2013 年 53 巻 4 号 p. 603-612
2013 年 53 巻 4 号 p. 603-612
A process model was proposed by Järvinen and co-authors for modelling the side-blowing decarburisation stage of the Argon-Oxygen Decarburisation (AOD) process. Here, a new model for the reduction stage has been derived and coupled with the earlier-developed model. The model considers mass-transfer controlled reversible reactions between the steel bath and the top slag. The effect of emulsification phenomena on the total reaction area and on the mass and heat transfer characteristics have been taken into account. The effects of various additions on the mass and heat balance have also been considered. The paper is divided into two parts: Part I presents the derivation of the model, while Part II considers validation of the model with full-scale production data from a 150 t AOD converter at Outokumpu Stainless Oy, Tornio Works, Finland.
The Argon Oxygen Decarburisation (AOD) process invented in 1954 by Union Carbide Corporation1) is the most widespread process used for making stainless steel, accounting for more than two thirds of total world production.2) In modern smelting plants the process is usually combined with an Electric Arc Furnace (EAF), in which the raw material or scrap is melted before the AOD treatment.2)
The main stages in the AOD process are decarburisation, reduction of the top-slag and desulphurisation. The objective of the decarburisation stage is to reach the target carbon content of the melt. At first, combined top and side-blowing is used for maximum oxygen delivery into the melt, because at high carbon levels, the decarburisation rate is controlled by the oxygen mass transfer in the bath.3) The turning point in the decarburisation rate of carbon is often referred as the critical carbon content,4) after which the decarburisation rate is controlled by mass transfer of carbon.3) Hence the combined blowing-stage is followed by a side-blowing stage, in which nitrogen and argon are used to dilute the blowing mixture in order to avoid excessive oxidation of chromium and other alloying elements.
In spite of the dilution of the blowing mixture during the side-blowing stage, some oxidation of alloying elements inevitably takes place and on this account, reduction of slag is conducted after the decarburisation stage. The reduction of top slag is stimulated by the addition of reducing agents and simultaneous argon blowing through tuyères, which not only enables efficient mixing of reductants, but also promotes emulsification of top slag at the steel-slag interface. Consequently, the steel-slag reaction surface area increases immensely, resulting in a rapid reduction of slag species of lower oxygen affinity by silicon. A typical composition of the top slag before and after reduction stage is shown in Table 1.
Järvinen et al.5,6,7) have presented a process model, which considers mass transfer -controlled reactions and locally varying conditions in the plume zone. This model can be used to describe decarburisation and slag formation during the side-blown decarburisation stage of the AOD process.
The objective of this paper is to derive a new reaction model for considering the reduction of top slag during the reduction stage. The partly simultaneous desulphurisation of the steel is excluded from this paper. Before the derivation of this model, the existing AOD models and their applicability to the modelling of the reduction stage are briefly discussed.
The AOD process has been studied extensively by many authors, which has led to the development of numerous models.3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27) Most of the developed models concern the decarburisation stage, which takes place at the gas/liquid-metal interface.3) Decarburisation models have been thoroughly reviewed by Wei & Zhu.19) However, many new studies have been published during the last 10 years and it is necessary to briefly discuss some new developments.
| Composition [wt-%] | |||||||
|---|---|---|---|---|---|---|---|
| Sample | FeO | Cr2O3 | MnO | SiO2 | CaO | MgO | Al2O3 |
| Before | 2.1 | 38.2 | 2.8 | 9.1 | 42.4 | 1.9 | 1.8 |
| After | 0.8 | 1.0 | 0.4 | 29.9 | 56.7 | 8.9 | 1.7 |
One of the most fundamental tasks of an AOD reaction model is to determine how oxygen is consumed in the process. For example, Fruehan3,10) suggested that the majority of blown oxygen is consumed initially by the oxidation of Cr in the vicinity of the tuyére zone and that during its rise in the bath, most of the chromium oxide is reduced by carbon. Excluding the model proposed by Asai & Szekely,8,9) most models take the rate-controlling behaviour of carbon at lower carbon contents into account. Many of the older models assumed that all the blown oxygen is consumed immediately in the oxidation reactions,13,14,15,16,17) although some allowed the accumulation of unabsorbed oxygen in the steel melt.3,8,9) The models proposed by Wei & Zhu19,20) and Järvinen et al.6,7) allow the oxygen to either react, accumulate or escape the bath, although Wei & Zhu employ a predetermined constant for the proportion of escaping oxygen. However, the most significant improvement provided by these models is the assumption that all the oxidation-reduction reactions take place simultaneously at the reaction interface.
