2013 年 53 巻 12 号 p. 2142-2151
2013 年 53 巻 12 号 p. 2142-2151
The formation and modification of non-metallic inclusions for Al-killed steel during Compact Strip Production Process (CSP Process) was studied by industrial experiments. The thermodynamics for the formation of Al2O3, MgO·Al2O3 spinel, various calcium aluminates and CaS bearing inclusion were analyzed with the help of calculation software FactSage. It is found that the oxygen activity in liquid steel during LF (Ladle Furnace) refining is confirmed to be determined by the equilibrium between dissolved Al and Al2O3 in inclusion. There are two manners for Al2O3 inclusion modification during LF refining: the first route is followed by Al2O3-low modified calcium aluminates-liquid calcium aluminates; and the other is as Al2O3– MgO·Al2O3 spinel-CaO–MgO–Al2O3 multi-component inclusion. In addition, the liquid calcium aluminates have the tendency to be multi-component inclusion. Two types of CaS bearing inclusion could precipitate in a slab during solidification. The favorable modified inclusions deform very well along with steel matrix during rolling process even if they are associated with CaS bearing layer, while the no or low modified Al2O3 based inclusion would be rolled into pieces and the micro-cracks might be generated around the inclusions.
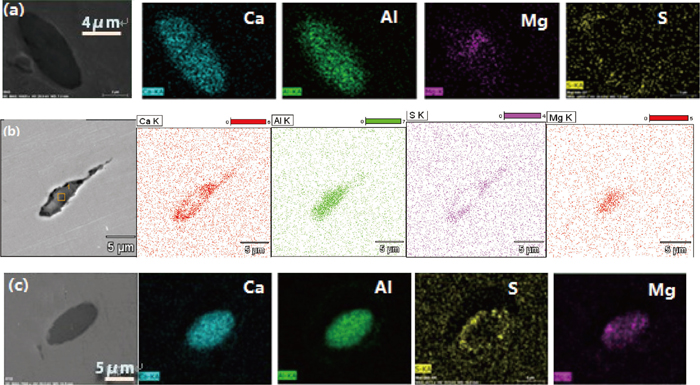
As a kind of secondary phase particle, non-metallic inclusions are detrimental to the continuity of steel and easy to cause stress concentration surrounding the inclusion during rolling process, in particular when the inclusion is irregular and hard. Consequently, inclusions are one of main causes for surface defects during rolling process. Figure 1 shows SEM-mapping images of a sliver defect observed on a cold strip, in which a large number of low modified calcium aluminates and MgO·Al2O3 spinel are detected, thus the defect is supposed to be caused by non-metallic inclusions. To modify these inclusions into ones with low melting point is beneficial to reduce stress concentration and improve product quality, thus many researchers have studied the inclusion formation and modification mechanism both in laboratory scale and plant scale.1,2,3,4,5) Ye et al.1) proposed that the inclusions in Al-killed steel were modified according to the following sequence as Al2O3–CA6–CA2–CA–CAx(liquid), where C means CaO and A denotes Al2O3 respectively, until the activity of Al2O3 was low enough that the precipitation of CaS in the outer layer was possible. Jiang et al.3) found that with reaction time increase, inclusions evolved from high melting point MgO·Al2O3 spinel or MgO based inclusions to lower melting point CaO–Al2O3–MgO multi-component inclusion which were characterized by high melting temperature inclusion cores surrounded by a lower melting temperature CaO–Al2O3 outer surface layer. Yang et al.4) proposed that it was difficult to modify MgO·Al2O3 spinel completely and a liquid calcium aluminate layer would precipitate surrounding the inclusion when the diameter of a MgO·Al2O3 spinel inclusion was larger than 5 μm. Deng et al.5) proposed that a CaO–Al2O3 layer would generate surrounding the inner CaO–Al2O3–MgO part during Al2O3–MgO system modified process based on their industrial trials.
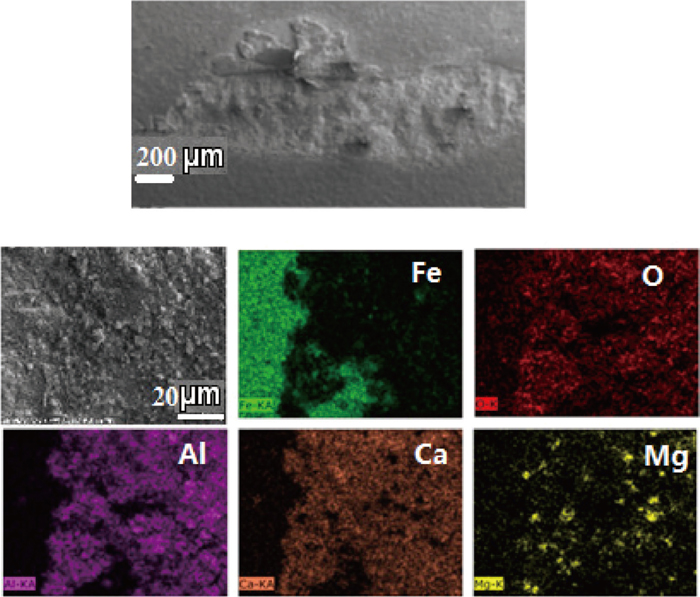
SEM-Mapping of a sliver crack appearing on a cold strip.
