2013 年 53 巻 10 号 p. 1894-1901
2013 年 53 巻 10 号 p. 1894-1901
Currently in Japan, 15 million tons of steelmaking slag as a by-product of the steelmaking process is produced annually. More than 60% of the steelmaking slag is used in civil construction. steelmaking slag has special properties which are presently under-exploited. Therefore, research into the greater utilization of the special characteristics of steelmaking slag in coastal environments has been undertaken over the last 20 years. It is known that steelmaking slag can reduce hydrogen sulfide in seawater. Hydrogen sulfide is highly toxic and fatal to benthic organisms. It also depletes oxygen and generates blue tide.
The purpose of this study is to evaluate and demonstrate the effects of removal of hydrogen sulfide in seawater by steelmaking slag. Both the laboratory and the field experiments showed that steelmaking slag removed the hydrogen sulfide from seawater and reduced the concentration of hydrogen sulfide in sediment. The field experiments also indicated that steelmaking slag changed the anaerobic condition of sediment into an aerobic condition. The results imply that effective utilization of steelmaking slag in coastal area restoration can significantly improve the surrounding marine environment.
Among the many by-products of the steel manufacturing process, Japan produces 40 million tons of iron and steel slag each year. Although near-100% reuse was achieved from an early date, research and development are underway aiming at effective utilization with higher added value. Concerning iron and steel slag, 25 million tons/year of blast furnace slag is produced in the ironmaking process, and more than 60% of this is used as a raw material for blast furnace cement, taking advantage of its hydraulic property. In addition to being a high value-added product, this also makes an important contribution to CO2 reduction. On the other hand, 15 million tons/year of steelmaking slag is generated in the steelmaking process, of which 60% is recycled in road construction and civil works. Although high value-added use has been realized blast furnace slag, similar applications that utilize the special properties and composition of steelmaking slag remain under-exploited.1) In response to this situation, recent research has focused on the iron content of steelmaking slag, which approaches 20%, and rocky shore denudation measures2) and bottom sediment improvement measures for enclosed sea areas3) using the Fe ions eluted from steelmaking slag have been reported.
In dredging hollows in enclosed sea areas and in sea areas with accumulated bottom sludge, hydrogen sulfide is generated as a result of decreased oxygen solubility accompanying higher water temperatures in summer, stagnation of water masses, and oxygen deficiency due to decomposition of organic matter deposited in the bottom sediment. Hydrogen sulfide causes problems such as deterioration of the biological environment. As one example, Tokyo Bay contains numerous former dredging sites where the dissolved oxygen concentration decreases remarkably in summer, and blue tides are an annual occurrence.4,5,6) Based on these conditions, when restoring dredging sites, which have become an origin of oxygen-deficient water masses that cause blue tide, the Council for Transport Policy of Japan’s Ministry of Land, Infrastructure, Transport and Tourism (MLIT) reported that it is necessary to actively promote the use of recycled materials, etc., in addition to soil and sand dredged from ports and harbors.7) Large amounts of sand capping material are necessary in order to backfill dredging hollows, which are one main cause of blue tide, and to improve bottom sediments. However, from the viewpoint of protection of the natural environment, it is not desirable to require use of natural mountain sand or crushed stone in these materials. Hence, use of industrial byproducts and recycled materials is considered necessary. In terms of supply capacity, steelmaking slag, which is a by-product with an annual amount exceeding 10 million tons, as mentioned above, and coal ash, which is generated by coal-fired thermal power plants at a rate of more than 10 million tons/year, are considered leading candidates.
