2013 年 53 巻 12 号 p. 2259-2265
2013 年 53 巻 12 号 p. 2259-2265
Carbon dissolution investigations were carried out at 1550°C on chars from waste CDs as an alternative resource for ironmaking. Waste CDs/DVDs consist of polycarbonate material (thermoplastics polymer) and these produced a significant volume of residual char (~19 wt%) during heat treatment at 1550°C for 15 minutes. The carbon content of chars was determined to be 89% C. XRD and RAMAN analysis were used for the structural characterization of chars. Carbon dissolution investigations using the sessile drop method showed a very rapid carbon pickup reaching 4.12%C within 2 minutes (overall rate constant K: 19.2 × 10–3 s–1). SEM investigations confirmed the absence of oxides and other ash impurities in the interfacial region. The contact angle was seen to change from 79° to 100° after 3 minutes and 105° after 15 minutes of contact. These studies have shown that carbon from waste material could prove to be a valuable carbon resource and be used to partially replace coal and coke during iron making for carburization.
The global crude steel production has seen a significant increase in recent years, reaching 1518 million metric tons in 2011, a growth of 78% in steel usage in the past ten years.1) Nearly, 70% of steel is produced using Basic Oxygen Furnaces (BOF) that required 770 kg of coal to produce 1 ton of steel. Nearly 29% of steel was produced in Electric Arc Furnaces (EAF) which used 150 kg of coal for producing a 1 ton of steel. The total world coal production reached a record level of 7678 million tons in 2011, an increase of 6.6% over the year 2010. Approximately 13% of total hard coal production is currently being used by the steel industry and over 60% of total global steel production is dependent on coal. Current coal resources are approximately 1004 billion tons equivalent to 130 years of global coal output.1) During steel making, both coal and coke are used as carbon sources for carburizing metal, slag foaming and iron oxide reduction.2,3) There is an urgent need to reduce the carbon footprint of steel making; it is therefore important to explore other carbonaceous resources as a replacement of coal/coke. Research is being carried out to reduce the consumption of traditional carbonaceous materials in steelmaking industries and on alternative sources of carbon.2,3,4,5,6,7,8)
More than 280 million tons of plastics are produced worldwide, with only 20% of this waste currently recycled.9) The rapid growth of waste plastics due to extensive utilization and short life span has created a massive problem. According to the Environmental Protection Agency – USA (EPA), nearly 5.5 million software and music CDs go to landfills and incinerators every year, and millions of CDs are just thrown away. These waste CDs have become a serious environmental issue due to their increased usage in daily life and improper methods of disposal.10) Polycarbonate (PC), thermoplastic polymer is used extensively in the manufacture of CDs. In the year 2000, the global production of polycarbonate was about 1.68 million tons which increased to 3.41 million tons in the year 2010; and is predicted to reach more than 6 million tons by the year 2020.11) The present study investigates the use of waste polycarbonate CDs as a supplementary carbon source for carburizing molten iron, and as a replacement of coal and coke in steelmaking industries.
The dissolution of carbon into molten iron and its associated kinetics controls the carbon content in iron-carbon alloys during iron and steel making processes. Several studies have focused on the use of graphite, coke and coal as carburizing materials to understand the fundamentals of carbon dissolution.12,13,14) During the carburizing process, carbon atoms get dissociated from crystal lattice sites, transfer into the iron/carbon interfacial region and pass through the interfacial boundary layer into the bulk liquid iron. These studies have provided information about the reaction kinetics and various factors affecting the carbon dissolution rate. Sulfur is also known to slow down the rate of carbon dissolution in some coals and cokes.12,13) In coke,14) the various metal oxides present in coke ash can form a viscous layer in the interfacial region which can act as a physical barrier for the transfer of carbon, and retard the dissolution of carbon. Basic characteristics such as sulfur content, residual carbon, ash content/composition, rate of volatile release, crystalline order of the carburizing material are key factors that influence the dissolution of carbon into molten iron.15)
In our previous study we have also used 100% melamine5) as a carbon source; this study showed that the carbon pickup by molten iron reached 5.65% C after a reaction of 45 minutes. Present study investigates the dissolution of carbon from waste polycarbonate (PC) based CDs chars into molten iron at 1550°C. Our results show that the high temperature pyrolysis of waste CDs produced ~19 wt% of solid char residue containing ~89% C that could be used as a potential carbon source for iron-carbon alloys. The kinetics of carbon dissolution and wettability has been investigated for chars produced from waste CDs at 1550°C.