The CSP (Compact Strip Production) process is with high casting speed and thus the steel cleanness is much more difficult to control. Therefore, the negative effects of inclusion on surface quality for a cold strip are more serious. However, currently there are rarely deep and systematic researches on the inclusion formation and modification during CSP process, in particular there are nearly no report about the deformation of inclusion during CSP rolling process. This manuscript’s author and colleagues have done some works concerning the inclusion control during CSP process,6,7) but it is still far away from enough. In this manuscript, steel samples at different points during whole CSP process and the evolution of inclusion were studied by SEM-EDS (Scanning Electron Microscopy-Energy Dispersive Spectroscopy). Moreover, the thermodynamic analysis for various inclusion formation is performed in order to obtain the favorable conditions for controlling inclusion with low melting point. Furthermore, deformation of kinds of inclusions during CSP rolling process is studied to understand how to reduce the negative effects of inclusion on the surface quality of cold strips.
The industrial trials were carried out for low carbon aluminum killed steel (LCAK steel) coil with composition listed in Table 1 by CSP process in Jiuquan Iron & Steel Corporation (JISCO) in Gansu province, P. R. China. The steel grade was produced by the following process. During basic oxygen furnace (BOF) tapping, Al–Fe alloys were firstly added into liquid steel for strong preliminary deoxidation and the synthetic slag powder was next dipped for preliminary desulfurization. There are three stations for LF (ladle furnace) refining. At the pre-treatment station Al wires were fed for deoxidation once again. Thereafter, the ladle was shifted to treatment station, where high basic synthetic slag was added rapidly and then graphite electrodes were switched on to adjust the slag composition and liquid steel temperature. At the post-treatment station, calcium wire was injected for inclusion modification. A 30 ton tundish and funnel type mould are equipped in the caster, where the slab thickness approximates to 70 mm. A six stands mill is applied to hot rolling, where the open rolling temperature, the final rolling temperature, and the coiling temperature approximates to 1373 K, 1123 K and 873 K, respectively. In addition, a five stands mill is used for cold rolling, where the thicknesses of cold strips are between 0.7 mm to 3.0 mm. In the present trials, steel samples were taken out after aluminium wire was fed (LF start), before calcium treatment (LF intermediate), several minutes after calcium treatment (LF end) of LF refining, in tundish, casting slab, hot rolled strips and cold rolling strips, respectively. Ten heats out of two casting were sampled during LF refining and continuous casting.
| C | Si | Mn | P | S |
|---|---|---|---|---|
| 0.040–0.055 | 0.025–0.040 | 0.135–0.180 | 0.0080–0.0150 | 0.002–0.006 |
The activity of dissolved oxygen in liquid steel and steel temperature during LF refining were measured by an oxygen probe. Then steel and slag samples were prepared for chemical analysis. The composition of steel and top slag at the end of LF refining were as shown in Tables 2 and 3. The composition of steel samples was analyzed by ICP-AES (Inductively Coupled Plasma - Atomic Emission Spectrometry) method as well as Carbon/Sulfur analyzer. The composition of the slag was analyzed by an X-ray fluorescence spectrometer. Inclusions on the cross-section plane of each steel sample were detected and analyzed by SEM-EDS to obtain information of inclusions such as morphology, size and chemical compositions, etc.
| LF end | In slab | ||||||
|---|---|---|---|---|---|---|---|
| T/°C | a[O]/10–6 | [S] | [Al]s | [Ca] | [S] | [Al]s | [Ca] |
| 1603 | 3.2 | 17 | 251 | 54 | 21 | 188 | 30 |
| 1598 | 2.2 | 35 | 471 | 40 | 36 | 395 | 23 |
| 1595 | 2.0 | 26 | 290 | 34 | 30 | 244 | 24 |
| 1595 | 2.0 | 25 | 253 | 29 | 28 | 247 | 21 |
| 1600 | 3.1 | 28 | 282 | 34 | 31 | 255 | 21 |
| 1613 | 2.4 | 27 | 323 | 46 | 30 | 259 | 20 |
| 1596 | 2.6 | 55 | 364 | 31 | 59 | 299 | 22 |
| 1608 | 1.8 | 30 | 364 | 38 | 33 | 258 | 17 |
| 1597 | 3.6 | 53 | 382 | 30 | 51 | 318 | 18 |
| 1603 | – | 17 | 587 | 31 | 22 | 554 | 23 |
| Al2O3 | SiO2 | CaO | MgO | TFe | MnO |
|---|---|---|---|---|---|
| 24.5–35.7 | 1.6–5.6 | 52.9–60.2 | 4.1–6.5 | 0.4–1.5 | 0.03–0.33 |
At the start of LF refining after aluminum feeding, the observed inclusion are mainly alumina-based irregular inclusion shown in Fig. 2. As shown in Fig. 3(a), it seems there are two routes for Al2O3 inclusion modification in composition during LF refining: in the first route the MgO component in the inclusion increases and forms MgO·Al2O3 spinel; and in the second route, the CaO component increases and generates low modified calcium aluminates. At the end of LF refining after Ca-treatment, CaO in inclusion increases obviously and many inclusions are in or close to the liquid region, but there are still a number of inclusions with high melting point shown in Fig. 3(b). As shown in Fig. 3(c), marjority of inclusions in tundish have been modified into liquid region.
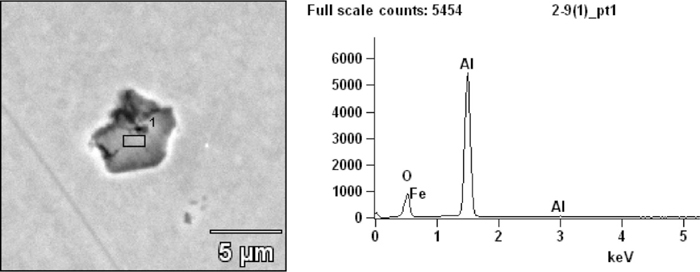
A typical Al2O3 inclusion observed after Al feeding.