The mathematical model proposed by Zhu et al.,21,22) a continuation of the work by Wei & Zhu,19,20) enables the modelling of the whole AOD process, including the top, side and combined blowing operation in the decarburisation phase and the modelling of the reduction practice. Later, Wei et al.23) refined the model with more verification work and a new heat transfer model. During the reduction stage, reactions are assumed to occur at the steel–slag interface. Reduction rates of the oxides in the slag are determined through oxygen supply ratios determined by Gibbs free energies of the reactions considered in the model.
Sjöberg proposed an AOD reaction model,17) which later evolved into two commercial models: TimeAOD28) and UTCAS.29) Relating to this work, Görnerup & Sjöberg18) studied the modelling of the reduction stage focusing on desulphurisation. The latest version of the TimeAOD model has been coupled with thermodynamic software Thermo-Calc, which is used for providing information about the equilibrium fraction of phases in the top slag.28) The Computational Fluid Dynamics (CFD) model proposed by Andersson et al.24,25,26,27) has been coupled likewise with the Thermo-Calc software, but has been applied only to the decarburisation period.
The AOD Converter Process Simulator proposed by Järvinen and co-authors6,7) is an extension of the earlier bubble reaction sub-model5) for the side-blowing operation. It considers all main reactions during the decarburisation stage and employs a novel approach for simultaneous solution of the local thermodynamic equilibriums and constraining mass-transfer onto and from the reaction surfaces. In addition to the oxidation-reduction reactions, the adsorption/desorption of nitrogen is taken into account with a sub-model developed by Riipi et al..30)
A new approach was adopted for considering the vertical distribution of composition, temperature, pressure and element activities in the plume zone. The process model represents the AOD converter as a combination of a Plug Flow Reactor (PFR), a Continuously Stirred Tank Reactor (CSTR) and a top slag zone. All the chemical reactions are assumed to take place in the plume zone simulated by the PFR, which is divided vertically into numerous computational cells. After the PFR, the slag phase ends up in a separate surface slag zone and the metal phase continues to the CSTR and eventually circulates back to the lowest computational cell of the PFR. This is a significant improvement over traditional approaches, which use average values for the whole reaction zone. However, the oxides in the surface slag were considered non-reducible and hence reactions between the metal bath and top slag were not taken into account.
As discussed above, the mathematical models for the side- and top-blown decarburisation stage have evolved from simplistic descriptions into complex process models capable of accurate predictions. However, much less attention has been paid to the reduction stage. Although numerous studies have been conducted on the emulsification of slag31,32,33,34,35,36,37,38,39,40,41,42) and reduction of slag18,43,44,45,46) as separate research fields, a phenomena-based model capable of combining the present knowledge of both phenomena has not been previously discussed in the literature.
In the previous work,6,7) the surface slag was considered non-reducible and hence had no active role during decarburisation. Therefore, it is obvious that the initial model cannot be used to observe the reduction stage. In this paper, a new reaction model is proposed for considering the reduction stage at the end of the AOD treatment.
During the reduction stage, two reaction interfaces can be identified: the steel–slag droplet interface and the steel–slag interface (see Fig. 1). In this context, emulsification of slag droplets is caused by the turning around of fluid flow at the top of the plume eye.47) When the critical interfacial velocity is exceeded, the surface area of the slag droplets is typically multifold compared to the plain interfacial area of the steel-slag interface.33) Moreover, the contribution of the steel–slag droplet interface is accentuated by the high mass transfer rate resulting from vigorous argon stirring and small slag droplet size. The steel–slag interface is handicapped by the slow mass transfer of slag species caused by inefficient mixing in the top slag.
