2013 年 53 巻 7 号 p. 1138-1142
2013 年 53 巻 7 号 p. 1138-1142
Lab-scale experiment and industrial test for direct alloying with ferrosilicon-MoO3 self-reducing briquette have been conducted. In order to avoid volatilization of MoO3 during direct alloying, the high-temperature volatilization characteristics of MoO3 has been studied, and it can inhibit the volatilization of MoO3 when adding ~25% MgO during the heating. Furthermore, lab-scale direct alloying experiments with self-reducing briquette were carried out to determine the optimum composition of briquette to get high yield of Mo in a medium frequency induction furnace. The Mo yield was related to the excess reducing agent (ferrosilicon) and the right flux addition (MgO, CaF2) inside the briquette. The results indicated that the amount of ferrosilicon maintaining the mixing molar ratio Si/O at 2 should be added. The flux addition must be controlled to form molten slag rapidly inside the self-reducing briquette. With the optimum composition of reducing agent and flux addition, the yield of Mo could reach as high as ~96%. When adding the self-reducing briquette into a 60 t ladle of Shijiazhuang Iron and Steel Co. Ltd. for industrial test, the average yield of Mo was 98.25%, and the change in the technology did not have an impact on the quality of products.
Ferromolybdenum is an additive of considerable importance for alloying steel in the steelmaking industry. However, the production of ferromolybdenum is quite material - and energy-intensive and entails relatively large losses of molybdenum. A novel technology is becoming an emerging process for direct alloying with molybdenum oxide as the alloying addition, these oxides normally being used in the production of ferroalloy. Z. B. Li et al.1) has tried to conduct a test in an electric arc furnace that carbon and molybdenum oxide powders were successively added into furnace at the reducing stage, and molybdenum would be reduced into the steel from molybdenum oxide by carbon which melted. But the yield of Mo was not sufficiently high. The literature2) shows that MoO3 has a relatively low melting point, 795°C. That would imply that it may be easy to volatilize at the steelmaking temperature. Inevitably, it will lead to a low yield of Mo during direct alloying.
As MoO3 is belong to acidic oxides, it can react with basic oxides to form stable compounds. Hence, the present work was carried out in order to investigate the effect of inhibiting the molybdenum oxide’s volatilization with addition of magnesia in a carbon tube furnace. In addition, as the technology of self-reducing briquette could accelerate the reducing process and improve the metal recovery,3,4,5,6) the briquette with ferrosilicon-MoO3 composite has been prepared and added into a medium frequency induction furnace for direct alloying experiment. At last, an industrial scale test has been carried out in a 60 t ladle of Shijiazhuang Iron and Steel Co. Ltd., and it has achieved a relatively good result.
Table 1 shows the chemical compositions of molybdenum oxide powders, and Fig. 1 gives the XRD pattern of molybdenum oxide powders. After being dried enough, a corundum crucible with molybdenum oxide powders (weight: 50 g) would be placed in a carbon tube furnace. The sample was heated to 1600°C in argon atmosphere and kept at the temperature for ten minutes. And then the sample would be taken out for cooling to the room temperature. The volatilization rate of MoO3 was calculated by the quality loss of the sample. In addition, in order to explore the effect of inhibiting the molybdenum oxide’s volatilization, the same heating experiments would be carried out for another five samples with a composite of molybdenum oxide powders and different proportions of magnesia powders (99 wt% MgO), which should be mixed uniformly. And in the five samples, the quality of magnesia respectively account for 10%, 15%, 20%, 25% and 30%. Likewise, the volatilization rate of MoO3 is calculated by the quality loss of the sample, and also the reaction products for the composites would be analyzed by X-ray diffraction (XRD).
| Sample | MoO3 | CaO | SiO2 | MgO | Al2O3 | Na2O | K2O |
|---|---|---|---|---|---|---|---|
| MoO3 powders | 86.4 | 2.05 | 1.44 | 0.045 | 0.08 | 7.38 | 0.14 |

XRD pattern of molybdenum oxide powders.
It is quite volatile for MoO3 powders at high temperature. When heating to 1600°C, the volatilization rate of pure molybdenum oxide powders reached as high as 20.12%. As illustrated in Fig. 2, the addition of MgO has a significant effect on inhibiting the volatilization of MoO3 powders. When the addition of MgO reaches 25%, the volatilization rate could be down to 4.26%. Figure 3 gives the XRD pattern of reaction products. It shows that a new phase MgMoO4 was present in the product after adding MgO. The more MgO adding, the less the free MoO3 is. And most of MoO3 has been transferred to MgMoO4.