The fact that these materials are more effective than natural materials in suppressing sulfide generation has also attracted attention.8,9,10) Asaoka and Yamamoto studied removal of sulfide ions by granulated coal ash, in which 10–15% of blast furnace slag cement was added to fly ash during granulation, and analyzed the mechanism of the decrease in sulfide ions not only by adsorption, but also by formation of pyrite (FeS2) and formation of sulfur (S0).8) The sulfide reduction effect of steelmaking slag has been evaluated by measuring the sulfides in artificial seawater after addition of slag to artificial seawater in which sodium sulfide was dissolved and shaking for a certain time.10) However, as this experiment was performed by adding slag to a solution with a sulfide ion concentration of 10 mg/L under conditions in which the solid-liquid ratio was from 1:100 to 1:10, the results are limited to conditions where the slag ratio is high in comparison with the case assuming use of steelmaking slag in actual seas. Moreover, knowledge of the mechanism of sulfide absorption by steelmaking slag and the sulfide suppression effect of introducing steelmaking slag in actual waters was still inadequate. In order to use steelmaking slag to backfill former dredging sites and improve bottom sediments in enclosed waters in the future, verification of its effect in suppressing hydrogen sulfide generation is indispensible. Research to elucidate the absorption mechanism and demonstration experiments to confirm the effect in actual sea areas are necessary.
To verify the applicability of steelmaking slag as a backfill material for dredging sites and a material for improvement of bottom sediments, in this research, sulfide adsorption tests using steelmaking slag were performed in the laboratory to confirm the absorption effect, and the mechanism was studied. A demonstration experiment was also performed, in which multiple types of steelmaking slag were placed on the sea bottom in the Asano Canal at the Port of Kawasaki. In that experiment, the sulfide concentration and dissolved oxygen concentration were measured, and the sediment and water quality improvement effect were verified as a step toward practical application.
First, 310 ml of artificial seawater (Aquamarine; manufactured by Yashima Pure Chemicals Co., Ltd.) was aerated with nitrogen gas to a dissolved oxygen (DO) concentration of 2 mg/L or less, followed by addition of 22 mg-S/L of Na2S·9H2O, and the pH was adjusted to 8.2±0.1 with 1 normal (1N) HCl. This artificial seawater was poured gently into 250 ml polyethylene bottles, and 0.08 wt% of the steelmaking slags A, B, C, D, and E (particle size≦0.5 mm) shown in Table 1 was added, respectively. The seawater containing the slag was then stirred at 30 rpm with a rotary shaker at 20°C, and the dissolved sulfide concentration was measured at designated time intervals using a Kitagawa-type gas detection tube (200SA, 200SB; manufactured by Komyo Rigaku Kogyo K. K.).
With slag E, when the sulfide concentration decreased to below the detection limit, 22 mg-S/L of Na2S·9H2O was added, the pH was adjusted to 8.2±0.1 with 1N HCl, and sulfide absorption per 1 g of slag was calculated.
For comparison, the same experiment was performed under a condition (X) in which steelmaking slag was not added and a condition (Y) in which natural sand (Toyoura standard sand; particle size≦0.5 mm) was added instead of steelmaking slag.
2.1.2. Composition Analysis for Reaction Product from Steelmaking Slag and Sulfide IonsNext, 1.0 wt% of slag E (Table 1) adjusted to a particle size of 0.15–8 mm was added to a solution prepared in the same manner as in 2.1.1 and stirred at 30 rpm with the rotary shaker. Over a 3 day period, the same amount of Na2S·9H2O as in the initial stage was added at 24 hour intervals, and the suspended matter that formed in the artificial seawater was filtered after 96 hours.
Mass spectrometric analysis of the specimen collected on the filter paper was performed for Fe, Ca, Mg, and Al using acidolysis/inductively coupled plasma atomic emission spectrometry (ICP-AES; ICPS-8000; manufactured by Shimadzu Corporation), for Si by acidolysis/molybdenum blue absorptiometry (UV-1240; manufactured by Shimadzu), for S and C by the combustion/infrared absorption method (EMIA-620; manufactured by Horiba, Ltd.), and for O by the inert gas fusion infrared absorption method (EMGA-650; manufactured by Horiba). In addition, non-reflective X-ray diffraction (X’Pert MPD for Si non-reflective specimens; manufactured by Philips) and X-ray absorption fine structure (XAFS) (installed in beamline BL-10 of the Ritsumeikan University Research Organization for Science and Engineering Synchrotron Radiation Center) to investigate those properties. With the -granular shaped steelmaking slag used in forming black precipitates, Electron probe microanalyzer (EPMA) elementary mapping of the slag cross section was performed, and XAFS (same as above) was conducted (Fig. 1).

