2013 年 53 巻 4 号 p. 557-563
2013 年 53 巻 4 号 p. 557-563
The effect of Fe–Si on the carbothermaic reduction of Panzhihua titanomagnetite concentrates were investigated under argon atmosphere by isothermal experiments at 1623 K and non-isothermal experiments in the temperature range from room temperature to 1723 K with a heating rate of 10 K/min, respectively. The morphology of reduced samples obtained by isothermal experiments was checked by scanning electron microscope. The results show that the addition of Fe–Si accelerates the carbothermic reduction rate of PTC. A part of silicon in the Fe–Si substitutes for carbon to participate the reduction of PTC. The addition of Fe–Si facilitates the nucleation and coalescence of metallic iron formed by reduction. A reaction mechanism for the carbothermic reduction of PTC with Fe–Si addition was proposed. The reduction process could be divided into three stages. In the first stage (lower than 1273 K), the solid phase reactions with carbon and silicon as reductants are dominant. The exdothermic reduction by silicon, to a certain extent, promotes the reduction of PTC. In the second stage (1273–1423 K), the rate of reduction by CO is much faster than that of reduction by silicon, resulting in little influence of Fe–Si on the reduction of PTC. In the final stage, the reduction by silicon markedly occurs again, which further facilitates the coalescence of metallic iron and the reduction of PTC.
Vanadium-titanium-bearing magnetite of Panzhihua, China, is a complex iron ore with the coexistence elements of vanadium and titanium. It accounts for more than 90% of the titanium reserves in China. By the beneficiation process of the ore, titanomagnetite concentrates and ilmenite concentrates are produced. Most of the titanomagnetite concentrates are used as the main materials for the blast furnace process in Panzhihua area now. Most of the iron and partly of vanadium can be reduced into the hot metal, however, almost all of the titanium remains in the slag, forming the high titanium slag with the content of TiO2 varying from 22 to 25%. There is no an appropriate and economic method to deal with such slag so far.1,2,3,4) During the past ten years, most of the studies were focused on developing an alternative route for extracting all the three useful elements together from the titanomagnetite concentrates, one of the potential choices is the rotary hearth furnace (RHF) process, which involves the reduction step of composite briquette of titanomagnetite concentrates with coal, and the smelting of the reduced specimen in an electric arc furnace.
It was observed by many researchers1,5,6,7,8) that the reduction of Panzhihua titanomagnetite concentrates (PTC) is slower than that of magnetite due to its special crystal structure of the ore with the existence of titanium, which results in a higher thermodynamic stability of titanomagnetite.9,10) This implies a longer residence time in RHF than that with the common iron ore. Therefore, it is important to enhance the reduction of PTC.
Enhancements of the reduction of PTC have been extensively studied, which refer to the pre-oxidation of PTC and the addition of alkali metal salt such as sodium sulfate, sodium carbonate and borax.11,12) It was found the reduction rate of PTC was accelerated by means of the both aforementioned methods. However, these were still some disadvantages of these methods, which are the extra investment for pre-oxidation process and the negative effects of the refractory lining for the addition of alkali metal salt. Recently, the Fe–Si was used as an additive in the carbothermic reduction of chromite.13,14) It was observed that the addition of Fe–Si enhanced the carbothermic reduction of chromite. In present study, an attempt was made to enhance the carbothermic reduction of PTC by the addition of Fe–Si. The influence of Fe–Si dosage on the carbothermic reduction process was investigated.
Chemical compositions and size distributions of raw materials were examined and are presented in Tables 1, 2, 3, 4. Figure 1 shows the X-ray diffraction (XRD) pattern of PTC. The mineral phases of PTC are magnetite and titanomagnetite in principal and with partial ilmenite. The PTC was mixed homogenously with coal and Fe–Si. The molar ratio of Cfixed/O(bonded with Fe) in mixture was fixed at 1.2. The mass ratio of Fe–Si to the PTC-coal mixture varied from 0% to 3% with the increment step of 1%.