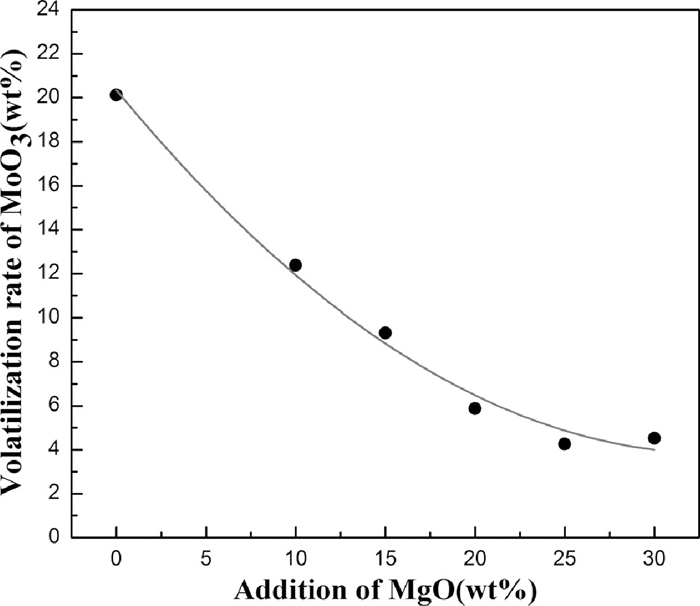
Effect of MgO addition on volatilization of MoO3 powder.
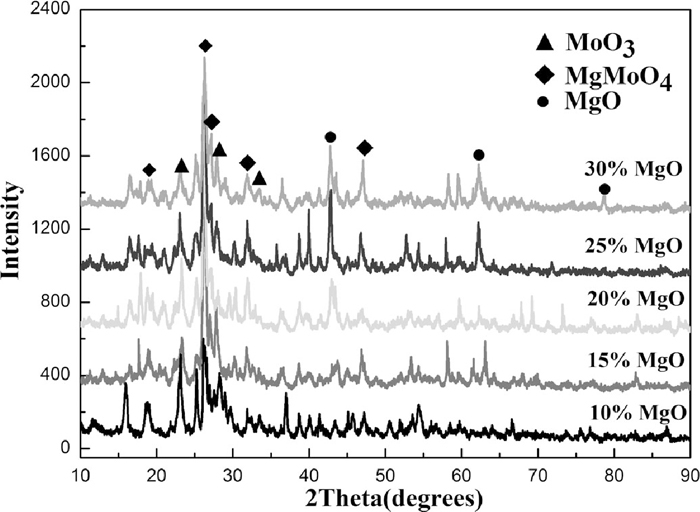
XRD pattern of reaction products.
The combination reaction takes place between MoO3 and MgO as shown in Eq. (1).7)
| (1) |
The ΔGo of above reaction indicates that MoO3 can spontaneously react with MgO into MgMoO4 at room temperature. The MgMoO4 with high melting point, is more stable during the heating process comparing to MoO3. Consequently, it is favorable for adding MgO to decrease the volatilization of MoO3 during the direct alloying, and the suitable proportion is ~25%.
For preparing the briquette, MoO3 powders as shown in Table 1, the reducing agent ferrosilicon (Fe-75%Si), magnesia (99 wt% MgO), and the flux (CaF2, 99+ wt%) were mixed. The particle size of the raw materials was <0.074 mm. After being mixed uniformly, the mixing powders would be pressed into a briquette of Φ20 mm × 20 mm. Eight different kinds of compositions of self-reducing briquettes would be designed and prepared for eight groups of direct alloying experiments, that being shown in Table 2. For the trial briquette 1, it maintains the mixing ratio [ferrosilicon mol(Si)]/[reducible oxygen mol (O)]=0.5 by the stoichiometric amount and excess of ferrosilicon amount in other trial briquettes.