Process of the experiments testing sulfide removal capacity of steelmaking slags.
The experimental location was at the Asano Canal at the Port of Kawasaki, as shown in Fig. 2. This is an enclosed body of seawater with approximately 1.9 m of the average difference in the height of the tide, a water depth of about 5 m, and few currents. According to the results of water quality measurements by Kawasaki City in fiscal year 2008, the DO of the sea area in the Asano Canal District was 5.0 mg/L in the upper layer and 0.8 mg/L in the lower layer in summer (August), and Chemical Oxygen Demand (COD) was 3.8 mg/L in the upper layer. As with the materials used in the laboratory experiments, the specimens were four types of steelmaking slag A, B, C, and D shown in Table 1, which were cut into fine particles (particle sizes 13–30 mm). For comparison, natural crushed stone (particle sizes 13–30 mm) was used. 2 tons of slag A, B, C, D and natural stone were installed respectively in the gabions.

Location of the field experiments and layout of gabions containing various types of steelmaking slag and natural stone.
In preparing the experimental materials, the steelmaking slag was filled in metal gabions 2 m in length, 1 m in width, and 0.5 m in height. As shown in Fig. 2, the gabions were placed on the sea bottom at 3 m intervals in Cases 1–4, and at a 5 m interval in Case 5 for comparison with Case 4. Polyvinyl chloride (PVC) pipes were inserted into the gabions filled with the material in advance to enable sampling of the interstitial water.
2.2.3. Water Sampling and Analysis MethodSampling was performed at various points. Seawater taken from immediately above the gabions (5 cm) and immediately above the natural seabed (5 cm) in the test area and comparison area was termed “overlying water,” and seawater sampled using the PVC pipes inserted in the gabions before the experiment was termed “interstitial water.”
The overlying water was sampled underwater by a diver using a sampling container. Samples for sulfide analysis were fixed with NaOH in accordance with JIS K 01023.3 and placed in a container filled with water on the ship at the site, transported to the laboratory while water-sealed, and analyzed promptly after delivery to the laboratory. Interstitial water was sampled from the water-sampling PVC pipes using a syringe, fixed with NaOH and sealed in the syringe in the same manner as the overlying water samples, and transported to the laboratory in a water-sealed condition.
Bottom sludge in contact with the sides of the gabions and sludge on the original ground were sampled by a diver. Sludge samples for sulfide analysis were placed in a 100 mL polyethylene container. The sulfides were fixed with a tetraammine zinc (II) ion solution on the site vessel in accordance with Bottom Sediment Survey Method II 17 (Environmental Agency, Water Quality Bureau, Water Quality Management Division No. 127, Sept. 1988), and other sludge samples were placed in 1.1 L polypropylene containers. These samples were transported to the laboratory in a cold dark condition, in a cooler box filled with ice, and were measured promptly after delivery to the laboratory.
The dissolved sulfide concentration, DO concentration, and pH of the overlying water and interstitial water were measured in accordance with JIS K0102 39.1, JIS K0102 32.1, and JIS K0102 12.1, respectively. The oxidation-reduction potential (ORP) of the overlying water and interstitial water was measured by the platinum electrode method (Water Pollution Survey Guidelines 5.14), and the hydrogen electrode standard value was calculated using the single electrode potential of a reference electrode (Ag–AgCl/3.3 mol/L KCl). The ORP of the original ground was measured by direct insertion of an electrode (PST-5721C; manufactured by DKK-TOA Corporation), as it was difficult to sample the interstitial water using a syringe.