| PTC | coal | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TFe | FeO | TiO2 | V2O5 | SiO2 | CaO | MgO | Al2O3 | C | volatile | ash | S |
| 52.62 | 32.0 | 12.0 | 0.6 | 4.2 | 1.3 | 2.6 | 3.9 | 71.41 | 12.02 | 16.57 | 0.58 |
| Components | Fe | Si | Mn | Cr | P | S | Diameter/µm | ≤74 | >74 |
|---|---|---|---|---|---|---|---|---|---|
| content | 33.84 | 65 | 0.6 | 0.5 | 0.04 | 0.02 | Content | 96.40 | 3.60 |
| Diameter/µm | <74 | 74–80 | 80–96 | 96–109 | 109–120 | 120–150 | > 150 | Total |
|---|---|---|---|---|---|---|---|---|
| Content/wt.% | 88.40 | 3.26 | 4.20 | 1.86 | 1.81 | 0.32 | 0.15 | 100 |
| Diameter/µm | <100 | 150–180 | 180–250 | 250–550 | 550–1700 | > 1700 | Total |
|---|---|---|---|---|---|---|---|
| Content/wt.% | 20.80 | 51.34 | 14.81 | 9.87 | 2.00 | 1.18 | 100 |
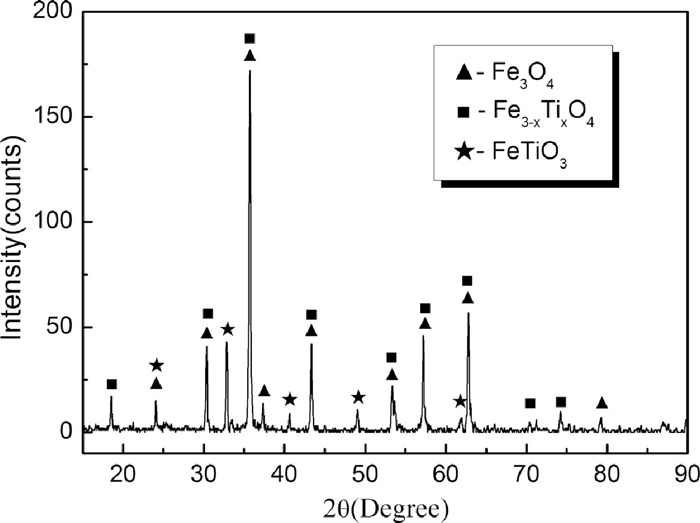
XRD pattern of PTC.
The mixtures were made into the spheroid briquettes under the pressure of 15 MPa with a briquette maker. The diameter of the briquette is about 30 mm and the total mass of the specimen was about 20 g. All the briquettes were dried at 393 K for 6 hours before the reduction experiments.
The reaction mechanism of the mixtures with various Fe–Si dosages was checked by thermogravimetric analysis method, which was carried out in a NETZSCH STA 449C thermal analyser. The heating rate is 10 Kmin–1 in argon atmosphere from room temperature to 1723 K. About 70 mg mixture was used for each experiment.
The isothermal reductions of the briquettes at 1623 K in argon were carried out in a vertical electric resistance furnace, whose schematic is shown in Fig. 2. A briquette was loaded in a basket made of nichrome wire and hanged up of the furnace. The furnace was purified by blowing argon at a high rate before each experiment, and then kept the flow rate of argon at 1 NL·min–1. The basket with briquette was loaded down quickly into the hot zone of the furnace when the furnace temperature reached a desired value. The briquette reduced for different time was cooled in the argon quickly after the specimen was took out from the furnace.
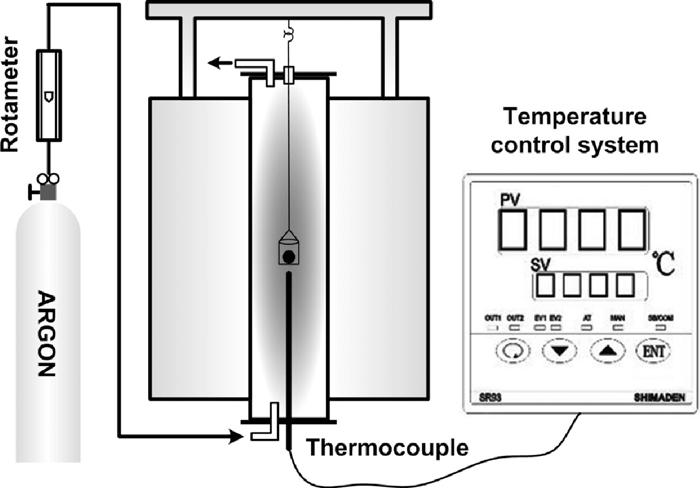
Schematic of experimental system.
