35 basicity) with pO2 of 1×10–16 at 1773 K. Even if MgO content is increased up to 10 mass% with 1 mass% TiO2, no spinel is found to be formed as long as Al2O3 content is less than 19.6 mass% at the basicity of 1.35 from FactSage evaluation. However, as shown in Fig. 8, if the MgO content is increased up to 15 mass%, spinel can be formed in the slag composition containing more than 14.7 mass% Al2O3 depending on the basicity. As Al2O3 content is increased, the basicity range to form spinel is enlarged. If Al2O3 is more than 19 mass%, spinel can be formed in the quite wide basicity range below 1.35. As shown in the projection for 1.35 iso-basicity line with pO2 of 10–16 in Fig. 3, the temperature range where spinel can exist as a crystal compound in the slag melt is increased with increasing Al2O3 content. Spinel formation estimated by the FactSage calculation was confirmed in terms of in-situ observation and EDS analysis as represented in 3.1. According to these results, spinel can be formed and exist even at 1823 K for the CaO/SiO2 of 1.25 and 1.35 when Al2O3 content is increased to more than 17 mass% in the condition of 15 mass% MgO and 1 mass% TiO2.
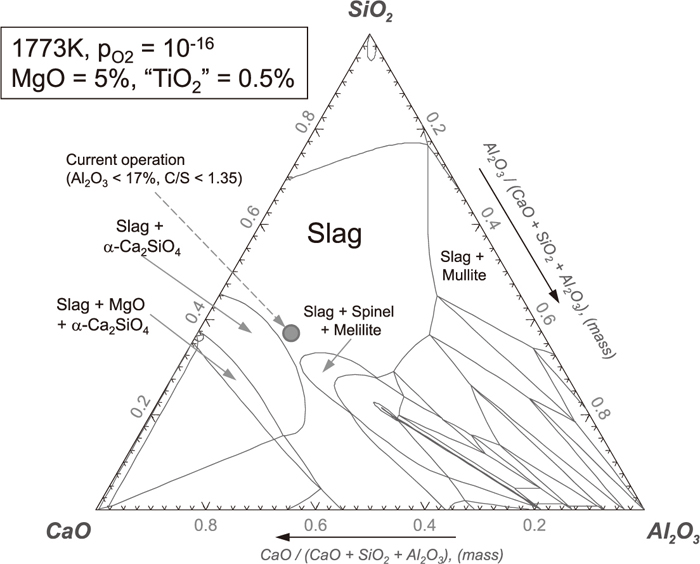
Phase diagram of CaO–SiO2–MgO–Al2O3–TiO2 slag system with 5 mass% MgO and 0.5 mass% TiO2 at 1773 K (PO2=10–16 atm).

Phase diagram of CaO–SiO2–MgO–Al2O3–TiO2 slag system with 15 mass% MgO and 1.0 mass% TiO2 at 1773 K (PO2=10–16 atm).
As already mentioned, it can be expected that TiO2 addition to CaO–SiO2–MgO–Al2O3–TiO2 slag may lower the slag viscosity because the TiO2 content in real BF slag is less than 2 mass%. The experimental results obtained in the current study are in good agreement with the previous works of less than 2 mass% TiO2 bearing slags.13,14,15) As already shown Fig. 5 and Table 3, slag viscosity was decreased with increasing TiO2 content from 0 to 5 mass% in the CaO-SiO2- 15 mass%MgO-17.3 mass%Al2O3-xTiO2 slag. Because TiO2 content in real BF operation is less than 2 mass%, TiO2 seems to act as a network modifier in BF slag.
In the previous section, the sutable range of the slag compositions for the spinel formation was found out. The formed spinel in the slag phase may deteriorate the slag fluidity. However, the spinel formation is supposed to manly occur at the refractry surface in the hearth as like the Ti bear since the wall temperature is a little bit lower than the slag temperature. Therefore, it is expected that the spinel formation in the slag phase will not practically influence the slag flow in the hearth.
4.3. Effect of TiO2 Content on the Critical TemperatureHandfield et al.9) said the increase of Ti2O3 made the liquidus temperature higher, however, Gruzdev et al.16) reported the opposite effect. In both cases, the ratio of Ti4+ to Ti3+ is the crucial factor to affect liquidus temperature. As shown in Fig. 9, a single liquid phase is existed for 1 mass% TiO2 and two liquid phases are supposed to coexist for 3 mass% and 5 mass% TiO2. Table 5 shows the relative ratio of TiO2 to Ti2O3 in the liquid with TiO2 contents at 1873 K, which was calculated from FactSage for three different slags. In both the liquid 1 and liquid 2, the ratio of TiO2 to Ti2O3 is decreased with TiO2 content. The calculated liquid miscibility for high TiO2 bearing slag, however, is not yet confirmed experimentally. Thus, further work will be necessary for the confirmation of the liquid miscibility in these slags. According to the calculation results, the ratio of (mass% TiO2/mass% Ti2O3) is decreased with TiO2 addition in slag melts with pO2 of 1×10–16. As shown in Table 4, the critical temperature was lowered as TiO2 content was increased. This means that the liquidus temperature is decreased with increasing Ti2O3 content or Ti3+ fraction in the CaO–SiO2–MgO–Al2O3–TiO2 slag. The experimental results are in good accordance of Gruzdev et al.16)