Waste CDs consist of a polycarbonate (PC) disc body (~95 wt%) coated with a dye and reflective layers of aluminum on outer surfaces. The dye layer typically contains an organic material that is used to store data while the reflective aluminum layer is used to reflect the laser beam. Waste CDs were buffed to remove the dye and reflective layers and then these CDs were soaked in nitric acid for 10 minutes and washed with ethanol to remove all residual impurities. The carbon content of this plastic material was measured by carbon & sulfur analyzer and was found to be ~75 wt% C. Fourier Transform Infrared (FTIR) with a spectral range of 400 cm–1 to 4000 cm–1 to identify the polymeric peaks of PC in waste CDs.
2.2. Pyrolysis of Waste CDsThe pyrolysis of waste CDs was carried out under isothermal conditions in a horizontal tubular furnace (Fig. 1) with a continuous flow of argon at 1 L/min. The hot zone of the furnace was preheated to 1550°C and the temperature was controlled by a thermocouple. A weighed amount (~1 g) of waste CDs sample (Fig. 2) was kept in an alumina crucible and placed on a graphite sample holder. Initially the graphite sample holder was placed at the cold zone (temperature: ~250°C to 300°C) to avoid thermal shock, held there for 5 minutes, then pushed into the hot zone (1550°C) for 15 minutes. The sample was then withdrawn from the hot zone. These experimental conditions were designed to expose the samples to the high temperature typically experienced by carbonaceous materials during their interaction with liquid iron. Pyrolysis time of 15 minutes was found to be sufficient for the complete degradation of polymer, as no further weight loss was observed. After high temperature pyrolysis, the residual char was collected and carefully characterized using carbon and sulfur analyzer, X-ray Diffraction (XRD), Scanning Electron Microscope, BET Surface Analyzer and Laser Raman Spectroscopy methods. Char materials obtained by pyrolysis were later used as a carburizer material for carbon dissolution investigations.

Schematic representation of the horizontal furnace.

Preparation of char from waste CDs at 1550°C.
Sessile drop method13,15,16,17) was used for investigating the interactions between the carbonaceous material (PC char) and molten iron at 1550°C. Interfacial phenomena including wettability of carbonaceous material and liquid metal were also investigated during this study. The pyrolyzed char was ground and sieved by 100–125 μm size. Approximately 1 g of PC char was put in a die and compacted under a load of 50 KN using a hydraulic press. The compacted char substrate was placed on a graphite sample holder and then 0.5 g of 99.98% pure electrolytic iron was placed on top of the substrate as shown in Fig. 3(a). Experiments were carried out under inert argon flowing at 1 L/min. The sample holder was first placed in the cold zone for 5 minutes to prevent thermal shock and slowly pushed into the hot zone (at 1550°C). This assembly was placed in the hot zone for predefined experimental time period for investigating the carburization behavior (45, 60, 90, 120, 600 and 900 sec). Charge-coupled device (CCD) camera along with video recorder was used to observe the melting/reaction behavior of metal droplet; the melting of iron was marked as beginning of the reaction time. Samples were quenched into the cold zone after predefined reaction time. All experiments were repeated several times to ensure the reproducibility of results. Carburized iron droplet (Fig. 3(b)) was collected, its surface was cleaned with ethanol to remove any undissolved carbonaceous particles; the carbon pickup by the metal droplets was measured using LECO carbon and sulfur analyzer.

PC char substrate and iron (a) before Reaction (b) after Reaction.
The wettability between the electrolytic pure iron and char substrate sample was studied using the sessile drop approach. The wetting image of the liquid melt on the solid substrate was monitored and recorded continuously by a CCD camera.18,19) These recorded images were converted to digital video and then various video images were converted to individual images for contact angle measurement. The individual image file (Fig. 4) was processed by specially designed software to measure contact angles as a function of time.19) To study the interface and distribution of carbon in the iron droplet, SEM/EDS investigation was performed. The carbonaceous substrate along with the iron droplet was carefully cold mounted in epoxy resin for 24 hr. The resin mounted substrate along with iron droplet was than cross sectioned in the middle and polished for SEM/EDS investigations.