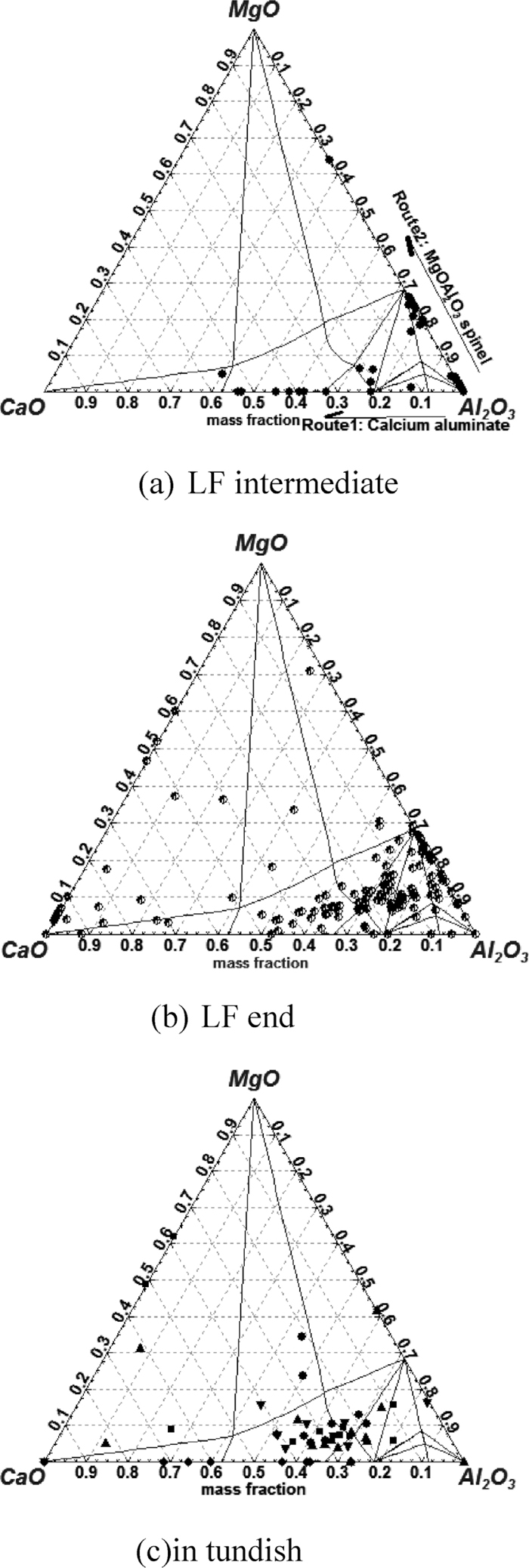
Inclusion composition prejected in CaO–MgO–Al2O3 ternary phase diagram at 1873 K.
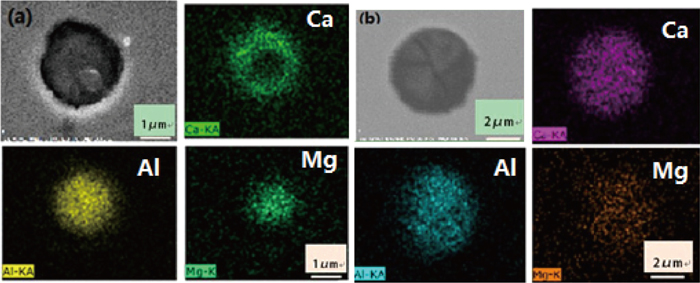
Figure 4 shows SEM-Mapping photos of two modified calcium aluminates after Ca-treatment during LF refining. As shown in Fig. 4(a), Ca distributes almost over the whole inclusion while Al mainly concentrates in the center of the inclusion. The EDS shows that the center is composed of lower modified calcium aluminate (mixture of CA6 and CA2) and the outer layer is better modified (mixture of CA2 and CA). As shown in Fig. 4(b) , the distribution of Al and Ca in this inclusions are both almost homogeneous. EDS results show it is composed of 48 mass% CaO and 52 mass% Al2O3, i.e., a typical well modified C12A7. In addition, it is interesting to find that there are some Mg concentrated regions in the inclusion surface. In the Mg bearing area, the Ca concentration is much less and the Al concentration is almost unchanged, so it seems that Ca rather than Al is reduced by Mg. The reasons will be explained in the following discussions.

SEM-mapping of calcium aluminates with different modified degree.
As shown in Fig. 5(a), Al distributes almost over the whole inclusions; relatively high Ca content concentrates in the outer surface while its content is obviously less in the center. Mg concentrated zone seems to be incompatible and complementary with Ca enriched layer. EDS result shows that the center is lower modified MgO·Al2O3 spinel, composed of 3.0 mass% CaO, 28.0 mass% MgO and 69.0 mass% Al2O3. The inclusion in Fig. 5(b) is composed of 45 mass% CaO, 1.0 mass% CaS, 7.0 mass% MgO, 43.0 mass% Al2O3 and 4.0 mass% SiO2, a well modified CaO–Al2O3–MgO–SiO2 multi-component inclusion with low melting point. In addition, elements distribute almost uniformly over the inclusion that shows a complete modification.
SEM-mapping of multi-component inclusion with different modified degree.