Projection of 1.35 iso-basicity line of CaO–SiO2–MgO–Al2O3–TiO2 slag system with 15 mass% MgO and 17.3 mass% Al2O3 (PO2=10–16 atm).
| Sample | (mass% TiO2/mass% Ti2O3) in the liquid at 1873 K | ||
|---|---|---|---|
| Slag | Slag’ | Ave | |
| Slag_T1.0_B1.35 | 1.389 | – | 1.389 |
| Slag _T3.0_B1.35 | 1.312 | 0.103 | 1.284 |
| Slag _T5.0_B1.35 | 1.241 | 0.098 | 0.796 |
Ito et al.17) reported that the (pct Ti3+)/(pct Ti4+) ratio increases with increasing the slag basicity at CaO/SiO2 ratios below unity while it decreases at CaO/SiO2 ratios above one.
On the other hand, Y. Morizane et al.7) proposed that the (mass% Ti4+)/(mass% Ti3+) ratios increased with increasing the slag basicity over the entire basicity range. Table 6 shows the relative ratio of TiO2 to Ti2O3 in the liquid at 1873 K under the pO2 of 1×10–16 atm which were calculated from FactSage for two slag samples with basicity of 1.25 and 1.35, respectively. The ratio of (mass% TiO2/mass% Ti2O3) is increased with increase of te slag basicity, which is in good agreement with those of Morizane et al.7) and Ito et al.17) Since pO2 was fixed, the change of the (mass% Ti4+)/(mass% Ti3+) ratio was is simply due to the effect of basicity.
| Sample | (mass% TiO2/mass% Ti2O3) in the liquid at 1873 K |
|---|---|
| Slag_T1.0_B 1.25 | 1.225 |
| Slag_T1.0_B 1.35 | 1.389 |
Figure 10 also shows the measured viscosities of those two samples. As shown in the figure, critical temperature was lowered from 1703 K to 1673 K with decrease of the slag basicity from 1.35 to 1.25. The Ti3+/Ti4+ is fixed by the the basicity under constant pO2. Thus, the decerase of the critical temeprature with decerase of basicity simply means that the critical temperature was also lowered with decrease of Ti3+/Ti4+. This result is well agreed with that in 4.3, which is also similar to the experimental results of Gruzdev et al.16)

Slag viscosities with the basicity of 1.25 and 1.35 (under 1 mass% TiO2) under the pO2 of 1×10–16 atm.
According to the experimental results under the constant pO2 of 1×10–16 atm, the increase of TiO2 in the CaO–SiO2–MgO–Al2O3–TiO2 slag with the same basicity of 1.35 lowered slag viscosity and critical temperature as shown in Fig. 5 and Table 3. These results can be explained by the relative ionic potential of Ti3+ and Ti4+. Because the ionic potential of Ti3+ is smaller than that of Ti4+, it is expected that Ti3+ is much stronger modifier than Ti4+. Thus, slag viscosity and critical temperature were lowered when the ratio of Ti4+ to Ti3+ was decreased. In the same manner, as shown in Table 5 and Fig. 10, the critical temperature was decreased with decreasing the ratio of Ti4+ to Ti3+ when slag basicity was decreased from 1.35 to 1.25. This indicates that Ti3+ acts as a relative stronger network modifier than Ti4+ does.
In this study, the spinel formation in slag melt was investigated by using in-situ observation and FactSage calculation to examine the possibility of protective layer formation from slag phase onto the wall and bottom of BF hearth. At the same time, the slag viscosity for CaO-SiO2-15 mass% MgO-17.3 mass%Al2O3-xTiO2 slag was continuously measured from 1773 K to 1623 K by a viscometer to clarify the effect of spinel formation by TiO2 addition on the viscosity and critical temperature. From the experimental results, the optimal slag composition to form spinel was proposed considering slag fluidity. From these findings, the following conclusions were obtained.
(1) Spinel (m.p. 2403 K) was formed in CaO-SiO2-15 mass%MgO-17.3 mass% Al2O3-1.0 mass%TiO2 (C/S 1.35 and 1.25) slag and it existed as crystalline phase at the slag temperature of 1823 K.
(2) The slag viscosities and critical temperatures for CaO-SiO2-15 mass%MgO-17.3 mass%Al2O3-xTiO2 with C/S 1.35 decreased with increasing TiO2 content. The ratio (%TiO2/%Ti2O3) is decreased with increasing TiO2 addition to slag. It means that the critical temperature is decreased as Ti2O3 content is increased in the CaO–SiO2–MgO–Al2O3–TiO2 slag system.
(3) With decrease of the slag basicity in CaO-SiO2-15.0 mass%MgO-17.3 mass%Al2O3-1.0 mass%TiO2 was decreased from 1.35 to 1.25, the critical temperature was lowered from 1703 K to 1673 K, and the estimated mass%TiO2/mass%Ti2O3 ratio in the liquid at 1873 K by FactSage are decreased.
(4) Since the ionic potential of Ti3+ is smaller than Ti4+, the slag viscosity and critical temperature are increased with increasing the relative ratio of Ti4+ to Ti3+.
(5) The suitable slag composition to form spinel might be placed in the slag basicity range of 1.25–1.28 with 15 mass% MgO and more than 17 mass% Al2O3.