The wetting behavior of char substrate in contact with molten iron as function of time.
Carbon pickup by molten iron from Polycarbonate (PC) char, associated kinetics and contact angle measurements are detailed below along with an in depth characterization of PC char obtained. These results have also been compared with corresponding results observed with various carbonaceous materials such as synthetic graphite, natural graphite, coke and coal chars.
3.1. Characterization of CharThe high temperature pyrolysis of polycarbonate CDs produced significant quantities of carbon rich char. The total amount of char produced was ~19 wt% and carbon content in char was determined to be ~89 wt% C. This result is in good agreement with published literature on pyrolysis of polycarbonates.20,21) During thermal degradation in an inert atmosphere, PC is known to loose carbonate, isopropylidene groups and hydrogen of the benzene rings during initial stages; the degradation of aromatic carbon occurs in later stages.20,22,23) A literature survey for estimating the pyrolysis residue of various thermoplastics polymers i.e. Poly Vinyl Chloride (PVC), Poly Styrene (PS), Poly Ethylene (PE), Poly Propylene (PP), Low Density Poly Ethylene (LDPE), HDPE has shown that high temperature pyrolysis generally did not produce significant quantities of char.24,25) Demribas24) observed maximum char generation of 9 wt% from PVC for a pyrolysis temperature of 740°C. Under similar conditions PS, PE and PP gave solid residues 0.6 wt%, 1.8 wt% and 1.6 wt% of initial mass respectively. Zevenhoven et al.25) also found that PVC produced 5.9% char under the nitrogen atmosphere at 850°C; LDPE and HDPE produced only 0.2 and 2.3% of char residues respectively. These thermoplastics polymers did not produce significant amounts of carbonaceous char during high temperature pyrolysis. Polycarbonate thermoplastic polymer however yielded ~19 wt% after pyrolysis at 1550°C, which is very high when compared to other thermoplastic polymers.
The structural characterization of char residue was performed using XRD, LRS, SEM and BET surface analyzer. X-Ray Diffraction pattern of the pyrolysis char sample is shown in Fig. 5. The diffraction profiles for PC char clearly show the presence of a prominent (002) peak around ~25°, which is attributed due to presence of graphitic carbon in residue. The peak at 2θ angle of ~43° can be assigned to (100) diffraction of hexagonal graphene carbons. The (002) and (100) peaks are due to the presence of π bonds between carbon (sp2 hybridized) atoms. The remaining peaks at around 35°, 42° and 60° are due to the presence of trace amounts of alumina contamination from the alumina crucible. The origin of the γ-band at 20° is due to disorder in the lattice structure of graphitic carbons and indicates defects in sp2 hybridized carbon. However a low intensity of γ-band indicates the presence of little amorphous carbon. The ratio of intensity of γ/π bands can be used as a measure of the degree of disorder in the polycrystalline of carbon.

XRD profiles of PC chars prepared at 1550°C for 15 minutes.
The crystallite size in the char of the carbon structure was determined from the width of the (002) peak. The crystallite height (Lc) corresponding to C (002) peak was calculated by Scherrer formula:
| (1) |
The structural features of PC char were also analyzed by Raman spectroscopy, where scattering process has contributions from various phonon vibration modes of materials. The spectrum is shown in Fig. 6 after excitation with 514 nm laser light. The spectrum has two peaks at around 1590 cm–1 and 1350 cm–1. The peak at 1350 cm–1 is D–band (disorder-induced feature), which indicates a typical sign for defects in graphitic structures. The strongest G–band (tangential) at 1590 cm–1 is characteristic peak highly graphitic structure. In general, the intensity ratio of IG/ID bands can be used to evaluate the degree of crystallisation or imperfections in the carbon structure. The IG/ID ratio of PC char was found to be more than unity indicating reasonably good ordering in the PC char.

Raman spectrum of PC char prepared at 1550°C for 15 minutes.
Scanning Electron Microscope was used to investigate the surface morphology of the char residue after pyrolysis. The SEM images obtained from the exterior char residue are shown in Fig. 7. The SEM images of PC char indicate a porous surface with macro-size pores into its interior mass. Due to rapid devolatilization during pyrolysis, a smooth and continual char layer dispersed with bubbles that folds into interior mass of char was observed. These images of PC char residue also indicate a highly porous structure.