The sulfide concentration in the bottom sludge was analyzed in accordance with the iodometric titration method (Bottom Sediment Survey Method II 17, as mentioned above). In determining the dissolved iron concentration in the overlying water, the iron concentration in seawater filtered through a 0.45 μm filter (hereinafter, this filtrate is referred to as dissolved iron) was captured and concentrated using a separation column (NOBIAS CHELATE-PA1; manufactured by Hitachi High-Tech Fielding Corporation) and analyzed using an electrically-heated atomic absorption spectrometer (AAnalysst600, PerkinElmer Inc.).
The experimental results are shown in Fig. 3. The sulfide concentrations of the solutions in the case of slag A, B and C addition decreased to below the detection limit after 24 h. With slag D, the sulfide concentration was 2 mg/L after 24 hr, and with slag E, the concentration was below the detection limit after 3 hr. On the other hand, the sulfide solution with no addition and the sulfide solution containing added natural sand both continued to show high values of 20 mg/L and 22 mg/L, respectively, after 24 hr. Thus, this experiment confirmed the sulfide reduction effect of steelmaking slag.

Change over time of dissolved sulfide concentration added with various types of steelmaking slag.
With slag E, an additional 22 mg-S/L of Na2S·9H2O was added under an initial condition which was below the detection limit. At 6 hr after addition, the concentration again decreased to below the detection limit. At this point, another 22 mg-S/L of Na2S·9H2O was added, and the concentration decreased to below the detection limit 15 hr after addition. The amount of S absorbed by slag E during this period was calculated at 73.7 mg/g-slag. This calculated amount of sulfide ion absorption is on a level near the saturation adsorption amount of 108 mg-S·g–1 of sulfide ions by granulated coal ash with addition of 10–15% of blast furnace slag cement to fly ash, as reported by Yamamoto and Asaoka.9)
Based on this result, as the reason why the sulfide concentration of the sulfide solutions containing added slag showed large decreases, while the sulfide solutions added nothing or natural sand both continued to show high sulfide concentrations after 24 hr, the possibility that insoluble sulfides and sulfur are formed by reaction of the iron component eluted from the slag with dissolved sulfides by the reactions shown in (1) and (2)11) is conceivable.
| (1) |
| (2) |
The result from chemical composition analysis for the reaction product (suspended matter captured on filter paper) formed when steelmaking slag (slag E) was introduced into artificial seawater in which the sulfide concentration had been adjusted using a reagent are shown in Table 2. The reaction product contains oxygen, iron, sulfur, calcium, and silicon. The results of non-reflective X-ray diffraction are shown in Fig. 4. Only peaks for simple S and crystals of CaCO3 could be observed. FeS and other crystals were not observed.

XRD pattern of suspended material after H2S absorption.
Where the slag is concerned, Fig. 5 shows the results of an investigation by EPMA elementary mapping of the cross sections of slag particles after reaction. While the distribution of S with Fe at the slag surface shows good agreement, the distribution of Ca shows virtually no agreement with the distributions of Fe and S.

SEM image and EPMA elementary mapping of the slag after H2S absorption.
The results of an XAFS analysis of the product on the filter paper and the surface of the slag particles after reaction are shown in Fig. 6. With the product on the filter paper, peaks that are agreement with the peaks of sulfuric acid ions (SO42–) and simple sulfur were confirmed, whereas in the slag particles after reaction, peaks consistent with FeS and sulfuric acid ions (SO42–) were observed.

Sulfur K-edge spectra of several sulfide compounds, slag particle with and without H2S absorption, and suspended material after H2S absorption.