The reduced briquettes were examined by chemical analysis method, the degree of metallization (η) of which was calculated according to Eq. (1):
| (1) |
It is widely accepted that the mechanism of carbothermic reduction of metal oxides (MxOy) is two-stage mechanism with the participation of gaseous intermediate (CO and CO2) according to the following equations:
| (2) |
| (3) |
| (4) |
The removals of oxygen from metal oxides are predominantly in the form of CO at high temperature. Carbon reacts with CO2 to generate CO continuously for keeping a strong reducing atmosphere.
Figure 3 shows the equilibrium atmosphere for main reactions occurring in the reduction of PTC. It indicates that titanium-bearing iron oxides (Fe2TiO4, FeTiO3, FeTi2O5) are more difficult to be reduced than iron oxides by CO and higher PCO/(PCO+PCO2) is required for the reductions of titanium- bearing iron oxides over 1273 K.
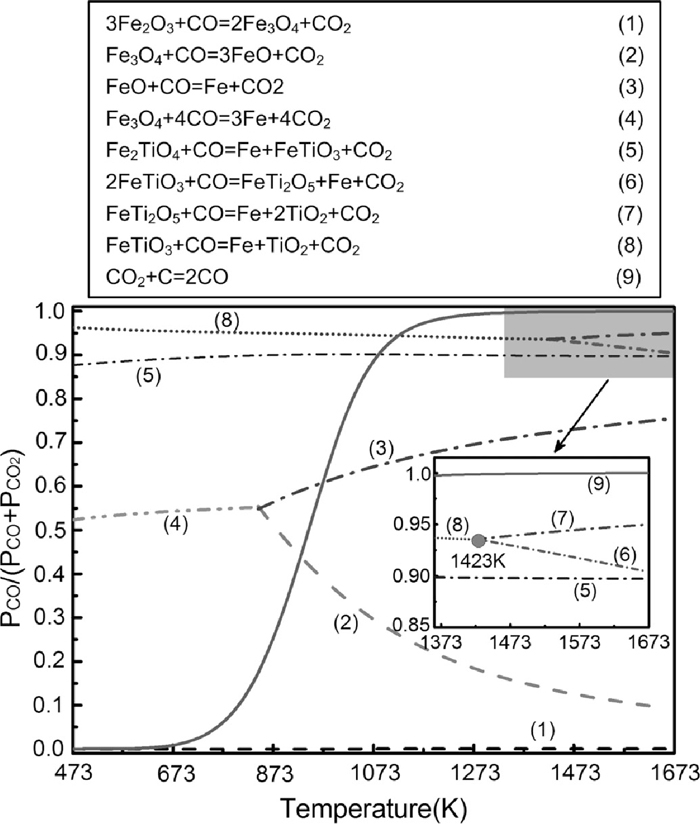
Dependency of equilibrium atmosphere for gas-solid reactions mainly occurred in the reduction of PTC on temperature.
A stepwise reduction of ilmenite was found from the calculation, which is also a “fork-like” reduction mechanism. The critical temperature is 1423 K, lower than which the ilmenite is reduced to iron and rutile, and higher than that the reduction of ilmenite becomes stepwise with ferrouspseudobrookite as the intermediate phase. This is because of the instability of ferrous-pseudobrookite at temperature lower than 1423 K according to the equilibrium binary phase diagram of FeO–TiO2.15)
When the Fe–Si are added, solid-solid reactions with silicon may occurs according to the Eq. (5):
| (5) |
Figure 4 presents the ΔGθ as the function of temperature for the main solid phase reactions probably occurred in the carbothermic reduction of PTC with Fe–Si. It is found that all of the solid phase reactions are thermodynamically feasible under the temperature in present study. All of reactions with carbon as reductant are endothermic, while the reactions with silicon as reductant are exothermal except the reaction (10).