In the model derived in this paper, it is assumed that the contribution of the steel–slag interface to the total reaction rates is negligible and that the dominating phenomena during the reduction stage can be accurately described by reactions occurring on the surface of the emulsified slag droplets. Furthermore, it is assumed that the species from the top slag, metal bulk and plume gas phase establish a thermodynamic equilibrium at the surface of the slag droplets, in which only one average composition, one average temperature and one average surface area are considered. From a modelling perspective, the steel–slag droplet interface is represented by a multi-phase surface element with zero thickness. The model derived in this paper is programmed with C/C++ and coupled with the previously proposed model for side-blowing decarburisation stages as illustrated in Fig. 2.
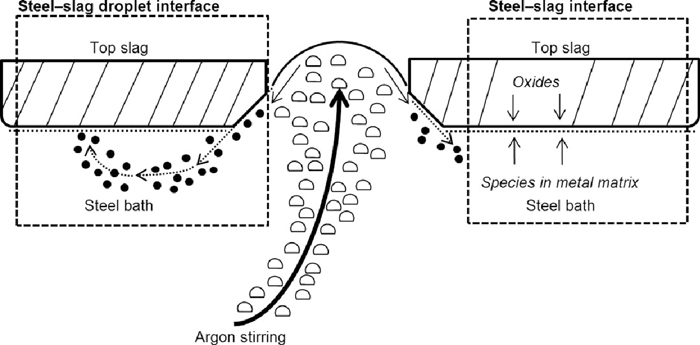
Schematic illustration of the two interfaces considered in the model.
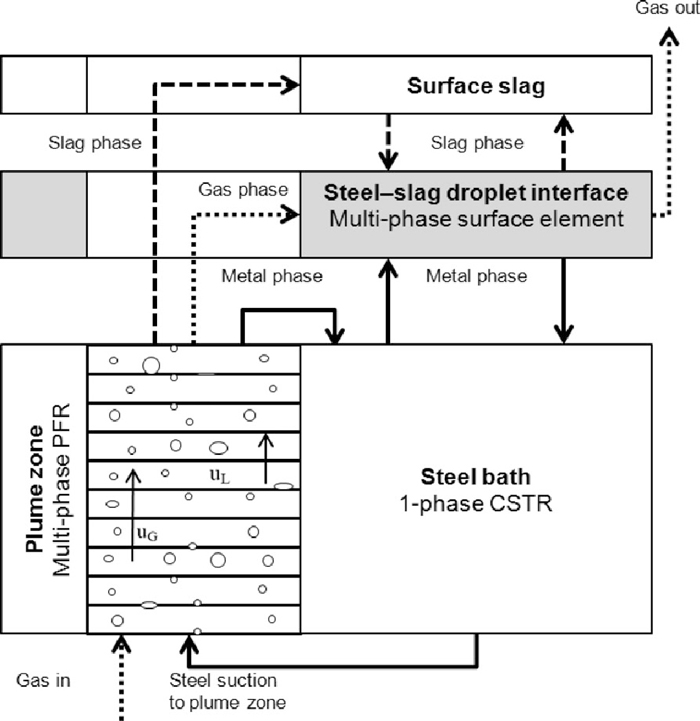
Schematic illustration of the modified process model.
The total number of species considered in the system is 21. The metal phase is a concentrated Fe–Cr–Mn–Si–C–O–N–Ni alloy, while the gas phase is considered to consist of O2, CO, CO2, N2 and Ar.
The liquid top slag is constituted by FeO, Cr2O3, MnO, SiO2, CaO, MgO, Al2O3 and MeOx, which is used to describe oxides that are excluded from the model. For all purposes, CaF2 was taken here as the calcium ion. Chromium oxide is assumed to exist only in its trivalent form, due to the high basicity of decarburisation slags.48) At first, it is assumed that direct accumulation of CaO into the slag takes place, to act as a slag former.
The approach proposed by Järvinen5,6) is employed here for solving the multi-phase system considered in the surface element. In this approach, it is assumed that if the chemical rate is sufficiently high, the reaction rates will be controlled by mass transfer only, and the exact value of the forward reaction constant kf becomes irrelevant as long as it satisfies the equilibrium condition. This enables the simultaneous solution of thermodynamic equilibrium at the interface and the mass transfer of species onto the interface. This approach has been tested successfully for modelling the plume zone of the AOD vessel during the decarburisation stage5,6,7) and for chemical heating in the CAS-OB process.49) The proof of this approach is not repeated here.