Crystals of FeS and FeS2 were not observed in the results of the X-ray diffraction analysis of the product. On the other hand, at the surface of the slag, where reactions occur readily, the distribution of Fe and S showed good agreement, and in the results of the XAFS analysis, a peak consistent with simple sulfur was observed in the suspended matter on the filter paper, while a peak consistent with FeS was observed in the reacted slag particles. These facts suggest that elementary sulfur and amorphous compounds of sulfides and Fe are formed on the slag surface and in the solution by the reaction between steelmaking slag and dissolved sulfides. According to research by Asaoka et al., the decrease in the sulfide ion concentration in the presence of granulated coal ash is considered to be caused by formation of pyrite and formation of S on the granulated particle surface by catalysis.12) Because the granulated coal ash material contains Fe2O3, CaO, SiO2, and other substances which are also found in steelmaking slag, formation of FeS and S by a similar mechanism is also considered possible in the present case.
It may be noted that a peak consistent with sulfuric acid ions was observed in the XAFS analysis. However, because artificial seawater has a high content of sulfuric acid ions, it is not clear whether the sulfuric acid compounds were newly-formed or not. An experiment in which sulfides and steelmaking slag are added to aerated seawater containing no sulfuric acid and judgment as to whether oxidation of S proceeds as far as sulfuric acid by XAFS analysis of the product is conceivable.
3.2. Experiments in Actual Sea AreaThe monitoring results are shown in Figs. 7, 8, 9, 10, 11.
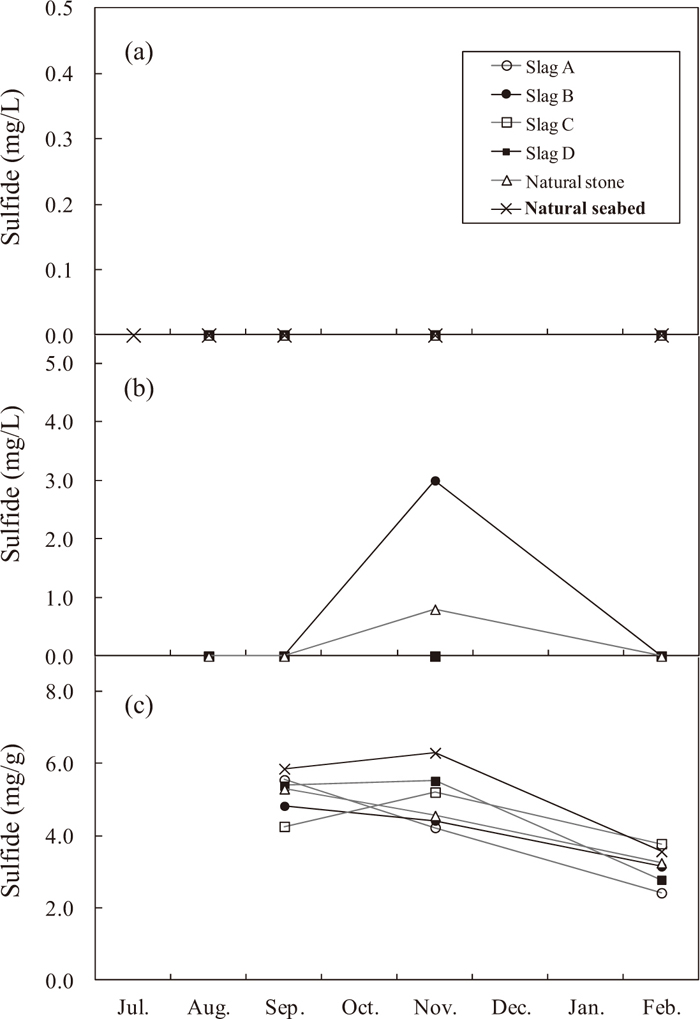
Monthly change of sulfide concentration (a) in the overlying water (5 cm above) and (b) in interstitial water of gabions containing various types of steelmaking slag and natural stone and (c) in surface sediment (5 cm to gabions containing various types of steelmaking slag and natural stone and natural seabed).

Monthly change of dissolved oxygen (a) in the overlying water (5 cm above) and (b) in interstitial water of gabions containing various types of steelmaking slag and natural stone.