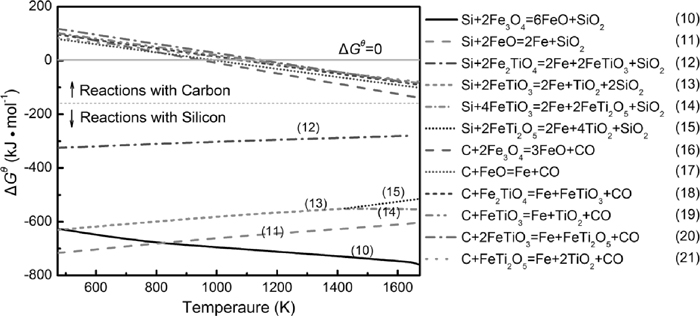
Dependency of ΔGθ on temperature for solid state reactions mainly occurred in the reduction of PTC.
The effect of Fe–Si addition on the carbothermic reduction of PTC was investigated by the isothermal method at 1623 K in argon atmosphere. Figure 5 shows the dependence of metallization degree of reduced briquettes on heating time. It was observed that the addition of Fe–Si accelerates the reduction rate of PTC. In the first 12 minutes of heating process, the metallization ratio of the samples with Fe–Si was higher than that without Fe–Si. This can be attributed to the following reason. During the heating process, part of silicon works as good reductant to participate in the reduction of PTC. The reduction of PTC by silicon (silicothermic reduction) is exothermic, which leads to the acceleration of reduction due to the local high temperature inside the sample. For the samples reduced for the same time, the more Fe–Si added, the higher degree of metallization achieved. However, after more than 12 minutes heating, the degrees of metallization increased insignificantly with the heating time increasing. This can be attributed to the lower concentration of reductants and reactants in the stage and the slow reduction rate resulting from the diffusion of reductant through product layer to the reaction surface. It is noticed that there was a little difference in the degree of metallization between the samples with Fe–Si and that without Fe–Si. This may be possible for two reasons. First, the concentration of ironbearing oxides became lower in the later reduction stage and some iron-bearing oxide particles were surrounded by reduction product, which resulting in the less solid-solid contact between silicon and iron-bearing oxide particles. Second, most of silicon probably was consumed in the early 12 minutes, leading to the lower concentration of silicon in the later stage.
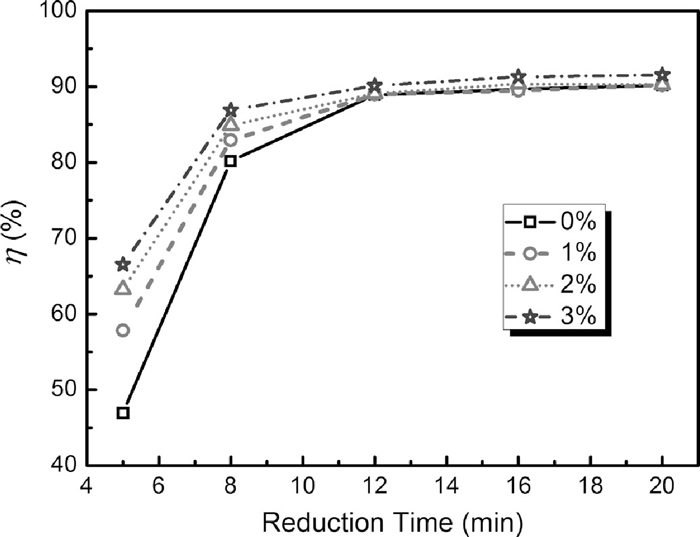
Degree of metallization vs reduction time for briquettes with different Fe–Si dosage reduced at 1623 K in argon.
The carbon residues in the reduced samples with various Fe–Si dosages as a function of the reduction time are presented in Fig. 6. It is found that the carbon residues of the samples increased with increasing the addition of Fe–Si. This indicates that part of silicon in the Fe–Si substitutes for carbon to reduce the PTC.