Taking these considerations into account, the chemical reactions and their corresponding rate expressions can be formulated as follows:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
Although reactions with refractory materials are excluded from this work, the effect of refractory wear on the slag composition has been taken into account with a fixed constant for dissolution rate of refractory material into the slag. Moreover, the effect of refractory wear on the converter geometry has been taken into account as a loss of refractory lining thickness per heat. The converter geometry affects, for example, the height of the steel bath and the thickness of the top slag layer. Reactions between the top slag and the refractory lining are taken into account with an average dissolution rate of refractory material into the top slag, which is calculated from the loss of refractory lining thickness per heat.
It should be noted here that the conservation of mass and heat are solved consequently, not simultaneously. However, if sufficiently small time steps are used, no significant inaccuracy is caused, while the stability of calculations is substantially improved.
3.2. Conservation of MassDiscretisation of the conservation equations is carried out with an implicit finite-difference method, using a 1st order Euler-method for time integration. Conservation of species i in the metal (8), gas (9) and slag phase (10) at the interface are expressed below:
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
| (16) |
| (17) |
The conservation of heat in the isothermal surface element is defined by heat transfer and reaction enthalpies (18).
| (18) |
| (19) |
| (20) |
| (21) |
| (22) |
| (23) |
As mentioned earlier, the surface area of the emulsified droplets is taken as the total reaction area in the model. By assuming a uniform droplet size, the total surface area of the droplets can be approximated using Eq. (24).
| (24) |
| (25) |
| (26) |
| (27) |
| (28) |
Based on results with CFD modelling,51) the plume diameter was set to 1.5 m. The interfacial velocity ui is defined by:47)
| (29) |
| (30) |
| (31) |
| (32) |
The angle between inertial and vertical force was set to α = 30° based on laboratory scale experiments.42) A linear approximation has been used for calculating the density of the steel bath with respect to temperature:
| (33) |
| (34) |
| (35) |
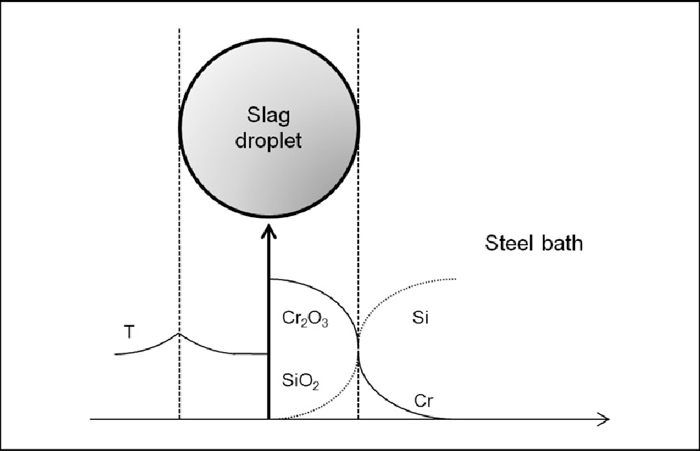
Schematic illustration of the slag droplet model.
