2013 年 53 巻 10 号 p. 1902-1904
2013 年 53 巻 10 号 p. 1902-1904
In heat-treated steels, austenite grain size (AGS) affects the phase transformation kinetics during cooling and the mechanical properties correlated with microstructure. Numerous experimental and theoretical investigations on austenite grain growth in various alloy steels have been reported.1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31) Typically, the formation of austenite grains is initiated as the reheating temperature rises above the Ac1 temperature; thereafter, the grain size of austenite rapidly increases with temperature. Austenite grains also grow gradually when the steel is held at a certain reheating temperature, because the austenite formation depends on a diffusion-controlled transformation. The relationship between the austenite grain size and reheating conditions can be expressed as the following Arrhenius-type equation:
| (1) |
Several empirical models based on Arrhenius-type equations have been reported to predict the austenite grain growth for plain-carbon and low-alloy steels:20,24)
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
Precipitate-forming elements such as Nb, Ti, V increase strength through the formation of carbides or nitrides and suppress the growth of austenite grains, improving strength through the Hall-Petch effect.1,2,3,4,5,6,8,9,10,11,12,13,14,15,16,18,23,25,26,27) Among the models previously reported to predict austenite grain growth, few have considered the influence of the precipitate-forming elements in alloy steels. In the present work, a model was developed to predict the austenite grain growth during reheating including the effects of various alloying elements.
Experimental data on AGS over wide ranges of chemical composition and reheating conditions were collected from literature;1,2,4,8,9,10,13,14,15,16,17,18,19,20,21,22,23,24,27,28,30,31) the 457 data collected are summarized in Table 1, including the minimum, maximum, and mean values of alloying element content, reheating temperature, holding time, and measured AGS. Based on this experimental data, a new empirical model based on an Arrhenius-type equation was proposed for predicting austenite grain growth:
| (8) |
| Min | Max | Mean | |
|---|---|---|---|
| C (wt.%) | 0.03 | 1.1 | 0.565 |
| Ni (wt.%) | 0 | 3.47 | 1.735 |
| Cr (wt.%) | 0 | 2.99 | 1.495 |
| Mo (wt.%) | 0 | 0.45 | 0.225 |
| Cu (wt.%) | 0 | 0.52 | 0.26 |
| Al (wt.%) | 0 | 0.485 | 0.2425 |
| V (wt.%) | 0 | 0.27 | 0.135 |
| Ti (wt.%) | 0 | 0.14 | 0.07 |
| Nb (wt.%) | 0 | 0.11 | 0.055 |
| N (wt.%) | 0 | 0.024 | 0.012 |
| T (°C) | 800 | 1300 | 1050 |
| t (s) | 0.01 | 18000 | 9000 |
| AGS (μm) | 4.7 | 157.2 | 81 |
| i | pi | qi |
|---|---|---|
| Ni | –1.729 | –16185 |
| Cr | *** | –1132 |
| Mo | 0.223 | 2540 |
| Cu | –7.449 | –41949 |
| Al | 18.996 | 222088 |
| V | 1.350 | 39721 |
| Ti | –22.399 | –208274 |
| Nb | 148.515 | 1721974 |
| N | –410.616 | –4321929 |
Nb, V, Ti are carbonitride-forming elements. Austenite grain growth is retarded both by the solute drag effect of solute Nb and by the pinning effect of NbC.23) Also, precipitate particles reduce the rate of grain boundary migration.27) The solute drag effect is weaker for V than for Nb, but the presence of V-carbide also brings about fine austenite grains.11,25) TiN formed in liquid steel is very stable and also suppresses the growth of austenite grains.3,12) These three strong carbonitride formers are added not only individually, but also in combinations of two or all together.
Additional elements such as Cu, Al, N were considered in the proposed model. Cu addition is reported to be effective in austenite grain refinement, since Cu is segregated during reheating5) and consequently promotes austenite nucleation rate.29) Al reacts with N to form AlN, adding a drag force that suppresses the growth of austenite grains.6,9) The abnormal austenite grain growth is commonly observed when AlN is dissolved. N addition contributes to the suppression of austenite grain growth by forming nitrides or carbonitrides with elements including Ti, V, Al, Nb.4,9,26)
Figure 1 compares previous models in predicting the literature data on AGS given in Table 1 that was used to derive Eq. (8). These models distinctly overestimate the AGS in steels containing precipitate-forming elements, implying that the previous models cannot represent the effect of precipitate-forming elements in inhibiting austenite grain growth. However, the new proposed model predicts these data well as shown in Fig. 2; that is, the model successfully reproduces the experimental data that was used to optimize its parameters. All experimental data are predicted within an acceptable error range of ±40°C and most of the data (401 of 457) are accurately predicted within a narrow error range of ±20°C.