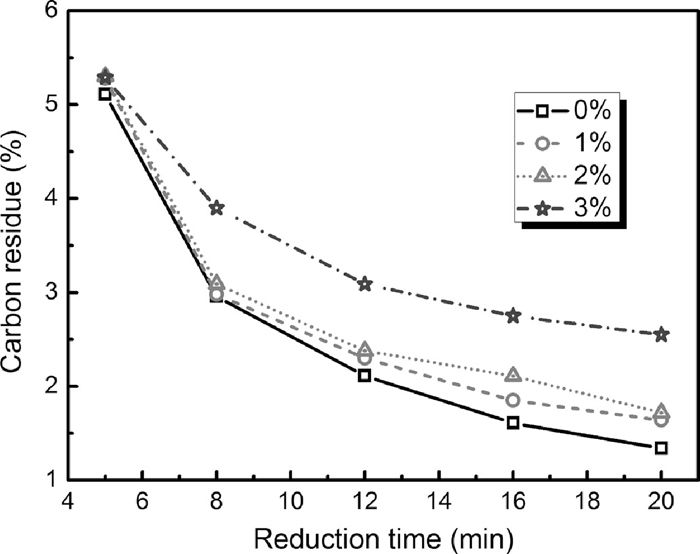
Carbon residue vs reduction time for briquettes with different Fe–Si dosage reduced at 1623 K in argon.
For the briquette without Fe–Si addition, the carbon residue (xRC (wt%)) can be calculated by the following equation:
| (6) |
For a certain value of metallization degree, mRC can be obtained according to Eq. (7):
| (7) |
where mTO (g) and mTFe (g) are the total mass of removable oxygen and that of iron element in the sample, respectively. mCC can be represented as follow:
| (8) |
here, mTC (g) is the total mass of fixed carbon in the briquette.
Combining Eq. (6) with Eqs. (7)–(8), carbon residue can be represented by Eq. (9):
| (9) |
Assuming that:
| (10) |
So, Eq. (9) can be rewritten as:
| (11) |
When Fe–Si was added, assuming that there is no effect on the degree of metallization for samples reduced under same conditions, the carbon residue for the reduced sample
| (12) |
where mFeSi (g) is the mass of Fe–Si added in the sample.
| (13) |
It is assumed that reduction by carbon and silicon occurs according to Eqs. (2) and (5), respectively. Thus,
| (14) |
| (15) |
| (16) |
| (17) |
Thus, combining Eq. (13) with Eqs. (14)–(17),
| (18) |
If no silicon acts as reductant, the value of
| (19) |
Based on the results of isothermal reduction of briquettes without Fe–Si addition, the relationships between degree of metallization and carbon residue for briquettes with various Fe–Si dosages were obtained, shown in Fig. 7. The shadow area represents the theoretical area where the carbon residues of reduced briquettes with Fe–Si should be located. It was observed that all of carbon residues of reduced samples, for all Fe–Si dosages, located in the theoretical area. It was noticed that the carbon residues of sample with 1% Fe–Si dosage, after reduced for 12 minutes, were more close to the theoretical minimum while that with 3% Fe–Si dosage were more close to the theoretical maximum. This indicated that more silicon replaced carbon acting as reductant when more Fe–Si were added under present experimental conditions. Thus, larger carbon residues for briquette with 3% Fe–Si dosage were observed in Fig. 6.
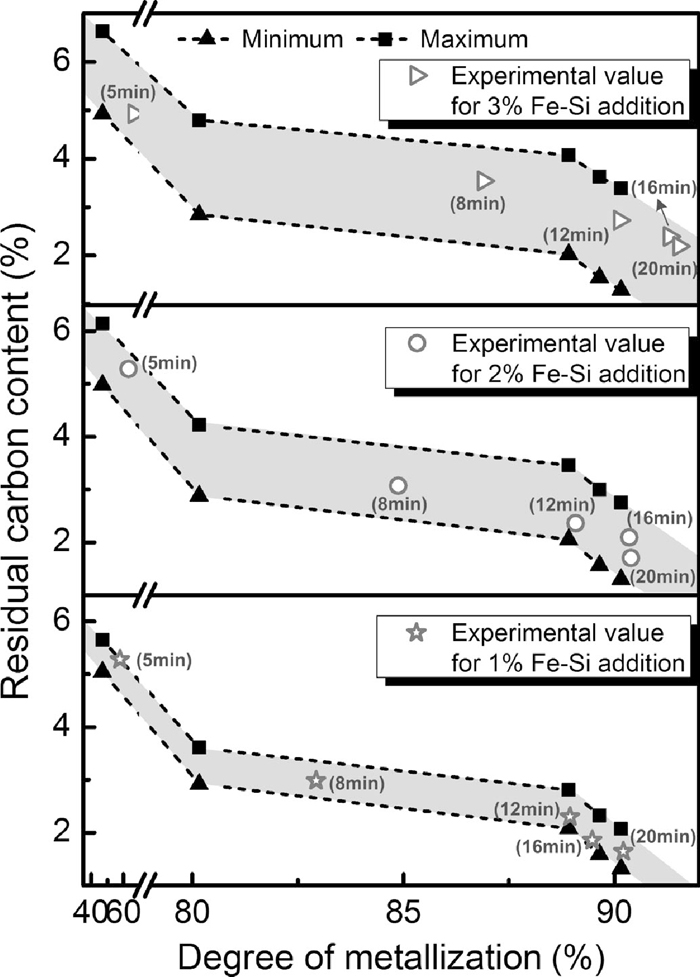
Relationships between degree of metallization and carbon residue for briquettes with various Fe–Si dosages reduced at 1623 K.