| No. | Si/O (molar ratio) | Compositions of briquette, wt% | |||
|---|---|---|---|---|---|
| MoO3 powder | Ferrosilicon powder | MgO powder | CaF2 powder | ||
| 1 | 0.5 | 48.85 | 16.62 | 11.86 | 22.67 |
| 2 | 1.0 | 36.49 | 24.83 | 13.29 | 25.40 |
| 3 | 1.3 | 33.70 | 30.57 | 12.27 | 23.46 |
| 4 | 1.7 | 31.30 | 35.50 | 11.40 | 21.79 |
| 5 | 2.0 | 26.49 | 36.05 | 12.87 | 24.59 |
| 6 | 2.3 | 24.99 | 39.68 | 12.14 | 23.20 |
| 7 | 2.0 | 35.13 | 47.81 | 17.06 | 00.00 |
| 8 | 2:0 | 22.31 | 30.37 | 16.26 | 31.07 |
In this experiment, a medium frequency induction furnace with 15 kg in capacity was used. Schematic illustration of the experimental apparatus is shown in Fig. 4. The self-reducing briquettes were placed in the feeder in advance. When the ingot iron in crucible was fully molten, the self-reducing briquettes would be added into the molten iron. After the briquettes melted fully into slag, the molten steel was poured into the ingot mold for cooling. The content of molybdenum of steel ingot was analyzed, and the following equation calculates the yield of molybdenum
| (2) |
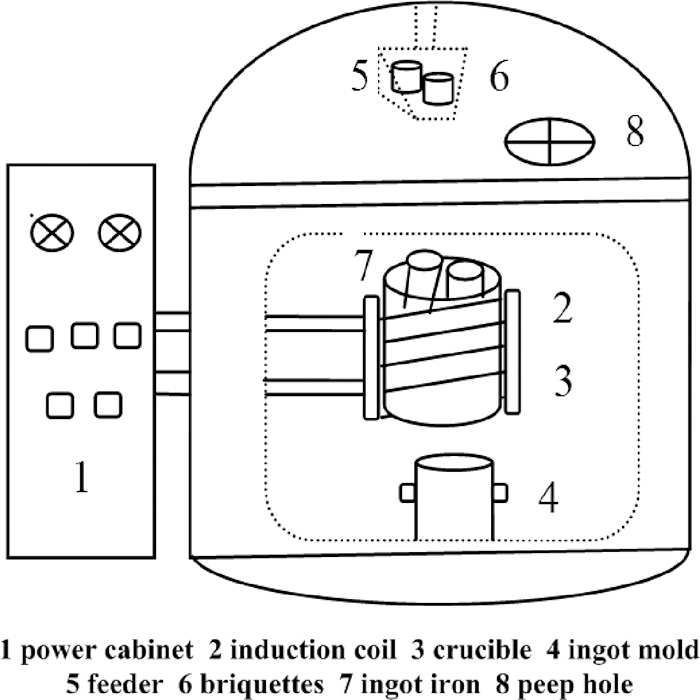
Schematic illustration of the experimental apparatus.
Where m1(g) is the mass of steel ingot; m2(g) is the mass of adding briquettes; ω1(%) is the mass percentage of Mo in steel ingot; ω2(%) is the mass percentage of Mo in the adding briquettes.
3.3. Results and Discussions 3.3.1. Process of Reducing and AlloyingFigure 5 shows the diagram of relationship between standard Gibbs free energy of chemical reactions and temperature based on the thermodynamic data.7) Once the briquette is added into the molten iron, the temperature inside the briquette became higher and higher. And Eqs. (3), (4), (5) show that the chemical reactions would take place in the briquette by the thermodynamic state diagram.
| (3) |
| (4) |
| (5) |
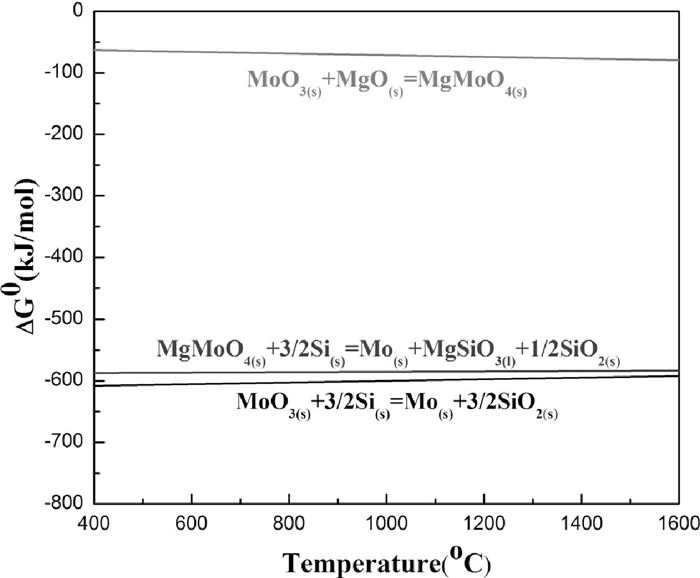
Diagram of relationship between standard Gibbs free energy and temperature.
In the self-reducing process, Mo generated gradually and diffused into the steel liquid. As the temperature rise continuously, the briquette began to melt. In that case, the activity of Si at liquid would increase and it was good for the reducing reaction. Meanwhile, it is easy for Mo to diffuse from briquette to steel liquid.