For Al-killed Ca-treated steel, the CaS bearing inclusion is another type of frequently observed inclusion. Figures 6 and 7 shows SEM-Mapping photos of two types of CaS bearing inclusion observed in slabs. Although they both appear a ring-shaped sulfur concentrated zone in the outer layer of inclusions, some difference will be found if one observes more carefully. As shown in Fig. 6, Ca concentrates in the outer layer of the inclusion and it is obviously weaker in the inner core, which is almost overlapped with the distribution of sulfur. Al and Mg concentrate in the inner irregular-shaped core and their areas are obviously smaller. The EDS for the inner core shows that the inner core is a low modified MgO·Al2O3 spinel, which is almost solid at casting temperature. As shown in Fig. 7, Ca, Al and O distribute almost over the whole inclusion and their areas are approximately the same in size and shape. The EDS for the inner core shows it is a well modified calcium aluminate, a mixture of CA and C12A7 and in liquid at casting temperature. In addition, oxygen also distributes in the outer layer of the inclusion that means outer layer is composed of not only CaS but oxides. The mechanisms of these two types CaS bearing inclusion will be discussed in the following.

SEM-mapping of a inclusion with CaS precipitation associating with low modified MgO·Al2O3 spinel based core observed in casting slab.

SEM-mapping of a inclusion with CaS precipitation associating with well modified calcium aluminate observed in casting slab.
Figure 8 shows some typical low modified inclusions observed in rolled strips with thickness between 0.8 mm to 2.0 mm. Some low modified calcium aluminates (Fig. 8(a)) and/or MgO·Al2O3 spinel (Fig. 8(b)) are rolled into two parts. Of course, some Al2O3 based inclusions (Fig. 8(c)) almost do not deform during rolling process, but micro-cracks generate associating with inclusion along the rolling direction. The micro-cracks are very harmful for the surface quality as well as steel service life as they are likely to be the origins of surface defects and fatigue crack. CaS bearing layer associating with oxides are likely to separate from the oxide core along the rolling direction (Figs. 8(d)–8(f)). This type of CaS bearing inclusion characterizes that the associated oxides are low modified inclusions with high melting points.

Morphology of some typical low modified inclusions during rolling process.
Figure 9 shows the deformation of three typical inclusions during rolling process. The first type (Fig. 9(a)) is a favorable modified multi-components inclusion. Its composition is 9.5 mass% MgO, 49.8 mass% Al2O3, 39.7 mass% CaO and 1.0 mass% CaS. The components are almost uniform over the whole inclusion excluding there is a small MgO concentrated region in the inclusion center. This inclusion have well deformed along with steel matrix. Figure 9(b) shows the deformation of the second type of typical inclusion, in which outer CaS-rich layer of the inclusion deforms obviously, whereas the inner core deforms very little. In addition, there is a CaS based tail behind the inclusion which is nearly taken off from the matrix inclusion. The EDS for the inner core shows it is a low modified MgO·Al2O3 spinel that is similar to that of the inclusion in Fig. 6. Therefore, this type of inclusion is supposed to originate from that type inclusion in Fig. 6. As shown in Fig. 9(c) the inclusion has well deformed and all the parts of the inclusion almost deform uniformly. The CaS rich layer is also observed clearly in the outer layer whereas it is not separated from the inner calcium aluminate core. The EDS for the inner core shows it is a well modified calcium aluminate, a mixture of CA and C12A7, which is similar to the inclusion in Fig. 7. Therefore, this type of inclusion originates from that type. Therefore, the well modified inclusion can also deform very well during rolling process even if CaS is precipitated with it.
Deformative performances of three types of inclusion during rolling process.
In order to better understand the inclusion control, the thermodynamic analysis for the formation and modification of various inclusions are performed. The activity coefficient of each element in liquid steel is calculated by Eq. (1) where
| (1) |
|
| C | Si | Mn | P | S | Al | O |
|---|---|---|---|---|---|---|---|
| S | 0.11 | 0.063 | –0.026 | 0.29 | –0.029 | 0.035 | –0.27 |
| Al | 0.091 | 0.0056 | 0.0065 | 0.033 | 0.03 | 0.045 | –6.6 |
| O | –0.436 | –0.131 | –0.021 | 0.07 | –0.133 | –3.9 | –0.2 |
| Mg | –0.24 | –0.09 | – | –1.38 | –0.12 | –289 | |
| Ca | –0.34 | –0.097 | –0.0156 | –0.097 | –336 | –0.072 | –9000 |
| i | S | Al | O | Mg | Ca |
|---|---|---|---|---|---|
| fi | 1.01 | 1.01 | 0.72 | 0.74 | 0.000131 |
The dissolved oxygen activity a[O] determined by the Al–Al2O3 equilibrium is expressed as,
| (2) 9) |
Thus, a[O] is obtained as
| (3) |
Usually, researchers assume the activity of Al2O3,
| Present work (estimating by FactSage) | Fujisawa et al. (experimental)11) | |||||
|---|---|---|---|---|---|---|
| Type | mass%CaO | a(Al2O3) | a(CaO) | mass%CaO | a(Al2O3) | a(CaO) |
| CA6 | 8.3 | 1.00 | 0.005 | |||
| CA2 | 23.3 | 0.29 | 0.10 | |||
| CA | 35 | 0.22 | 0.14 | 40 | 0.17 | 0.18 |
| C12A7 | 48 | 0.05 | 0.45 | 50 | 0.027 | 0.53 |
| C3A | 62 | 0.0087 | 1 | CaO-sat | 0.01 | 1 |
As show in Fig. 10, with Al2O3 inclusion modification to liquid calcium aluminates, the a[O] in equilibrium decreases gradually. This is as the Al–O reaction (2) will be boosted with decreasing activity of Al2O3 in inclusion. The measured a[O] at the start of LF refining almost all locate in the Al2O3 saturated region and that at the end of LF refining locate in the regions in equilibrium with calcium aluminates composed of between CA6 to CA. In fact, as shown in Fig. 2, the inclusions at the start of LF refining are almost pure Al2O3. As shown in Fig. 3(b), at the end of LF refining, most of the inclusions are modified into various calcium aluminates and majority of them are between CA6 to CA in composition. Those accord with theoretical calculations very well.