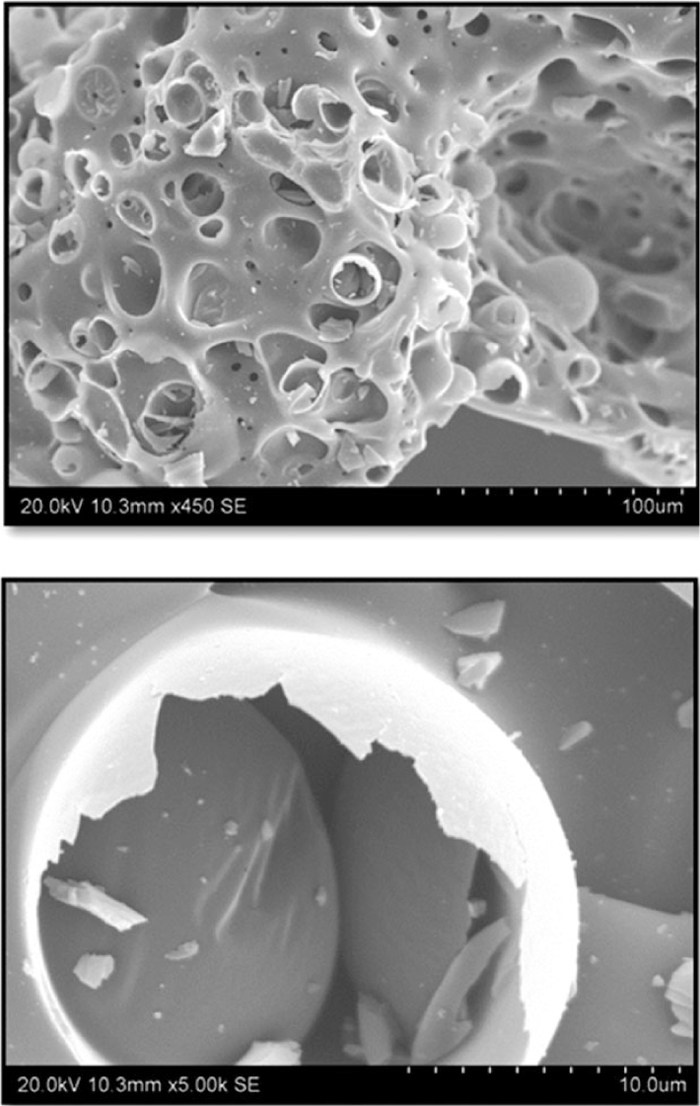
SEM images of pyrolysis PC char at 1550°C for 15 minutes.
The SEM results are in good agreement with the surface analysis carried out for BET surface area and pore distribution analysis. PC char residue showed BET surface area 27.33 m2/g of with average cumulative volume of 0.025 cm3/g; the average pore size was determined to be 5.38 nm. Gupta et al. observed that26) coal blends with 20% LDPE plastics had four times the surface area in chars as compared to pure coal char samples, i.e. 25 m2/g as compared to 6.5 m2/g. The increase in micropore surface area of polycarbonate char can be attributed to pores opening up during the pyrolysis process; escaping volatile matter27) also resulted in the formation of additional pores.
3.2. Carbon DissolutionThe dissolution of carbon into iron is a key step in the making of iron-carbon alloys. A number of the investigations have focused their attention on the dissolution behavior of graphite, coke and coal as carbon sources; a few studies have also been reported on polymers.5,17) Both experimental and theoretical studies have provided information about the reaction kinetics and various factors affecting the dissolution rate. The dissolution kinetics of carbon from highly ordered materials such as graphite was generally found to be mass transfer limited.28)
Figure 8 shows the dissolution of carbon from PC chars at 1550°C as function of time; the pickup was very rapid during the initial contact reaching 3.66% within 1 minute. The rate slowed down after that and the total carbon level in molten iron reached a value of 4.62 wt% after 15 minutes of contact. The first order rate constant for carbon dissolution was obtained using following equations.17)
| (2) |
| (3) |

Variation in carbon pickup from PC char by molten iron at 1550°C with time.

Plot of ln ((Cs – Ct)/(Cs – Co)) vs. time for first 2 minutes.