| (36) |
The surface renewal model proposed by Higbie59) has been used for determining heat and mass transfer constants in the boundary layers on both sides of the slag droplet as illustrated in Fig. 3. The mass and heat transfer coefficients for metal, gas and slag phase are given by:
| (37) |
| (38) |
| (39) |
Some diffusion coefficients in liquid steel are illustrated in Table 2. However, as the model employs an effective diffusion model, only one effective diffusion coefficient is determined for each phase. The molecular diffusion coefficients for the steel and slag phases are set to Dm, L = Dm, S = 4.0×10–9 m/s2, which are roughly in the same range as the values provided in Table 2. The temperature-dependency of diffusion coefficients is excluded in this work. For the gas phase, the following equation is used:
| (40) |
| (41) |
| (42) |
| Species | Dm x 10–9 [m2/s] |
|---|---|
| Cr | 3–5 |
| Mn | 3.5–20 |
| Si | 2.5–12 |
| C | 4–20 |
| O | 2.5–20 |
| N | 6–20 |
| Ni | 4.5–5.6 |
| (43) |
| (44) |
| (45) |
It has been shown that both bubble size and gas-metal slip velocity vary strongly within the AOD vessel.63,64) Iguchi et al.63) have reported a bubble size of 25 mm and a corresponding slip velocity of 0.5–0.7 m/s in the Fe–Ar system in the plume zone of the vessel. Tilliander et al.64) have reported slip velocities in the order of 0.01 m/s outside the plume area during oxygen-blowing. Moreover, bubble size increased slightly towards the surface of the plume eye, being approximately 45 mm in the vicinity of the surface. In this paper, bubble size and corresponding velocity difference with the liquid phase were estimated by the following equations:65,66)
| (46) |
| (47) |
Equilibrium constants are calculated using the enthalpy and entropy data presented in Table 3. The average reaction enthalpies and entropies at 1800–2100 K were first calculated for pure species using the thermodynamic database of the HSC Chemistry67) and then converted to the selected standard states using literature data.68) The Henrian reference state (1 mol-%) is employed for the liquid species, while the reference states of the gaseous and slag species are pure gaseous species and pure liquid species, respectively. The equilibrium constant of reaction k is defined by:
| Reaction | ΔH [J/mol] | Δh [J/kg] | ΔS [J/(mol·K)] |
|---|---|---|---|
|
|
–234304.0 | –7322274.6 | –50.0 |
|
|
–141141.4 | –11751011.4 | 67.0 |
|
|
–419249.5 | –34905457.7 | –16.9 |
|
|
–250982.1 | –4494101.0 | –56.8 |
|
|
–1045107.1 | –10049879.4 | –188.1 |
|
|
–371418.3 | –6760681.3 | –90.9 |
|
|
–806109.5 | –28701981.7 | –178.3 |
| (48) |
There are relatively well-established models for calculating activities in a steel melt, such as the Unified Interaction Parameter Model (UIP),69) which is in principle a generalisation of Quadratic Formalism70,71) and Wagner-Lupis- Elliott formalism.72,73) Here, the UIP model is used for calculating the activity coefficients for the liquid metal species at the steel-slag interface, employing the Henrian standard state (1 mol-%) and the molar interaction parameters
| (49) |
| (50) |
Silicate slag poses a challenging area for thermodynamic modelling due to its netlike structure and complex interactions between the slag species. Sophisticated slag models are indeed fairly difficult to incorporate into computationally efficient process models, and therefore many authors have chosen to use simplistic thermodynamic models or semi-empirical equations.
| ij / jk | Fe | Cr | Mn | Si | C | O | N | Ni |
|---|---|---|---|---|---|---|---|---|
| Fe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cr | 0 | –4.974) | 0.975) | 3.276) | –4.975) | –4.8477) | –9.875) | –0.002775) |
| Mn | 0 | 0.975) | –0.64274) | –3.375) | –1.975) | –4.777) | –4.575) | –1.875) |
| Si | 0 | 3.276) | –3.375) | 126.676) | 9.875) | –7.177) | 6.175) | 1.275) |
| C | 0 | –4.975) | –1.975) | 9.875) | 1375) | –20.077) | 7.275) | 2.475) |
| O | 0 | –4.8477) | –4.777) | –7.177) | –2077) | –10.777) | 0 | 2.477) |
| N | 0 | –9.875) | –4.575) | 6.175) | 7.275) | 0 | 0.7575) | 1.675) |
| Ni | 0 | –0.002775) | –1.875) | 1.275) | 2.475) | 2.477) | 1.675) | 0 |
Activity coefficients of the slag species at the steel-slag interface are calculated with equations given by Wei & Zhu.19) These equations are based on the slag model proposed by Wei.78) Unlike in the PFR used to describe the plume zone, the influence of CaO, MgO and Al2O3 has been taken into account in the surface element. CaF2 was considered here as the calcium ion, similar to Nakasuga et al.45) The relevant equations for activity coefficients of FeO (51), Cr2O3 (52), SiO2 (53) and MnO (54) are presented below:
| (51) |
| (52) |
| (53) |
| (54) |
At the beginning of the reduction stage, reducing agents are fed into the converter in order to maximise reduction of chromium oxides in the slag. The feeding time of additions is taken into account by a employing a constant feed rate separately for each batch of additions.