Monthly change of Oxidation-Reduction Potential (a) in the overlying water (5 cm above) and (b) in interstitial water of gabions containing various types of steelmaking slag and natural stone (c) in surface sediment of natural seabed.

Monthly change of content of Fe ion in the overlying water (5 cm above).

Monthly change of pH (a) in the overlying water (5 cm above) and (b) in interstitial water of gabions containing various types of steelmaking slag and natural stone.
In the overlying water, sulfides were below the minimum limit of determination at all survey points (Fig. 7(a)). As the reason why the sulfide ion concentration was below the minimum limit of determination in all test areas (slag), the comparison area (natural stone) and the natural seabed, because the Fe ion concentration (Fig. 10) in this sea area was high, at 20–140 ppb, even though hydrogen sulfide evolved from the bottom sludge, it is considered that this H2S formed iron sulfide by reaction with Fe ions in the seawater, and furthermore, because the overlying water contained dissolved oxygen and had a high ORP, the sulfide ions were oxidized to sulfur and sulfuric acid ions.
The sulfide concentration of the interstitial water increased in the slag B area and the controlled area (natural stone) in November, as shown in Fig. 7(b), but was below the minimum limit of determination in all other months. As the reason why the sulfide concentration of the interstitial water was below the minimum limit of determination except in the areas containing slag B and natural stone in November, the following two possibilities are conceivable.
(1) Water permeability was good due to crevices in the slag and natural stone, resulting in an increased supply of oxygen, which accelerated the reaction with sulfides and reduced the sulfide concentration. (2) The sulfides reacted with Fe eluted from the slag, reducing the sulfide concentration.
Although the sulfide concentration of the interstitial water in slag B increased during November, there was no effect on the overlying water, and in comparison with the other test areas and the comparison area, the sulfide concentration in the bottom sludge in contact with the gabion containing slag B did not increase. As shown in Fig. 7(c), the sulfide concentration in the bottom sludge in contact with the sides of the gabions in the test areas (slag) and the comparison area (natural stone) showed low values in comparison with the concentration in the bottom sludge of the natural seabed (area where neither slag nor natural stone was placed), with the exception of slag C in February.
The following three points are conceivable as the reason why the sulfide concentration in the sludge in contact with the sides of the gabions in the test areas and the comparison areas was low in comparison with that in the natural seabed. (1) Water permeability was improved by the crevices in the slag and natural stone, and the seawater around the gabions formed an aerobic environment (Fig. 9). (2) The sulfide concentration in the vicinity was reduced by reaction of sulfides with Fe ions eluted from the slag. (3) pH increased in the vicinity of the slag (Fig. 11), suppressing the activity of sulfate-reducing bacteria living in the bottom sludge near the slag, thereby reducing generation of hydrogen sulfide.13)
3.2.2. Dissolved Oxygen (DO) and Oxidation-Reduction Potential (ORP)As shown in Fig. 8(a), in the entire study area, the DO of the overlying water was low in summer, at 3 mg/L or less, and increased to 4 mg/L or more from autumn. In August and November, the DO of the test areas was higher than that of the comparison areas. In general, no significant changes were observed in the DO of the interstitial water, which was 2 mg/L or less. However, in February the DO of slag C increased to 4 mg/L (Fig. 8(b)).
As shown in Fig. 9(a), no differences were seen in the ORP of the overlying water in the test areas and comparison areas, but the ORP of slag C increased in February. As shown in Fig. 9(b), the ORP of the interstitial water was high in the test areas in comparison with the comparison area in November. The ORP of the natural seabed trended in the range of approximately –200 to 0 mV, while in contrast, that of the test areas and the comparison area was 200 to 500 mV in both cases, confirming that the anaerobic atmosphere of the bottom sludge was improved by placement of the materials.