The carbon pickup by molten iron from char was significant within 2 minutes of reaction time as shown in Fig. 8 and slowed down afterwards. A similar behavior was observed in the wetting experiments. The contact angle between the substrate and molten iron was measured and is shown in Fig. 10. During initial contact from 0 to 10 seconds, a small decrease in contact angle was observed from 79 to 70°, possibly due to the transfer of carbon and also due to a change in interfacial energy due to interaction between char and molten iron. Wu et al.13) observed a similar behavior while using graphite as a carbon source. During later periods, an increase was observed in contact angles that reached 100° after 3 minutes and stabilized at 105° after 15 minutes thereby indicating a much poorer wetting with the carbon level in molten iron approaching saturation. This result point too much lower driving force for carbon dissolution as expected.

Contact angle as a function of time.
Carbon distribution at interface was also examined by SEM/EDS by analysing the cross sectional image of iron droplet (Fig. 11) in PC char substrate/Fe system. A number of cavities and voids were observed (point #2) in the interfacial layer between molten iron and carbon char. The EDS spectra at point #2 clearly indiactes that a large number of voids were made up of only carbon. The existance of these voids resulted in an increased area of contact in the interfacial region. Transfer of carbon particulates from the substrate into the iron mass through these voids was also observed and could be one of the possible way for the dissolution of carbon into liquid iron, and particulates of char separated from the char substrate which was clearly seen in Fig. 11 at point #2 and this char particulates penetrated into the iron mass. The EDS results at iron mass shown as point #1 also confirms the presence of carbon into iron bulk.

SEM images coupled with EDS of metal/carbon interface for PC char substrate and iron after carbon dissolution.
A high rate of carbon dissolution was observed for PC char, which was much higher than rates typically observed for metallurgical coke. Cham et al.31) observed a large difference in the overall carbon dissolution rate constant for two different cokes: coke-1, K = 14.7 × 10–3 s–1 and coke-2, K = 1.1 × 10–3 s–1; this difference was attributed to differences in the chemical composition of mineral matter present in two cokes. Our previous study32) also reached a similar conclusion regarding the influence of mineral matter such as silica, sulfur and calcium on carbon dissolution while using coke as a carburizing material. In addition to the interfacial blockage, the in-situ reduction of silica is also known to consume some of the solute carbon. Khanna et al.29) used different coal chars as a carbon source and observed that the carbon concentration in the melt pickup was quite slow and reached a maximum value of ~ 3 wt% after 15 minutes of contact, thereby concluding that the deposition of reaction products in the interfacial region strongly hindered dissolution of carbon in iron. A number of researchers13,14,15,17,32) have confirmed the formation of interfacial layer formed due to ash, mineral matter and other oxides which can partially block the contact between the iron droplet and carbon source, and cause a decrease in the rate of carbon transfer into iron. McCarthy et al.15) investigated the dissolution of coal chars using the sessile drop approach; the carbon pickup reached only upto 0.12 wt% and 0.28 wt% respectively for char 1 and char 2 after three hours of contact time. In the case of PC char, the EDS spectra showed peaks for only carbon and iron at the interfacial region between iron and char substrate (point #2 - Fig. 11). The negligible presence of other elements in this region indicates the absence of ash blockage, therefore offering minimal resistance to carbon dissolution. In addition, if there is poor wetting between iron and ash compounds or other changes occur in wettability between iron and carbon material that would also affect the contact surface area and influence the dissolution behavior. We did not observe an ash layer in the interfacial region, and the initial decrease in contact angles indicated a good wetting (Fig. 10). The increase in contact angle in later stages is associated with slower carbon dissolution at the char/iron interface. Previous investigations on natural graphite19) showed that increasing carbon level in metal had an influence on wettability; the contact angle was seen to decrease during initial contact and then reached a higher but stable value after extended contact. A similar trend was also observed in this study with C level in the droplet reaching 4.12%C after 2 minutes, the contact angle reached a stable value of ~105°; not much change was observed later on. The interfacial region was characterised by large particulates of carbon floating in molten iron (Fig. 11 at point #2). This indicates that several pieces of porous carbonaceous material had got detached from the substrate and made their way into molten metal. Partial dissociation of the substrate occurred during the interaction between molten iron and char substrate; highly porous nature of the substrate allowed a large area of contact and aided dissolution. It has been reported33,34) that cokes with high porosity exhibited a larger surface area for reaction, which in turn resulted in a higher dissolution of carbon into molten iron. A few studies were also carried out on raw PC and PC soot collected during the charring process; the carbon pickup reached a value of 5.5%C and 5.3%C respectively from these materials after 15 minutes of contact with molten iron.
