2013 Volume 53 Issue 4 Pages 551-556
2013 Volume 53 Issue 4 Pages 551-556
Recovery of Nd from used products is a crucial problem to be solved for a stable supply of Nd resource. In the present work, the use of two immiscible liquids, i.e. a Cu-rich phase and an Fe-rich phase, in an Fe–Cu–C system was regarded as one of the solutions for this purpose and the distributions of Nd between two immiscible liquids in an Fe–Cu–C system were measured. We found Nd is distributed in the Cu-rich phase more than in the Fe-rich phase in an Fe–Cu–C system and Nd is more concentrated at low temperatures than at high temperatures. In addition, the thermodynamic parameters for Nd were obtained based on the experimental results.
In the past two decades, the use of two immiscible liquids in Fe–Cu–C systems in several kinds of metal recycling has been investigated. It is known that a uniform liquid in an Fe–Cu system separates into two immiscible phases, i.e., a Cu-rich phase and an Fe-rich phase, when C is added to the system.1,2,3,4,5) Based on this phenomenon, the recovery of Cu and Fe from Fe–Cu mixed scraps such as motor cores and polychlorinated biphenyl transformers has been investigated by Marukawa and Tanaka et al.6,7) It has also been reported that the minor metals contained in an Fe–Cu–C system are distributed between the Cu-rich and Fe-rich phases.8,9,10,11,12,13) This behavior can be used to recover the minor metals from the phase in which they are concentrated, and to remove impurities from phases in which impurities are concentrated. From this viewpoint, Yamaguchi et al.8) have conducted the fundamental experiments on the copper enrichment of low grade copper scraps such as ashes of shredder dust and trash, and the recovery of precious metals, i.e. Au, Ag, Pd, Pt and Rh. The recovery of valuable metals from speiss generated in the smelting process of metals based on this phenomenon has been investigated by Voisin et al.9,10) Gonzalez et al.11) made an suggestion that molybdenum is concentrated in the Fe-rich phase by applying the two immiscible liquids to the Cu slag. Recently, Lu et al.12) have focused on municipal solid wastes as the target material which the two immiscible liquids are applied to. The removal of Ni from low-grade Cu scraps has been studied by Sano et al.13) with respect to impurity removal.
The recovery of valuable metals from used products is important for stable supplies of metal resources. In this study, we focus on the use of two immiscible liquids in an Fe–Cu–C system as the seed technology for the treatment of used products because the motors in automobiles, electric appliances and so on consist of Fe, Cu, and various valuable metals. Nd is a particularly significant metal since Nd-based magnets are used in many electronic devices and Nd supplies are unstable. In this study, the temperature dependence of the distribution of Nd between the two immiscible liquids in an Fe–Cu–C system was investigated. In addition, the interaction parameters for Nd were evaluated based on experimental data on the distribution of Nd in the two immiscible liquids in an Fe–Ag–C system, as well as that in an Fe–Cu–C system.
An Fe–Csat alloy, Cu–Nd alloy, and Cu were used in the experiments. The Fe–Csat alloy was prepared by melting 100 g of electrolytic Fe (Toho Zinc Co., Ltd.: C 36 ppm, P <10 ppm, 12 ppm, Si <5 ppm, Mn 1 ppm, Cu 1 ppm, N 10 ppm, O 200 ppm) and 4 g of C powder (Sigma-Aldrich Co. LLC) in a carbon-crucible at 1573 K under Ar gas for 3 h. The Cu–Nd alloy was prepared by melting 27 g of Cu (Nilaco Corp.: > 99.99%) and 3 g of Nd (Mitsuwa Chemicals Co., Ltd: > 99.9%) in a carbon-crucible at 1523 K under Ar gas for 3 h. The weight of Fe–Csat alloy in the sample was 10 g and the total weight of Cu–Nd alloy and Cu in the sample was 10 g; the proportion of Nd in the Cu–Nd was varied by varying the amount of Nd. The sample was melted in a carbon- crucible at a target temperature under an Ar gas flow 50 ml/min (s.t.p.) for more than 10 h. The target temperatures were 1523, 1623, and 1723 K. After the holding time, the sample was quenched with water, and the compositions of the Cu-rich and Fe-rich phases were analyzed using inductively- coupled plasma atomic emission spectrometry (ICPAES) and infrared absorptiometry.