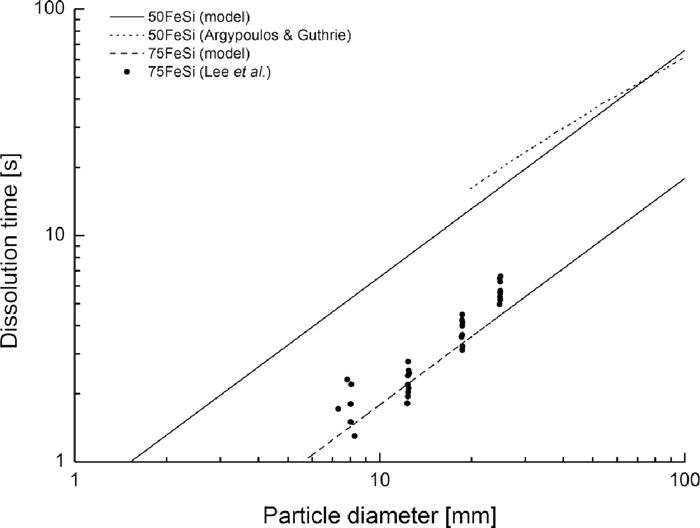
Given the amounts of FeSi typically fed into the vessel during the reduction stage, it is essential to consider related melting and dissolution behaviours. Experimental data for dissolution of 75FeSi particles is shown in Table 5. It is apparent that the dissolution time of FeSi is far shorter in the steel bath than it is in the slag bath. Simulations of fluid dynamics reported by Guthrie et al.79) suggest that FeSi additions do not surface but rather dissolve in the mixing zones located in the steel bath. Moreover, typical mixing times are estimated to be in the order of seconds due to vigorous argon stirring.80) Thus, it may safely be assumed that the limiting factor in the dissolution of additions in the steel melt is the melting of the material itself.
| Particle size [mm] | Average dissolution time in steel bath [s] | Average dissolution time in AOD slag [s] |
|---|---|---|
| 25.4 | 5.6 | 136.9 |
| 19.1 | 3.7 | 87.4 |
| 12.7 | 2.2 | 47.1 |
| Property | 75FeSi | 50FeSi | SiMn | Steel scrap |
|---|---|---|---|---|
| Fe [wt-%] | 23.8 | 50 | 10.3 | 99.0 |
| Si [wt-%] | 75 | 50 | 29.1 | 0.35 |
| Mn [wt-%] | 0 | 0 | 60.2 | 0.30 |
| C [wt-%] | 0.04 | 0.04 | 0.036 | 0.20 |
| dp [m] | 0.050 | 0.050 | 0.050 | 0.050 |
| ρp [kg/m3] | 280081) | 610085) | 612081) | 775084) |
| Tm [K] | 158981) | 168385) | 148881) | 179384) |
| lm [J/kg] | 144861781) | 83946885) | 49447381) | 27200084) |
| λ [W/(m·K)] | 2.9381) | 9.6281) | 4.18481) | – |
| λe [W/(m·K)] | 9979.4×dp | 5126.9×dp | 1153.6×dp | 1167.9×dp |
In this model, the dissolution time of the particles is estimated in its simplest form by observing the melting time of a one-dimensional spherical form.49) An analytical solution for the melting time is given by:
| (55) |
| (56) |
For particle sizes 10–50 mm, a good agreement with experimental results was achieved by assuming a linear dependency of effective heat conductivity from particle size (Figs. 4–5). The effective heat conductivities were substantially higher than the measured thermal conductivities as they include the effect of the exothermic reactions with the solid iron steel shell.81)
The temperature of the steel bath is updated upon additions according to the following equation:
| (57) |

The objective of the numerical solution method is to solve the thermodynamic equilibrium and the set of conservation equations for mass and heat for each time step. Mass transfer coefficients, droplet size and droplet formation rate are held constant during each time step. In the first iteration cycle, the thermodynamic equilibrium and conservation of mass are solved simultaneously, while temperature is held constant in order to improve numerical stability. This does not cause any significant modelling error when small time steps are used.