The PTC-coal mixtures with various Fe–Si dosages were heated from room temperature to 1723 K at a heating rate of 10 K/min under argon atmosphere, the results are presented in Fig. 8. It shows that Fe–Si has no influence on the mass loss of mixture when the temperature is below 1148 K (Fig. 8(a)). During this heating period, the devolatilization reactions of coal mainly occurred. When the temperature was higher than 1148 K, the mass loss of mixture with Fe– Si decreased with the increase of the Fe–Si dosage, and became slightly smaller than that of mixture without Fe–Si. There is no mass loss was detected for all specimens at temperature of higher than 1673 K. The maximum mass loss is approximate 33.6% for all specimens except for the specimen with a Fe–Si dosage of 3%, which has a smaller mass loss, about 31.7%. This can be attributed to the silicothermic reduction reactions occurred during the heating process, which results in no mass loss due to the all of reaction products formed are solid. For the specimen without Fe–Si, PTC was reduced by carbon (or carbon monoxide) with the gaseous products (CO and CO2) formation. Therefore, the mass loss detected during heating process consists of the volatile release, carbon and oxygen loss by the reduction. When Fe– Si is added, the PTC can be partially reduced by silicon, resulting in that the removed oxygen from iron-bearing oxides remain in the specimen. Moreover, the substitution of carbon by silicon as reductant, to a certain extent, makes the consumption of carbon decreased.
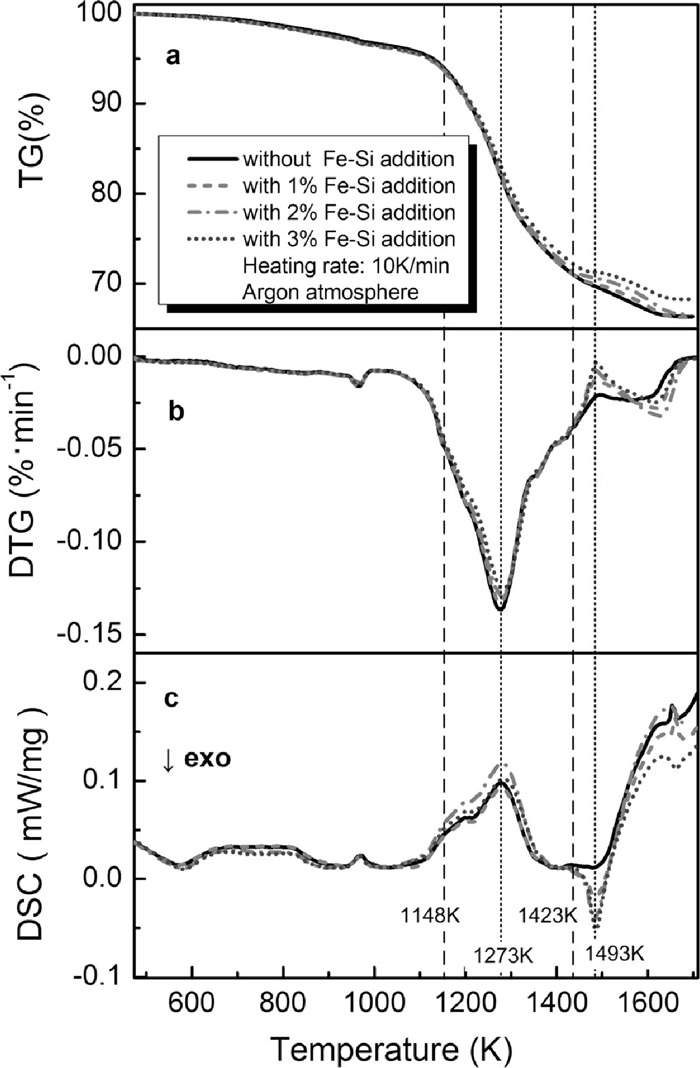
TG-DTG-DSC curves for PTC-coal mixtures with different Fe–Si dosages during non-isothermal heating process.