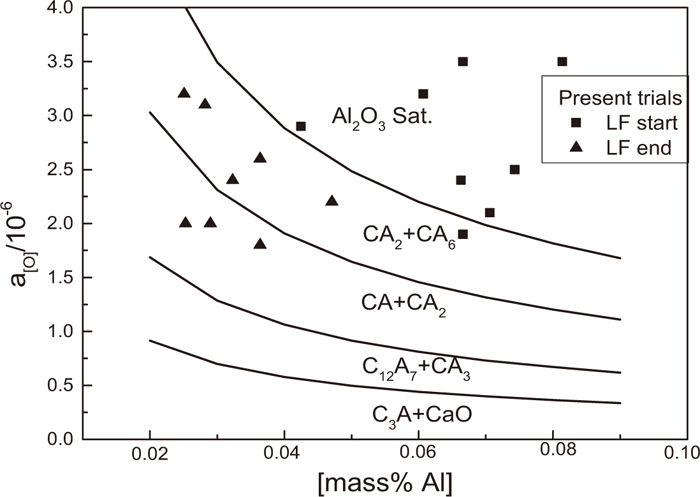
Relation between dissolved Al and activity of O at 1873 K in equilibrium with various calcium aluminates.
As the ladle refractory is lined with MgO–C brick, it is likely to form MgO·Al2O3 spinel during LF refining. Many researchers3,4,5,12,13) have studied the formation and modification of MgO·Al2O3 spinel. Equations (4) and (5) can be used to calculate the stability diagram of MgO, MgO·Al2O3 and Al2O3,
| (4) 12) |
| (5) 12) |
In the calculation, the activities of MgO·Al2O3 in MgO and Al2O3 saturations are taken as 0.8 and 0.47 respectively, and the activities of MgO and Al2O3 at saturated state are 0.99 and 1 respectively.13) It should to be pointed out that only the first order interaction coefficients are taken into account to calculate the activity coefficients of Mg and Al in present work, thus the present stability diagram shown in Fig. 11 appears somewhat different from that in Refs. 12) and 13). The stability phase diagrams can be applied to predict the correct steel composition for the formation of different oxides. For present LCAK steel (with composition shown in Table 1) with about 0.03 mass% dissolved Al, MgO·Al2O3 spinel inclusions are formed when the dissolved Mg in the steel ranges from approximating 0.2 × 10–4 to 10×10–4 mass%, and Al2O3 inclusions are formed only when the dissolved Mg lower than 0.2 × 10–4 mass%. It is very easy to form MgO·Al2O3 spinel during LF refining. In addition, Jiang et al.’s3) and Deng et al.’s5) experimental data were also plotted in the diagram. Jiang et al.’s data locate at the boundary between MgO and MgO·Al2O3 regions and Deng et al.’s data locate at the MgO·Al2O3 region. Interestingly, a number of MgO based inclusions were observed as well as MgO·Al2O3 spinel reported in Jiang’s experiments while only MgO·Al2O3 spinel without MgO based inclusion were reported in Deng et al.’s results due to the dissolved Mg content difference. Therefore, it shows the present calculations are very reasonable.
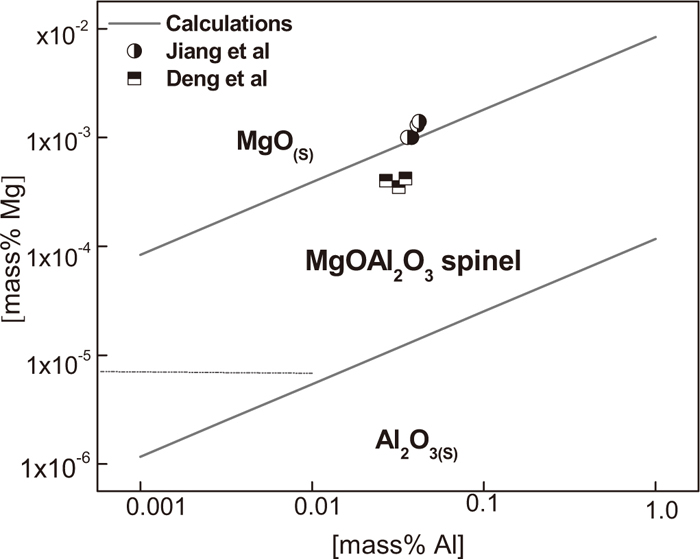
Stability diagram of Al2O3, ·Al2O3 spinel and MgO.