In total, the conservation of mass in the system is defined by 45 equations: 21 equations for conservation of species in the surface element, 21 equations for conservation of species in the bulk phases and 3 equations for conservation of total mass in the steel bath, plume and the top slag, respectively. Contrary to the Newton-like method applied in the plume zone, the full Newton’s method was applied for numerical solution in the reduction model. This algorithm can be expressed as follows:
| (58) |
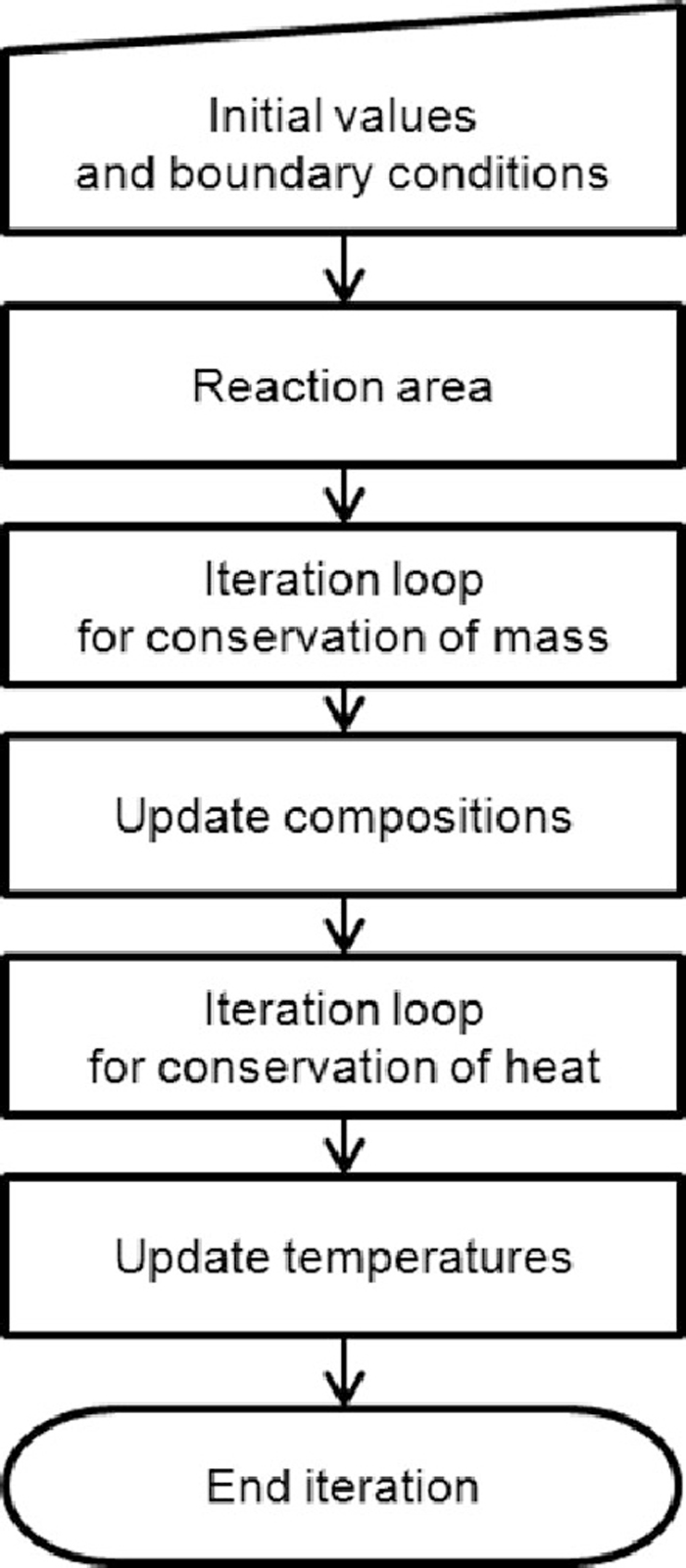
Flow sheet for the numerical solution of the surface element.
Figure 6 illustrates the flow sheet for the numerical solution of the surface element. The iteration loop starts with the calculation of a residual vector with the initial values. The Jacobian matrix is formed of differentiates with respect to all relevant species and masses, as illustrated below:
| (59) |
Although numerous mathematical models have been proposed for modelling the decarburisation stage, the reduction stage has received much less attention. The model derived in this paper is a systematic attempt to construct a mathematical model for describing the dominating reaction phenomena during the reduction stage and also an extension to the previous model derived for side-blowing operation.