Wu et al.13) found that the overall dissolution rate constant (K) for synthetic graphite (24 × 10–3 s–1) was more than twice that of natural graphite (10.68 × 10–3 s–1); this difference was attributed to physical interfacial blockage from natural graphite. Cham et al.30) reported that the dissolution of carbon increased with increasing crystalline order (Lc value) of coke. In their studies, coke with Lc value of 1.5 nm showed the overall dissolution rate constant (K) of 2.68 × 10–3 s–1. Previous studies12,30) have also pointed to a interdependence between structural ordering and carbon dissolution rate suggesting that carbonaceous materials with higher Lc values generally had a higher rate of dissolution of carbon. For a graphitic carbonaceous material, the dissociation of carbon atoms is faster due to the arrangement of carbon atoms in two dimensional arrays as compared to coke. A high Lc value will therefore aid the dissociation of carbon atoms.12,29) Khanna et al.29) investigated carbon dissolution rates from different types of coal chars having small Lc values; these showed much slower rates of carbon dissolution when compared to synthetic and natural graphite. In their study, four different types of coal chars were investigated with Lc valued ranging between 0.95 nm to 1.27 nm; corresponding first order carbon dissolution rate constant (k´) were found to range between 0.08 × 10–3 s–1 to 0.64 × 10–3 m·s–1. It was concluded that the slower dissociation rate of carbon atom from chars slowed down the kinetics of dissolution.
As discussed in earlier section both XRD and RAMAN analysis indicates moderate graphitization for PC char. An Lc value (calculated from XRD analysis) of 3.1 nm for PC char, which is higher than typical values for coke and coal chars, represents a moderate structural order and it could be one possible reason for higher dissolution of carbon into molten iron. The overall rate of carbon dissolution into liquid metal also depends on the experimental approach used as well as the characteristics of carbonaceous material. Overall Carbon dissolution rate constants (K) for various coke and coal chars was reported in the range of 0.08 × 10–3 s–1 to 14.7 × 10–3 s–1 using carburizer cover method. Different values of rate constant for various carbonaceous materials using sessile drop approach is shown in Table 1. The rate of carbon dissolution (19.2 × 10–3 s–1) observed for PC char within the first two minutes of contact was much higher than typical values observed for coke and coal chars.
An in depth investigation has been carried out on the dissolution of carbon into molten iron at 1550°C using 100% waste PC char as a carburising source. This material is seen as a promising alternative carbon resource for ironmaking. The major findings from this study are:
(1) Pyrolysis of waste polycarbonate CDs produced ~19 wt% char residue which was rich in carbon (~89%C). The char generation capacity of PC at 1550°C was found to be significantly higher than the typical yields from polymers including PVC, PS, PE, PP, LDPE and HDPE.
(2) The overall carbon dissolution rate constant (K) was determined to be 19.2 × 10–3 s–1 and a carbon pickup of 4.12%C was observed within initial 2 minutes and reached to 4.62% C after 15 minutes of reaction. This level of carbon pickup from PC char was found to be better than typical results when coke, coal chars, natural garphite have been used as carburising agents.
(3) Both XRD and Raman analysis indicated a moderate graphitic structural order in PC char; the Lc value was determined as 3.1 nm. This graphitic nature of char is possible reason for higher carbon dissolution into iron.
(4) The SEM images and BET surface area measurement clearly showed a porous nature for the PC char. In the interfacial region, porous particulates of carbon were seen in molten iron thereby offering high reaction surfaces; these large contact areas suggest a possible means for transfering significant amounts of carbon into molten iron and a rapid dissolution rate. Due to the lower levels of oxides impurities in PC char, formation of ash layer between iron droplet and char substrate was not observed.
(5) An improvement in wetting behaviour was observed during initial stages of carburization; this result is in accordance with a good carbon dissolution observed during this time period. During later stages (after 2 minutes) with increased level of carbon in molten iron, the system changed towards non-wetting behaviour.