To analyze the formation and evolution of various calcium aluminates, the stability phase diagram of various calcium aluminates inclusion was drawn as shown in Fig. 12 following Taguchi et al.’s methods,14) where the solid dots denotes the present trial results. As shown, with the Ca content in liquid steel increase, Al2O3 can be modified to calcium aluminates with higher CaO content. In the case of dissolved Al is 0.03 mass%, CA2 starts to form when [Ca] is more than 0.5 × 10–4 mass%, CA starts to form when [Ca] is more than about 10.0 × 10–4 mass%, the liquid calcium aluminates starts to form when [Ca] is larger than about 20×10–4 mass%. Ref. 5) reported that the Ca during LF refining before Ca treatment is between 0.3–5.0 × 10–6, thus it is possible to form low modified calcium aluminate. The measured data in present industrial trials after Ca-treatment locate in liquid region on the whole approaching CA line. In addition, the measured a[O] between 1.8 × 10–6 to 3.6 × 10–6 are in good accordance with calculated values, i.e., approximating to 2.0 × 10–6 as well. As a result, it shows that the composition of liquid steel has been adjusted into the proper region in equilibrium with liquid calcium aluminates.
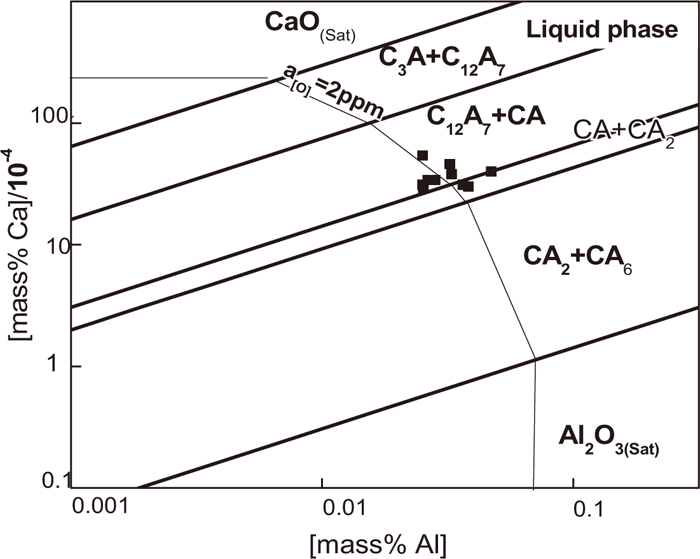
Stability phase diagram of various calcium aluminates.
CaS precipitation due to direct reaction between dissolved Ca and S after Ca-treatment can be expressed as
Thus the relationship between Ca and S for CaS precipitation is as
| (8) |
Figure 13 shows the relationship between dissolved Ca and S for CaS precipitation where the αCaS is considered as 1 in the calculations. It can be seen that majority of measured data at the end of LF refining locate below the curve of 1873 K, while the measurement in the slab are higher than the curve of 1823 K, i.e., casting temperature. As a result, it is more likely for CaS to precipitate directly in the mold during solidification with the temperature decrease. Furthermore, S concentration is higher in liquid core of a slab during solidification due to sulfur segregation.

Equilibrium between S and Ca for CaS precipitation at different temperature.
The well modified calcium aluminates have high sulfide capacity, thus they can also desulfurize and form CaS bearing inclusion. With this manner for CaS bearing inclusion precipitation, the chemical reaction is generally expressed as reaction (10) for Al killed steel. The standard Gibbs energy is obtained by coupling the reactions (2), (6) and (7).
| (9) |
Thus the relationship between S and Al for CaS bearing inclusion precipitation is as
| (10) |
Figure 14 shows the relationship between dissolved Al and S for CaS bearing inclusion precipitation with various calcium aluminates. It can be seen that majority of experimental results at the end of LF refining locate between the curves of C12A7+CaS and that of C3A+CaS, and more measured data in mold locate between the curves of C12A7+CaS and CA+CaS. So it is much easier to precipitate CaS bearing inclusions in this manner in mold during solidification as well. However, very high dissolved Al is required for this type of CaS bearing inclusion precipitation associating with CA so it is unlikely to precipitate CaS in this manner when alumina inclusion is not favorable modified.
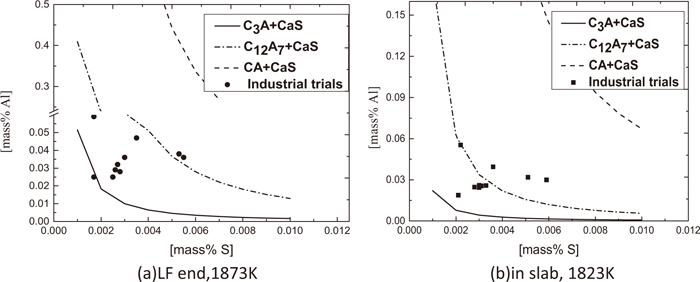
Relationship between Al and S for CaS bearing inclusion precipitation by combining with various types of calcium aluminates.
Therefore, it can be concluded that the type of CaS bearing inclusion shown in Fig. 6 forms due to direct reaction between S and Ca surrounding the low modified oxide as a solid core; another CaS bearing inclusion shown in Fig. 7 form due to the reaction between dissolved Al, sulfur and well modified liquid inclusions.