From a modelling perspective, a surface element with zero thickness is used to represent the reaction interface through which all reactions are assumed to occur. More specifically, an approach based on the law of mass action has been applied here for the simultaneous solution of the thermodynamic equilibrium of species and the governing masstransfer onto the reaction interface. Thermodynamic submodels have been employed for considering the activities of metal and slag species.
In this model, it was assumed that the reactions between emulsified slag droplets and the steel bath account for the dominating rate phenomena during the reduction stage. Hence the reaction interface consists of the total surface area of the emulsified droplets residing in the steel bath at a given moment. The effects of various physical parameters on the emulsification phenomena, including viscosity and density of steel and slag phases, have been taken into account.
The graphical user interface of the converter model has been updated to include relevant user functions for the mathematical model for the reduction stage. The user interface allows the addition of scrap, reductants, slag formers and fluxes and considers different feed rates for each batch of additions. In addition, a simple melting model is employed for describing the melting time of 75FeSi, 50FeSi, SiMn and scrap in the steel bath. Validation of the reaction model proposed in this paper is discussed thoroughly in Part II.86)
Symbols
A: Surface area [m2]
Cd: Drag coefficient
cp: Specific thermal capacity [J/(kg·K)]
D: Diffusion coefficient [m2/s]
d: Diameter [m]
F: Force [N]
f: Conservation equation
g: Standard gravity [9.81 m/s2]
h: Mass transfer coefficient [m/s]
h: Specific enthalpy [J/kg]
Keq: Equilibrium constant
kf: Forward reaction rate constant
M: Molar mass [g/mol]
p: Partial pressure [Pa]
q: Heat flux [W/m2]
R: Universal gas constant
rm: Melting rate [1/s]
Re: Reynolds number
R″: Reaction rate vector [kg/(m2·s)]
T: Temperature [K]
Tm: Melting temperature [K]
tres: Residence time of slag droplets [s]
S: Molar entropy [J/mol·K]
u: Velocity [m/s]
ui: Interfacial velocity [m/s]
x: Mole fraction
y: Mass fraction
[Δx]: Correction vector
[J]: Jacobian matrix
Indices
a: Addition
B: Buoyancy
b: Bubble
dis: Dissolution
D: Slag droplet
D: Drag
G: Gas phase
G: Gravity
Bath: Steel bath (CSTR)
i: Species i
j: Species j
k: Species k
k: Reaction k
L: Liquid phase
S: Slag phase
s: Slip
SE: Surface element
Slag: Slag zone
p: Particle
Plume: Plume zone (PFR)
Greek symbols
α: Heat transfer coefficient [W/(m2·K)]
α: Interfacial angle [°]
Γ: Binary phase indicator
γ: Activity coefficient
ΔG: Change of reaction in Gibbs free energy [J/mol]
ΔH: Enthalpy of reaction [J/mol]
ΔS: Entropy of reaction [J/(mol·K)]
ε: 1st order interaction parameter
η: Dynamic viscosity [Pa·s]
λ: Thermal conductivity [W/(m·K)]
λe: Effective heat conductivity [W/(m·K)]
νi, k: Stoichiometric coefficient of species i in reaction k, mole basis
π: Mathematical constant
ρ: Density [kg/m3]
σ: Interfacial tension [N/m]
τm: Melting time [s]
τr: Characteristic relaxation time [s]
Φ: Heat loss rate [W]
This research is a part of the Energy Efficiency & Lifecycle Efficient Metal Processes (ELEMET), a research program coordinated by the Finnish Metals and Engineering Competence Cluster (FIMECC). Outokumpu Stainless Oy, Finnish Funding Agency for Technology and Innovation (TEKES), Graduate School in Chemical Engineering (GSCE) and Academy of Finland (projects 258319 and 26495) are gratefully acknowledged for funding this work.
The authors would like to thank Dr Paavo Hooli, Mr Pentti Kupari and Mr Veikko Juntunen from Outokumpu Stainless Oy for their support and productive discussions regarding the paper. In addition, Mr Tommi Kokkonen, Mr Aki Kärnä and Mr Tuomas Alatarvas are acknowledged for their support. Dr John Braidwood and Mr Aaron Bergdahl are acknowledged for revising the language of this paper.