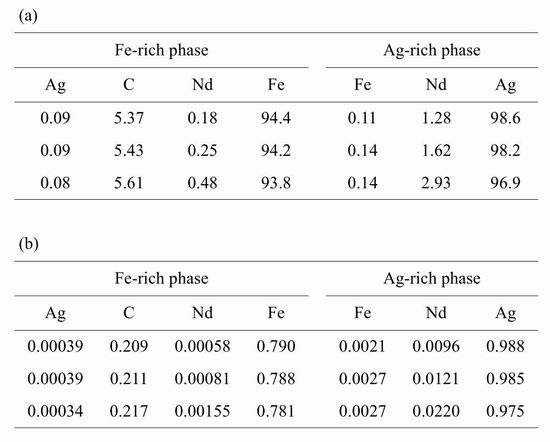
2.2. Distribution of Nd between Ag-rich and Fe-rich Phases in Fe–Ag–C SystemThe Fe–Csat alloy, Ag (Nilaco Corp.: > 99.99%), and Nd were used in the experiments. The Fe–Csat alloy (6 g), Ag (6 g), and different amounts of Nd were melted in a carboncrucible at 1623 K under an Ar gas flow 50 ml/min (s.t.p.) for more than 10 h. After the holding time, the sample was quenched with water, and the compositions of the Ag-rich and Fe-rich phases were analyzed using ICP-AES and infrared absorptiometry.
The compositions of the Cu-rich and Fe-rich phases as mass percentages and mole fractions are shown in Table 1. The Nd content in the Cu-rich phase is higher than that in the Fe-rich phase, regardless of the temperature and the amount of Nd. We found that Nd is distributed in the Curich phase more than in the Fe-rich phase. Figure 1 shows the relationship between the mass percentage of Nd in the Cu-rich phase and that of Nd in the Fe-rich phase. The plots for different amounts of Nd at 1523 K and at 1623 K give straight lines. This means that the distribution ratio of Nd between the Cu-rich and Fe-rich phases does not change in the Nd concentration range used in the present experiments. The distribution ratio of Nd, defined as [mass%Nd]in Cu-rich/ [mass%Nd]in Fe-rich, is determined by the slope of the fitted line through the origin for the plots. The distribution ratios are 18.8 at 1523 K, 10.4 at 1623 K, and 6.2 at 1723 K. The distribution ratio of Nd between the Cu-rich and Fe-rich phases increases with decreasing temperature; i.e., Nd is more concentrated at low temperatures than at high temperatures. Sano et al.12) reported that the distribution of Ni between the two immiscible liquids in an Fe–Cu–C system has the similar tendency as that of Nd in the present work. They found that Ni is more concentrated in the Fe-rich phase at low temperatures than at high temperatures.

Relationships between mass percentages of Nd in Cu-rich and Fe-rich phases in two immiscible liquids in Fe–Cu–C system.
The compositions of the Ag-rich and Fe-rich phases as mass percentages and mole fractions are shown in Table 2. The content of Nd in the Ag-rich phase is higher than that in the Fe-rich phase, regardless of the amount of Nd, which means that Nd is distributed in the Ag-rich phase more than in the Fe-rich phase. The mass percentage of Nd in the Agrich phase is plotted against the mass percentage of Nd in the Fe-rich phase in Fig. 2. The plot shows a linear relationship. This suggests that the distribution ratio of Nd between the Ag-rich and Cu-rich phases is constant under our experimental conditions. The distribution ratio of Nd, defined as [mass%Nd]in Ag-rich/[mass%Nd]in Fe-rich, is 6.3 at 1623 K, so Nd is concentrated in the Ag-rich phase in the two immiscible liquids in the Fe–Ag–C system.

Relationship between mass percentages of Nd in Ag-rich and Fe-rich phases in two immiscible liquids in Fe–Cu–C system at 1623 K.