To explain how the three typical types inclusions corresponding to Fig. 9 deform, the iso-thermal phase diagram for CaO–Al2O3–CaS ternary system, which is also calculated by FactSage, is analyzed as shown in Fig. 15, in which the data points are the observed inclusion components projected in the ternary phase diagram. For the first type inclusion, their composition locates at low melting point region (lower than 1873 K), which is liquid at the refining temperature even at the casting temperature. Thus, it can deform quite well during rolling process. The second type inclusion is a duplex CaS bearing inclusion, the central solid core locates in Al2O3 based region e.g., Al2O3, MgO·Al2O3 spinel or low modified calcium aluminates, with very high melting point; the outer layer locates in CaS based region, another corner in the phase diagram. There are nearly no interaction between the outer CaS based layer and inner solid core during its precipitation. The deformative abilities of CaS based layer and Al2O3 based core differ very much as their physical properties, such as their modulus of elasticity and resistance to deformation, are quite different. Therefore, the outer CaS rich layer is easily separated from the inner core during rolling process. In addition, for the third type of inclusion correspondingly, as the chemical reactions between dissolved S, Al and liquid calcium aluminate take place thus the composition of outer CaS bearing layer which contains calcium aluminate as well approaches that of the liquid inclusion center, which means they have similar properties. Moreover, as the outer layer also has a relatively low melting point thus it can also deform during rolling process. i.e., it is not easily separated from the inner core.
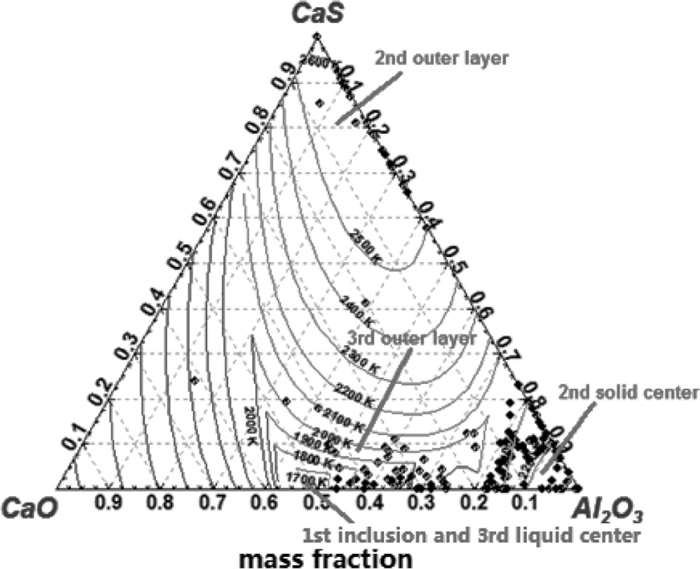
Composition of three types of inclusion projected in 1873 K CaO–Al2O3–CaS ternary phase diagram.
According to the analysis above, there are two routes for Al2O3 inclusion modification during LF refining: the first manner is followed by Al2O3 to various calcium aluminates; the second manner is as Al2O3–MgO·Al2O3 spinel-CaO–MgO–Al2O3 multi-component inclusion, respectively. Many researchers have already studied the two mechanisms of Al2O3 based inclusion modification, but majority of them only reported one of them separately. In fact, as shown in present trials, the two manners both exist during LF refining. As shown in Figs. 11 and 12, with very low Mg and/or Ca content in liquid steel, the MgO·Al2O3 spinel and/or low modified calcium aluminate will be generated. Deng et al.5) proposed that as MgO was less stable than CaO so it would be firstly reduced by dissolved Al and MgO·Al2O3 system inclusion generated prior. But Ca was also detected in liquid steel after tapping and at the start of LF refining during their trials, thus it shows the reaction (10) can also take place and compete with reaction (11) after Al feeding. According to reactions (11) and (12), the Al2O3 based inclusions will continue to generate during LF refining process, and they contact with Mg or Ca to form MgO·Al2O3 spinel or low modified calcium aluminate respectively.
| (10) |
| (11) |
After Ca-treatment, the Ca content in liquid steel increases quite a lot, thus the modified reactions (12) and (13) will speed up until the Al2O3 inclusions are modified into liquid inclusion.
| (12) |
| (13) |
Very interestingly, as shown in Fig. 4(b), there are some Mg concentrated regions at the inclusion surface. It seems that the following reaction (14) takes place when calcium aluminates have been well modified and enough high dissolved Mg is in liquid steel. In the Mg bearing area, the Ca concentration is much less and the Al concentration is almost unchanged, so it is verified that Ca rather than Al is reduced by Mg. This is because the activity of CaO is much higher than the activity of Al2O3 in liquid calcium aluminates such as C12A7 as shown in Table 6 and the activity of dissolved Ca is much lower than that of dissolved Al in liquid steel. It turns out much easier to reduce CaO in liquid calcium aluminates rather than Al2O3. Therefore, it is inferred that the MgO bearing layer expands from the surface to the center of inclusion and finally to form a multi-component inclusion if there is enough time and proper steel composition. This is not reported yet in the mechanism for Al2O3 inclusion modification proposed previously.
| (14) |
In fact, as the presence of Ca, Al and Mg in liquid steel during LF refining, the following reaction will also take place and contend with reaction (13)
| (15) |
At the beginning, there is relative high MgO activity in spinel and relative low Mg in liquid steel, the modification of MgO·Al2O3 spinel mainly appears as reaction (13). But with modification of MgO·Al2O3 spinel by the manner of reaction (13), the MgO activity in inclusion is so low and the Mg in liquid steel is relative higher, thus it is difficult for reaction (13) to take place any more. Thereafter modification of MgO·Al2O3 spinel is mainly by the manner of reaction (15) to form liquid multi-component phase. For a typical MgO·Al2O3 spinel, if it is only modified by reaction (13), it can be only modified into CaOAl2O3 ultimately, which is still with relative high melting point, i.e. in solid state at the steelmaking temperature. So it is also necessary to take place for reaction (15) to ensure the MgO·Al2O3 spinel modification into liquid phase.