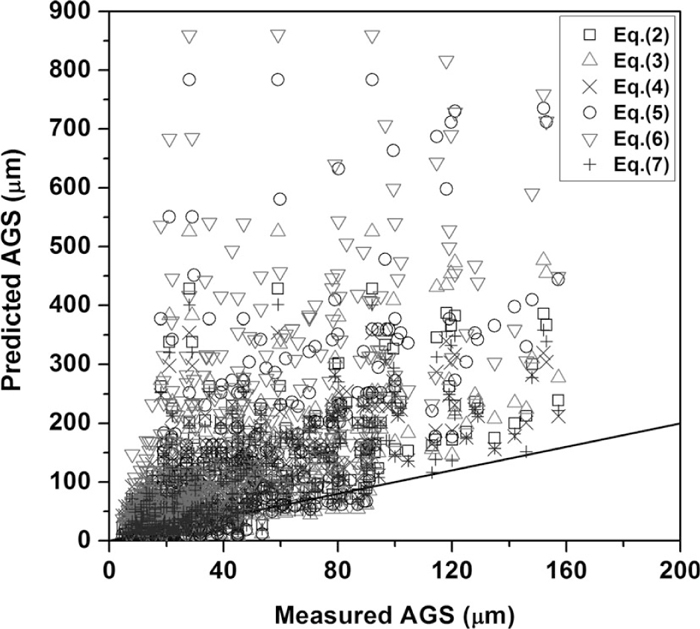
AGS predicted by previous models derived for carbon and low-alloy steels, compared to the experimental data referred in Table 1.
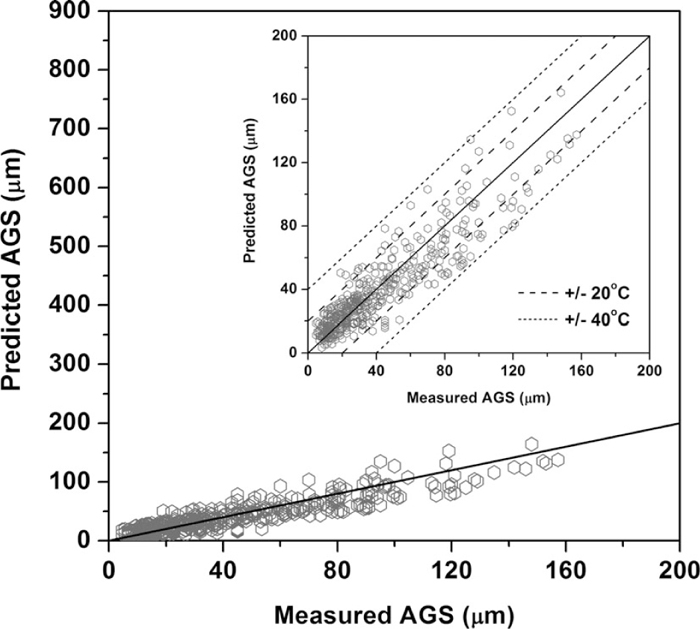
AGS predicted by the model proposed in the present study compared with experimental data.
Figure 3 shows the dependencies of austenite grain growth and growth rate on reheating temperature and holding time in a binary Fe–C steel with an average C content as indicated in Table 1. At a higher reheating temperature, the austenite grain growth rate was higher, and thus the resulting AGS was also higher. The austenite grain growth rate gradually decreased with holding time; this is because the driving force for austenite grain growth decreased with time, as the austenite grain growth continued and the total surface energy diminished at the grain boundary area.28) The proposed model expressed the physical behavior of the austenite grain growth well according to the reheating temperature and holding time.