3.3.2. Effect of the Amount of Reducing AgentFor the trial briquette 1–6, of which the Si/O (molar ratio) varies from 0.5 to 2.3, the yield of Mo in the direct alloying is shown in Fig. 6. It can be observed that the amount of reducing agent has a significant effect on the yield of Mo during the direct alloying. The figure shows that the higher the molar ratio of Si/O, the higher the yield of Mo in direct alloying. When the molar ratio of Si/O reaches 2, the yield could be as high as ~96%. Obviously, only when excess of ferrosilicon is added can MoO3 be reduced entirely in self-reducing briquette. With excess addition of ferrosilicon, the MoO3 powders would be fully surrounded by ferrosilicon powders. In that case, it could increase contact area between MoO3 powders and ferrosilicon powders, and MoO3 would be fast reduced out. Only when the reducing process goes faster than the volatilization of free MoO3 (some has not formed into MgMoO4), the volatilization loss of MoO3 will decrease as much as possible. Hence, in order to accelerate the self-reducing reaction and decrease the volatilization of MoO3 during direct alloying, the excess of reducing agent should be added into the briquette. However, the excess of ferrosilicon will lead to the increase of [Si] in the steel liquid during the direct alloying. Accordingly, for the adding amount of ferrosilicon, it is not just designed for the fully reduction, but also considering the specific type of steel.

Effect of the amount of reducing agent on yield of Mo.
Table 3 gives the final slag composition that being calculated in the case of fully reduction of MoO3. According to SiO2–MgO–CaF2 phase diagram8) shown in Fig. 7, the final slag composition of the trial briquette 1 is marked at point a, and point b, c, d, e represent for the trial briquette 2–4, 5–6, 7 and 8 respectively. For the trial briquette 3–6, the final slag compositions all stay at the low liquidus temperature (<1100°C), which marked point b and c in the phase diagram. And their yields of Mo are quite high as 89.65–96.25%. But for the trial briquette 7 with no addition of CaF2, the yield of Mo is just as low as 70.26%, of which the final slag composition stays at a high liquidus temperature marked point d. In trial briquette 8, in spite of adding more CaF2, a yield (94.56%) nearly with that in trial briquette 4 and trial briquette 5 was obtained. There was no effect on improving the yield by adding excess of CaF2. It had been enough for adding ~24% CaF2 in briquette to form into a low melting point final slag, and a high yield of Mo would be obtained. Hence, it indicates that the slag forming component may have an effect on the yield of Mo during direct alloying.
| No. | Final slag compositions of briquette, wt% | ||
|---|---|---|---|
| SiO2 | MgO | CaF2 | |
| 1 | 43.27 | 19.23 | 37.50 |
| 2 | 33.71 | 22.47 | 43.82 |
| 3 | 33.71 | 22.47 | 43.82 |
| 4 | 33.71 | 22.47 | 43.82 |
| 5 | 27.61 | 24.54 | 47.85 |
| 6 | 27.61 | 24.54 | 47.85 |
| 7 | 52.94 | 47.06 | 00.00 |
| 8 | 20.27 | 27.03 | 52.70 |
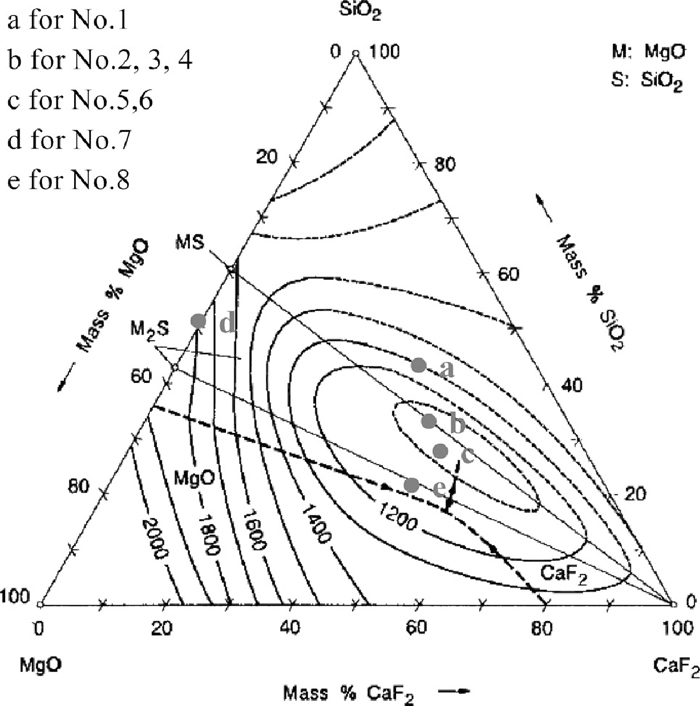
SiO2–MgO–CaF2 phase diagram.