The DTG curves for mixtures are shown in Fig. 8(b). It was found that the mass loss rates of specimens with Fe–Si became slower than that of specimen without Fe–Si at the temperature of higher 1148 K, owing to the occurrence of reduction reactions with silicon. The mass loss rate for all of specimens reached the maximum at 1273 K. When the temperature was higher than 1273 K, the mass loss rates became almost the same, which slowed down as heating proceeding, until the temperature reached 1423 K. This is because that reduction by CO becomes significant due to the fast carbon gasification rate when the temperature is higher than 1273 K. Although it is thermodynamically feasible, the rate of reduction by silicon is kinetically much slower compared to that of reduction by CO due to the limitation of contact between iron oxides and silicon particles and diffusion of reactants through the product layer to reaction surface. So, the reduction by CO made more contributions to the overall reduction of PTC in the temperature range from 1273 K to 1423 K. Therefore, the mass loss rates are almost same for samples with Fe–Si and those without Fe–Si.
It was noticed that there was difference in the DTG curves caused by the addition of Fe–Si when temperature is higher than 1423 K. The mass loss rate of specimen without Fe–Si decreased to 0.021%/min at 1493 K and then kept constant and then decreased gradually to 0 when the temperature higher than 1613 K. However, for specimens with Fe–Si, the mass loss rates reached a lower value than that of specimen without Fe–Si at 1493 K and then increase until the temperature reached 1618 K. This can be attributed to the occurrence of exothermic reduction reactions with silicon. The DSC curves of mixtures are shown in Fig. 8(c). For specimens with Fe–Si, the height of the exothermic peak at 1493 K increased with increasing the Fe–Si dosage, meaning more heat generated. As the heating proceeds, the reduction rate became slow and the specimen sintered. These factors improve the solid/solid contact inside the specimen, resulting in the feasibility for the reduction reactions with silicon as reductant. The mass loss rate for samples with Fe–Si decreased due to the silicothermic reduction dramatically occurred at this temperature range. When the temperature was higher than 1493 K, the mass loss rate for samples with Fe–Si increased. This is because that the carbothermic reduction rate became significant, due to the depletion of silicon and the acceleration of carbothermic reduction reactions, resulting from the heat release of silicothermic reduction reactions.
3.3. Microstructure EvolutionFigure 9 shows the SEM micrographs (backscattered electrons) of briquettes with different Fe–Si dosages reduced at 1623 K for 12 minutes. It is observed that the metallic iron phase (bright area) in the Fe–Si free briquette is zonal structure and that in the briquette with 1% Fe–Si addition coalesces and forms the bread-like structure. The granular structure metallic iron phases are found in the briquettes with the Fe–Si dosages of higher than 1%. The greater the Fe–Si dosage, the larger the metallic iron particle. This is an indication that nucleation and coalescence of metallic iron was facilitated in the briquettes with Fe–Si. During the reduction process, the iron particles introduced by Fe–Si works as nucleation particles to promote the nucleation of iron and the occurrence of reduction reactions with silicon as reductant, which is exothermal, facilitates the coalescence of iron particles.