As the reason for the high DO of the overlying water in the test areas in August and November in comparison with that in the comparison area and natural seabed, it is considered that the supply of DO was increased by the improved water permeability resulting from placement of the slag, and the amount of hydrogen sulfide generation decreased, resulting in a decrease in DO consumption accompanying the oxidation reaction of hydrogen sulfide. The large increases in the ORP of the interstitial water in both the test areas and the comparison area in comparison with the natural seabed through the entire test period are attributed to the increased supply of oxygen from the seawater due to the improved water permeability resulting from placement of the slag and natural stone.
3.2.3. Fe Ion ContentAs shown in Fig. 10, variations were seen in the Fe ion concentration of the overlying water in the test areas, comparison area, and natural seabed in September and November. However, in February, there were no differences among the three areas, and no clear effect of the difference in the materials on the Fe ion concentration could be observed. The Fe ion concentration in this sea area was found to be high, at 20–140 ppb, in comparison with the concentration of 5–28 ppb in the East Oogishima Inlet sea area, where a seaweed cultivation experiment was carried out during the same period.14) Therefore, the fact that no remarkable differences were apparent in the waters around the slag test areas, even though Fe ions were eluted from the slag, is considered to be due to the extremely high background Fe ion concentration in the sea area studied here.
3.2.4. pHThe pH of the overlying water and interstitial water is shown in Figs. 11(a) and 11(b), respectively. No increase in the pH of the overlying water could be observed in either the test areas or the comparison area. Although the pH of the interstitial water in the test areas (slag) exceeded that of the comparison area (natural stone), the level was comparatively low, at no more than 9.5. The following are considered to be possible reasons why there was no difference in the pH of the overlying water in the test areas and comparison area.
(1) The relative surface area per unit weight of the steelmaking slag was small because the slag used here had been cut to a fine size (≦13 mm), thereby suppressing CaO elution from the slag, (2) The alkali content eluted from the slag was dispersed by currents of seawater.
3.3. Comparison of Results of Laboratory Experiment and Actual Sea ExperimentThe laboratory experiment was performed under an O2 free condition in order to simulate the interior of the bottom sludge bed. Based on the analysis of the reaction product, the fact that H2S decreased when steelmaking slag was added under this condition is considered to be due to the above-mentioned reactions (1) and (2). When natural stone was added under the same anoxic condition, these reactions did not occur, and there was no decrease in H2S.
In contrast, at the Asano Canal, where the actual sea area experiment was carried out, a decrease was observed in the sulfide concentration of the bottom sludge which was in contact with the sides of the comparison area (natural stone area). It is conceivable that these results were also influenced by the high Fe ion content in this sea area. However, based on the fact that the concentration of dissolved oxygen increased, it is considered that the oxygen supplied from the seawater as a result of the increased water permeability due to crevices on the natural stone side had a large effect in accelerating reaction (3) or (4)15) and thereby reducing the content of sulfides in the bottom sludge in the vicinity of the natural stone.
| (3) |
| (4) |
In the test areas (slag part) of the Asano Canal, the concentration of dissolved oxygen in the overlying water tended to be high. Therefore, in addition to promotion of the reactions ((3), (4)) with sulfides by the improved permeability provided by the slag, which is the same as the effect of the natural stone, and the resulting decrease in sulfides, it is thought that the reaction of Fe ions contained in the slag with sulfides reduced the concentration of HS– without consuming oxygen, and as a result, the dissolved sulfide concentration of the interstitial water and the dissolved sulfide concentration of the sediment zone under the test area were reduced.
The demonstration experiment at the Asano Canal confirmed the effects of steelmaking slag and natural stone in suppressing generation of H2S. However, in examining the durability of these effects, it is necessary to consider different reaction modes. Namely, in the process in which floating mud settles to the bottom and the process in which the slag or natural stone settles into the bottom sludge after these materials are placed, it can be supposed that mud penetrates into the crevices in the slag or natural stone, the permeability of the materials is reduced, and oxygen is no longer supplied. In this anoxic state, a decrease in sulfides cannot be expected even if natural stone exists in the environment. However, if Fe ions are available, the results of the laboratory experiment suggest that generation of sulfides will be suppressed by reactions (1) and (2).