Jiang et al.3) proposed MgO in MgO·Al2O3 spinel would be reduced. While Park et al.18) proposed Al2O3 in MgO·Al2O3 spinel would be reduced by Ca during modification for stainless steel. The differences in their experiments are mainly of different dissolved Al in the liquid steel. Deng et al.5) observed a calcium aluminate layer in the out of CaO–MgO–Al2O3 multi-component layer within a modified MgO·Al2O3 spinel inclusion. In fact, in Deng et al.’s5) observation, low MgO content also was detected in the so called calcium aluminate layer, so it is also in favor of the present viewpoint.
It can be concluded that although there are two manners for Al2O3 based inclusion modification, but they will both be modified into CaO–MgO–Al2O3 multi-component inclusion finally if the modified time is long enough and the liquid steel composition is suitable. The top slag is composed mainly of CaO–MgO–Al2O3 ternary system including a small quantity of SiO2 and sulfide that is very similar to the composition of final inclusion in tundish. Suito et al.8) believed that the inclusion composition would be the same to the top slag if perfect slag-steel equilibrium attained ultimately. It is difficult to achieve this state but it shows the important effects of top slag composition on that of inclusion.
With the thermodynamic analysis above, as the temperature decreases and sulfur segregates, it is more easily to precipitate CaS bearing inclusion. In addition, there are also two types of CaS bearing duplex inclusion: the first type forms due to direct reaction between S and Ca surrounding the low modified oxide as a solid core; the other CaS bearing inclusion forms due to the reaction between dissolved Al, sulfur and well modified liquid inclusions, corresponding to reactions (7) and (9) respectively. Consequently, they show different deformation ability during rolling process.
By summarizing the analysis above, the formation and evolution of inclusion during CSP process can expressed as,
(1) When Al is added in the tapping process, a lot of Al2O3 inclusions are formed quickly. And Al–O equilibrium establish with inclusion soon. MgO as well as CaO in slag and refractory is reduced by Al to form dissolved Mg and Ca correspondingly. And then MgO·Al2O3 spinel and low modified calcium aluminates are generated.
(2) After Ca-treatment, the Ca content in liquid steel increases quite a lot, the MgO·Al2O3 spinel inclusions are modified into CaO–MgO–Al2O3 multi-component inclusion in a faster rate. And the low modified calcium aluminates are further modified into liquid ones. In tundish, most of inclusions are modified into liquid region in composition. In addition, the well modified calcium aluminates will be also changed into multi-component inclusion by reacting with dissolved Mg. The schematic diagram is as shown in Fig. 16.
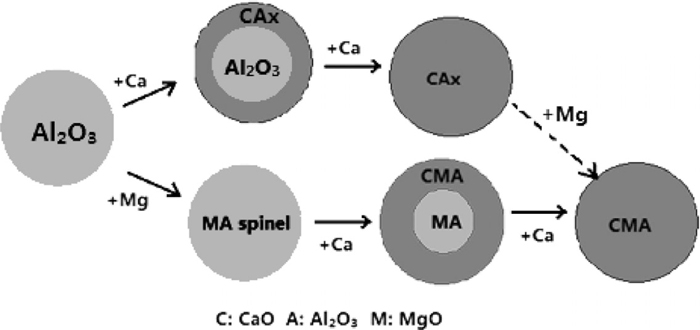
Schematic diagram of two manners of Al2O3 inclusion modification during LF refining for Al killed and Ca-treated steel.
(3) With the temperature decrease and sulfur segregation, it is more likely to precipitate CaS bearing inclusion. There are two types of CaS bearing duplex inclusion: the first type forms due to direct reaction between S and Ca surrounding the oxide as a solid core; another CaS bearing inclusion forms due to the reaction between dissolved Al, sulfur and well modified liquid inclusions. The schematic diagram is as shown in Fig. 17.

Schematic diagram of two manners of CaS bearing inclusion precipitation.
During rolling process, the low modified Al2O3 based inclusion may be broken into pieces and micro-cracks might be generated while the well modified multi-component inclusion can be well deformed. The two types of CaS bearing duplex inclusion also have different deformative ability during rolling process: the outer CaS rich layer of the first type is easily separated from the inner core and the second type can deform well and the outer layer is not easily separated from the inner core.
The formation and modification of inclusion during CSP process as well as their deformation during rolling process for a LCAK steel were studied in the present manuscript. It can be concluded as follows, Firstly, the oxygen activity in liquid steel during LF refining is determined by the equilibrium between dissolved Al and Al2O3 in inclusion.
Secondly, there are two manners for Al2O3 inclusion modification during LF refining: the first manner is Al2O3-low modified calcium aluminates-liquid calcium aluminates, and liquid calcium aluminates would final be multi-component inclusion when the steel composition is proper and time is long enough; the other is Al2O3–MgO·Al2O3 spinel-CaO–MgO–Al2O3 multi-component inclusion.
Thirdly, two types of CaS bearing duplex inclusions could precipitate in a slab during solidification: the first was with CaS precipitating directly surrounding solid inclusion core and the second were with liquid calcium aluminates reacting with dissolved S and Al during solidification process due to the sulfur segregation as well as temperature decrease.
Finally, the favorable modified inclusions deformed very well along with steel matrix during rolling process even if they are associated with CaS bearing layer, while the no or low modified inclusion will be rolled into pieces and the micro-cracks might be generated accordingly. In addition, if the CaS precipitates surrounding the low modified solid inclusion, the outer CaS based layer is easy to be separated from the inner core even taken off from it.
The authors express their thanks to National Natural Science Foundation of China for their kind financial support (No. 61271303).