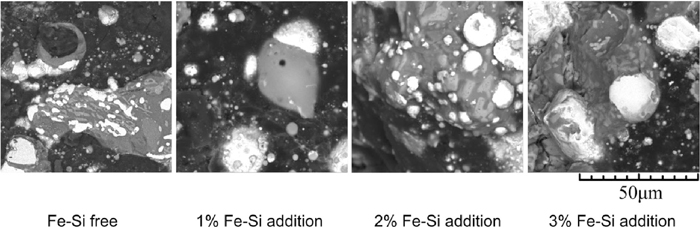
SEM images (backscattered electrons) of briquettes with different Fe–Si dosages reduced at 1623 K for 12 minutes. The bright area is metallic iron.
Based on the results obtained from isothermal and nonisothermal heating experiments, the reaction mechanism for carbothermic reduction of PTC with Fe–Si addition is proposed and schematically shown in Fig. 10. The whole reduction process can be described as follows.
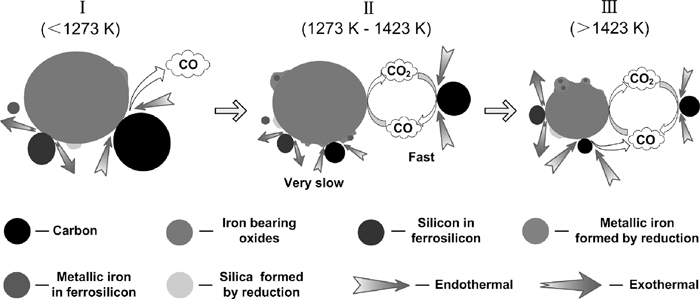
Schematic of the reaction mechanism of carbothermic reduction of PTC with Fe–Si addition.
(I) In the first stage (lower than 1273 K), the iron-bearing oxides are predominately reduced by carbon and silicon. This process is the solid phase reaction between iron-bearing oxides and reductants, whose reaction rate depending on the solid-solid contact is greatly slow. The endothermic reduction by carbon is locally enhanced by the heat supply from the exothermic reduction with silicon.
(II) In the second stage (1273–1423K), as the reduction proceeds, the reduction of iron-bearing oxides with CO as reductant becomes significant due to the fast carbon gasification rate when the temperature is higher than 1273 K. The rate of reduction by silicon is much slower than that by CO, in despite of the thermodynamic feasibility. The overall reduction rate is mostly contributed by the rate of reduction by CO. Therefore, the Fe–Si addition has little influence on the reduction rate in this stage. However, a few of iron particles in Fe–Si, acting as the nucleation particles, promote the nucleation of iron obtained by reduction.
(III) In the final stage (higher than 1423 K), the reduction rate become slower due to the limitation of diffusion rate of gases through the porous product layer and the briquette gets denser owing to the sintering mechanism. Thus, the solid- solid contact is improved and the reduction by carbon and silicon markedly occurs again. The heat released from the exothermic reactions with silicon makes the temperature in neighboring area higher, which facilitates the carbothermic reduction and promotes the coalescence of metallic iron.
The effect of Fe–Si on the carbothermic reduction of Panzhihua titanomagnetite concentrates was investigated by isothermal and non-isothermal experiment, respectively. The conclusion can be summarized as follows:
(1) The addition of Fe–Si accelerates the carbothermic reduction rate of PTC. A part of silicon in the Fe–Si substitutes for carbon to participate the reduction of PTC.
(2) The addition of Fe–Si facilitates the nucleation and coalescence of metallic iron formed by reduction. The greater the ferrosilicon dosage, the larger the metallic iron particle.
(3) A reaction mechanism for the carbothermic reduction of PTC with Fe–Si addition was proposed. In the first stage (lower than 1273 K), the solid phase reactions with carbon and silicon as reductants are dominant. The exdothermic reduction by silicon, to certain extent, promotes the reduction of PTC. In the second stage (1273–1423), the rate of reduction by CO is much faster than that of reduction by silicon, resulting in little influence of Fe–Si on the reduction of PTC. Fe–Si has little influence on the reduction of PTC. In the final stage, the reduction by silicon markedly occurs again, which further facilitates the reduction of PTC and the coalescence of metallic iron.
The authors are especially grateful to Scholarship Award for Excellent Doctoral Student granted by Ministry of Education (Grant No. 0903005109081-8) and Key Project of Chinese National Programs for Fundamental Research and Development plan (No. 2013CB632603).