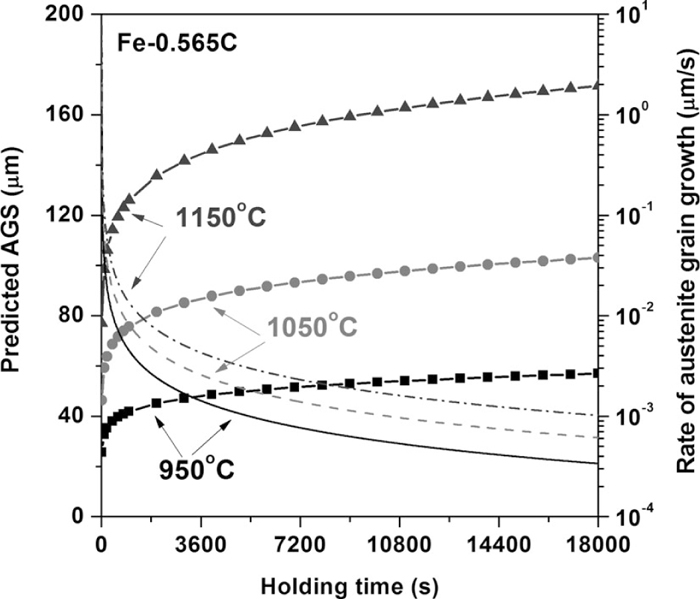
Predicted grain size variation (with symbol) and growth rate (without symbol) of austenite at different reheating temperatures.
The effects of various alloying elements on the variation of the austenite grain growth are compared in Fig. 4. The mean amounts of the alloying elements as indicated in Table 1 were used and the average reheating temperature of 1050°C was chosen. The addition of Ni, Cr, Mo reduced AGS approximately 50% relative to the binary Fe-0.565%C steel. Other elements are considered on the basis of the mean composition of low-alloy (L.A.) steel (Fe-0.565%C-1.735%Ni-1.495%Cr-0.225%Mo). The addition of 0.26% Cu was confirmed to be more effective in suppressing austenite grain growth than the addition of 0.2425% Al and 0.012% N. However, the composite addition of 0.055% Nb, 0.135% V, 0.07% Ti, and 0.012% N is expected to be most effective in suppressing austenite grain growth. This analysis was not an exact quantitative comparison because the real dissolution of precipitate is entangled with the amount and kind of carbonitride-forming elements as well as the reheating conditions. Nevertheless, this analysis provides a comparative analysis about the correlation between the austenite grain growth and the alloying element.
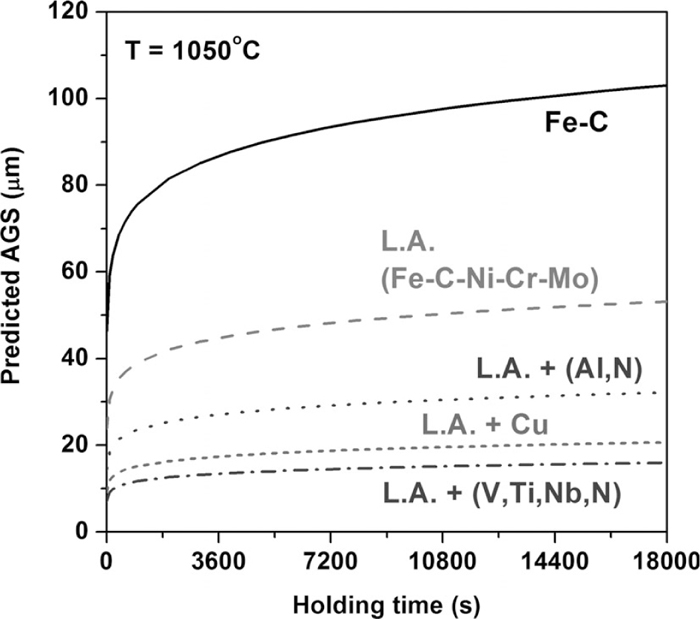
Effect of the alloying element on suppressing austenite grain growth.
To summarize, an empirical model has been proposed to predict the grain growth of austenite for reheating alloy steels by considering the effect of various alloying elements. The proposed model confirms that the austenite grain growth is suppressed by adding different alloying elements, whereas higher reheating temperatures increase the growth rate of austenite grains. In modeling the austenite grain growth, it is extremely difficult to fully account for precipitation phenomena because the thermodynamic kinetics of precipitate dissolution and formation is totally different depending on the kinds and amounts of carbonitride-forming elements. However, the new predictive model is a function of composition and reheating conditions only, regardless of precipitate dissolution and formation. It is expected that the present model will be useful to predict the austenite grain growth of alloy steels adequately without requiring any thermodynamic calculations for the precipitation and dissolution of carbonitride-forming elements.
This paper was supported by research funds of Chonbuk National University in 2012.