The reaction between Nd in the Cu-rich phase and Nd in the Fe-rich phase is expressed by Eq. (1).
| (1) |
The Cu-rich and Fe-rich phases are denoted by the subscripts (Cu) and (Fe–C), respectively. Both phases have the same Nd chemical potentials at equilibrium. The activity of Nd in the Cu-rich phase, aNd(Cu), is equal to the that in the Fe-rich phase, aNd(Fe–C), when the standard chemical potential of Nd is the same in both phases:
| (2) |
Using the relationship ai = γiXi, where ai is the activity of i, γi is the activity coefficient of i and Xi is mole fraction of i, Eq. (2) is rewritten as Eq. (3).
| (3) |
γNd(Cu) is the activity coefficient of Nd in the Cu-rich phase, based on Raoult's law, XNd(Cu) is the mole fraction of Nd in the Cu-rich phase, γNd(Fe–C) is the activity coefficient of Nd in the Fe-rich phase, based on Raoult's law, and XNd(Fe–C) is the mole fraction of Nd in the Fe-rich phase. γNd(Fe–C) and γNd(Cu) are given as Eqs. (4) and (5), based on Taylor expansions.14)
| (4) |
| (5) |
By combining Eqs. (3), (4), and (5) and using a relation
| (6) |
Here, we considered that the influence of
| (7) |
Equation (7) shows that
| (8) |
| (9) |

Relationship between left-hand side and XCu(Fe–C)/XFe(Cu) in Eq. (7) at 1623 K.
| (10) |
The dependence of distribution of Nd between Cu-rich and Fe-rich phases in Fe–Cu–C system on temperature was evaluated by using the thermodynamic parameters obtained in the present work. Here, we assumed that the temperature dependence of

Relationship between ln(XNd(Cu)/XNd(Fe–C)) and 1/T.
In our experiment, several % of Nd is contained in the Cu-rich phase in Fe–Cu–C system and in the Ag-rich phase in Fe–Ag–C system. The maximum Nd contents in the Curich phase and the Ag-rich phase were as high as 5.89 and 2.93 mass%. This magnitude of Nd content reminds us of the possibility of NdC2 formation. Therefore, we discussed the formation of NdC2 under our experimental conditions. Here, the binary M–Nd alloy (M=Cu or Ag) is treated for simplicity. The reaction of Nd in M–Nd alloy (M=Cu or Ag) and C to form NdC2 is expressed as below:
| (11) |
Equation (12) is changed into Eq. (13).
| (13) |
Here, both aC and aNdC2 are defined as 1. By using aNd(M) = γNd(M) · XNd(M), Eq. (14) was obtained.
| (14) |
This equation means the solubility of Nd in M–Nd alloy (M=Cu or Ag) is calculated when the activity coefficient of Nd in M–Nd alloy (M=Cu or Ag) is known. When Eq. (8) is used as the activity coefficient of Nd in Cu–Nd system, the Nd solubility in Cu–Nd system in the presence of C and NdC2 at 1523, 1623 and 1723 K are estimated to be 8.9, 9.5 and 10.2 mass% from Eqs.11, 13 and 14, respectively. It is expected that NdC2 does not appear in the Cu-rich phase in Fe–Cu–C system in our experiment even if the effect of Fe on the solubility of Nd is considered. This is because γNd(Cu) decreases with the content of Fe in Cu–Nd system due to the negative value of
On the other hand, there is no data on the activity coefficient of Nd in Ag–Nd–C system with the presence of C and NdC2. Thus, the formation of NdC2 in the Ag-rich phase in Fe–Ag–C system was discussed based on the activity coefficient of Nd in Ag–Nd system. The activity coefficient of Nd in an infinitely dilute solution of Ag,
In this study, the distribution of Nd between the two immiscible liquids in an Fe–Cu–C system was investigated at 1523, 1623, and 1723 K. In addition, the distribution behavior of Nd between the two immiscible liquids in an Fe–Ag–C system was investigated at 1623 K. The activity coefficient of Nd in an infinitely dilute solution of C-saturated Fe, the interaction parameter of Fe with Nd in Cu, and the interaction parameter of Cu with Nd in C-saturated Fe were determined from the experimental results. We found the following. Nd is concentrated in the Cu-rich phase in the two immiscible liquids in an Fe–Cu–C system and the tendency of Nd to concentrate in the Cu-rich phase becomes strong at low temperatures. In the two immiscible liquids in an Fe– Ag–C system, Nd is distributed in the Ag-rich phase more than in the Fe-rich phase. The thermodynamic parameters obtained in the present work are
This work was supported by the New Energy and Industrial Technology Development Organization (NEDO). We wish to thank them for financial assistance. The authors also are grateful to Dr. Hideki Ono (Associate Professor, Graduate School of Engineering, Osaka University), and Dr. Katsuhiro Yamaguchi (formerly with the Graduate School of Engineering, Osaka University is now with Kobe Steel, Ltd.) for their valuable discussions on thermodynamic assessment.