Initially, SiO2 generated by the solid-solid reaction between MoO3 and ferrosilicon, which could impair the self-reducing process. And it can decrease the melting point of the briquette that appropriate proportion of initial slag composition (MgO and CaF2) is added, and the molten slag could generate quickly inside the self-reducing briquette. As the molten slag wetting accelerates the mass transfer of reactants and makes them come into contact with each other,9,10) molten slag can play a critical role in the self-reducing process. Meanwhile, it is easy for Mo to quickly diffuse into the steel melt. The lower the liquidus temperature of slag composition, the better the dynamic condition of self-reducing briquette, and the higher the yield of Mo in the direct alloying could be.
In Shijiazhuang Iron and Steel Co. Ltd., the traditional method of molybdenum alloying was adding ferromolybdenum for smelting 42CrMo steel. It was tested that direct alloying with the self-reducing briquette partially instead of ferromolybdenum. And the technical process was that the self-reducing briquette was added into the ladle during converter tapping for direct alloying. The selected self-reducing briquette is no. 5 in Table 2.
4.2. ResultsTable 4 gives the test results of direct alloying with the self-reducing briquette. The yields of direct alloying with the self-reducing briquette respectively are 97.98%, 98.62% and 98.15%, and the average yield is 98.25%. Obviously, the Mo yield in the industrial test is higher than that (95.44%) in lab-scale experiment when alloying with the same composition self-reducing briquette. In industrial test, the deoxidier and self-reducing briquette were added into the ladle after one third of steel liquid was poured from converter to ladle, and subsequently two-thirds of steel liquid would flush on the briquette in ladle. In that case, the temperature of self-reducing briquette would rise up quickly. What is more, the converter tapping temperature is higher than that in induction furnace of laboratory. And so it resulted in a faster reduction and a less volatilization loss of MoO3. In addition, it was observed that briquette gathered around the crucible wall when added into the induction furnace. That resulted from steel liquid moving from the center to the periphery under the effect of electromagnetic stirring. When the briquette stayed around the colder crucible wall for a long time, it would lead to a more volatilization loss of MoO3. Therefore, the Mo yield in ladle should be higher than that in induction furnace.
| Test | Converter endpoint | Ladle Furnace entering station | Mass of Liquid Steel (kg) | Mass of briquettes (kg) | Yield of Mo (%) | |
|---|---|---|---|---|---|---|
| Mo (wt%) | Mo (wt%) | Si (wt%) | ||||
| 1 | 0.013 | 0.138 | 0.290 | 59800 | 500 | 97.98 |
| 2 | 0.007 | 0.089 | 0.149 | 55050 | 300 | 98.62 |
| 3 | 0.005 | 0.083 | 0.143 | 57600 | 300 | 98.15 |
It is observed that the [Si] in steel liquid rises up obviously, especially for 0.290% when adding 500 kg briquette in Test 1.
As shown in Table 5, the amount of MoO3 in the slag of ladle furnace entering station was quite small, which indicates that the MoO3 inside the briquette had been reduced out except for a small quantity of volatilization loss.
| Test | FeO | CaO | SiO2 | Al2O3 | MgO | MoO3 |
|---|---|---|---|---|---|---|
| 1 | 0.62 | 50.28 | 16.90 | 17.70 | 10.74 | 0.003 |
| 2 | 0.44 | 55.88 | 13.93 | 18.80 | 7.22 | 0.009 |
| 3 | 0.32 | 57.93 | 15.70 | 18.02 | 5.12 | 0.007 |
Inspection of the surface of continous-cast semifinished products showed that the change in the technology for alloying steel with the self-reducing briquette of MoO3 did not increase the number of defects. And the inclusion appraisal indicates that they were in the consenting range of non-metal inclusion level, the same with the traditional technology.
It is practicable for direct alloying with the self-reducing briquette instead of ferromolybdenum. And the technical process is adding self-reducing briquette into the ladle during converter tapping.
The composition of the self-reducing briquette has a significant effect on the yield of Mo during the direct alloying.
(1) It is quite volatile for MoO3 powders at high temperature. The addition of MgO can react into stable MgMoO4, which can decrease the volatilization of MoO3 during the heating process.
(2) The excess of reducing agent can increase the yield of Mo in the direct alloying. However, the excess of the stoichiometric amount will lead to the increase of [Si] in the steel liquid.
(3) In the self-reducing process of briquette, the forming of liquidus slag can optimize the dynamic conditions of reducing reaction. And it can accelerate the self-reducing reaction and increase the yield of Mo during the direct alloying.