Based on the fact that the existence of FeS and S0 was confirmed by XRD and XAFS in the laboratory experiment in the anoxic state, there is a high possibility that reactions (1) and (2) occurred. In this case, if oxygen is supplied, it is considered that the FeS formed by reaction (1) will be oxidized to iron oxide and S0. In reaction (2), if the ORP is high, it is thought that the Fe2+ that is formed here will be reoxidized to 3+, and reaction (2) will occur again. This means that Fe functions substantially as a catalyst in suppressing sulfides, which is in agreement with the suggestion by Asaoka.12) In the experiment involving absorption of sulfides by granulated coal ash, even though the amount of decrease in sulfides greatly exceeded the decrease due to formation of FeS by Fe, it is possible to explain this larger decrease in sulfides if the catalytic action of Fe is taken into account. If the bottom sludge has a high content of Fe and/or FeO, it is thought that the H2S formed by sulfate-reducing bacteria will react rapidly with the Fe and/or FeO, producing FeS, and the dissolved sulfide content in the seawater will decrease. This decrease in sulfides contributes to a decrease in consumption of dissolved oxygen, and the accompanying rise in ORP and increase in the oxygen supply result in oxidation of FeS and formation of iron oxides and Fe ions, S0, S2O32–, and SO42–. Accordingly, if a large amount of Fe ions exists, it is thought that a large amount of HS– will be consumed to form S0, S2O32–, and SO42–, and generation of H2S in the bottom sludge will be suppressed over a relatively long period. This supposition also agrees with the results of measurements of iron-rich lagoons in various parts of world showing low contents of sulfides in the bottom sludge.16,17,18,19)
Based on the foregoing discussion, it is assumed that generation of H2S can be suppressed stably over the long-term by adding steelmaking slag, which has high contents of Fe and FeO, to bottom sediments, which have a high content of organic matter that readily forms H2S. In particular, by laying steelmaking slag in the bottom sediments in inner harbors, under cultivation rafts, and at former dredging sites, where the low content of Fe and large volume of organic matter are conducive to reductive formation of H2S, it would be considered the generation of H2S can be suppressed over the long term, contributing to prevention of blue tide and, in turn, recovery of habitats for benthic organisms.
A laboratory experiment on removal of sulfides using steelmaking slag was performed, and a demonstration experiment of this method was conducted in an enclosed sea. As a result, the following conclusions were obtained.
(1) The laboratory experiment clarified the fact that steelmaking slag is effective in reducing the content of sulfides in artificial seawater.
(2) The demonstration experiment in an enclosed sea showed that steelmaking slag reduces the concentration of sulfides in interstitial water in the steelmaking slag and in the bottom sludge in contact with the slag, and also improves the anaerobic atmosphere in bottom sediments in contact with the slag.
(3) The results of EPMA and XAFS analysis suggested the possibility that Fe in steelmaking slag absorbs sulfides in seawater.
(4) The possibility that Fe has a catalytic function in removal of sulfides from bottom sediments is conceivable. However, verification of this point and examination of the persistence of the sulfide removal function of steelmaking slag are issues for future research.
Following construction experiments in actual sea areas where H2S is generated in large quantities and verification of the results, use of steelmaking slag in improvement of bottom sediments at former dredging sites and in enclosed sea areas is planned.
Part of this research was carried out as a Low Carbon Model Project of Japan’s Ministry of Economy, Trade and Industry (METI). Kawasaki City provided cooperation in implementation of the demonstration experiment. In the XAFS measurements, the synchrotron radiation equipment of the Ritsumeikan University Research Organization of Science and Engineering SR Center was used and cooperation was provided by Mr. Misaki Katayama and Mr. Koji Nakanishi of the SR Center. The authors wish to take this opportunity to express their thanks to all concerned for their generous assistance.