2013 年 53 巻 8 号 p. 1358-1364
2013 年 53 巻 8 号 p. 1358-1364
Recently a special attention is being paid on the ferroniobium production worldwide, especially in China. In present work, direct reduction followed by magnetic separation for Nb2O5-bearing ore is investigated. Reflected light microscope, scanning electron microscope with EDX, and high performance X-ray diffraction were used for fundamental analysis. The experimental results show that, (1) the optimum reduction parameters are 0.9 of C/O, 1200°C and 20 min. At these optimum parameters, the degree of metallization of ore-coal composite pellet is more than 90%. (2) In direct reduced pellets, metallic iron and slag have gathered respectively, and they can be magnetically separated. Nb and Ti are associated together and inserted in slag, and stay in slag phase after magnetic separation. (3) Based on the process of direct reduction followed by magnetic separation, 7.71% of Nb2O5-enriched slag is obtained, which is a good feed for producing ferroniobium or metallic niobium in electric furnace. The Nb2O5 content in Nb2O5-enriched slag is 1.8 times of the original ore, and the recovery of Nb is about 85%. These experimental results can give some theoretical references for industrial application in future.
Niobium is an important element for MRI (Magnetic Resonance Imaging) machines, high technology, and the steel industrial. Over three-quarters of the world’s niobium production currently comes from Brazil, with most of remainder coming from eastern Canada. Recently a special attention is being paid on the ferroniobium production worldwide, especially in China.1,2,3,4,5,6) China has been a top importer of ferroniobium in recent years, and its imports have increased over the last decade (Fig. 1).

Chinese imports for niobium.
In tradional process of ferroniobium production in China include, (1) the raw mineral is processed primarily by physical processing technology to get Nb2O5-bearing concentrate. (2) The Nb2O5-bearing concentrate is used to produce sinter or pellet. (3) Sinter or pellet is feed into blast furnace (BF) to produce Nb-bearing liquid iron. (4) Nb is oxidized to niobium oxides in converting process (BOF), and enriched in slag. In this long Sinter-BF-BOF route, particularly in BF, some Nb presents in liquid iron, and some Nb oxides present in slag because the selective reduction of ferrous oxides and niobium oxides can not be achieved in BF. So the recovery of Nb is very low (about 72%) and the energy consumption per unit Nb produced is very high.7)
As we all know, the grade of Nb2O5 content is an important factor for producing ferroniobium or metallic niobium. Compared with Sinter-BF-BOF route, reduction followed by magnetic separation route has the following advantages.8,9,10,11) (1) At the condition of BF, the reducing potential and reducing temperature are uncontrollable, and they are strong enough to reduce both ferrous oxides and niobium oxides. At direct reduction process, ferrous oxides can be reduced, but niobium oxides can not be reduced by controlling reducing potential and reducing temperature. So, direct reduction is an effective process to achieve selective reduction. (2) In BF and BOF, the Nb2O5-enriched slag is liquid. But in magnetic separation, the Nb2O5-enriched slag is solid, so the energy consumption is relatively lower. Therefore, aimed for producing Nb2O5-enriched slag, direct reduction followed by magnetic separation is investigated in present work, which can give the theoretical references for industrial application in future.
Almost over 90% of Nb2O5-bearing resources in China are found in Baiyunebo deposit in the Inner Mongolia Autonomous Region. In this study, Baiyunebo ore is used as the Nb2O5-bearing raw material. The Nb2O5 content of original Baiyunebo ore is very low, only 0.4–0.7%. But with the development of beneficiation technology, the grade of Nb2O5 can be increased to 4.32% (Table 1).12,13) From Table 1, in addition to the ferrous oxides and niobium oxides, there are some SiO2, TiO2, CaO, MgO and some alkalis (Na2O and K2O).
| TFe | FeO | Nb2O5 | CaO | SiO2 | MgO | Al2O3 | MnO |
| 35.52 | 1.6 | 4.32 | 2.42 | 21.87 | 1.05 | 0.26 | 0.31 |
| F | S | P | Na2O | K2O | TiO2 | Sc2O3 | TREO |
| 0.73 | 0.73 | 0.07 | 2.42 | 0.18 | 6.71 | 0.032 | 2.72 |
Niobium does not occur freely in nature. It primarily coexists in form of pyrochlore, a niobium-rich, complex mineral containing some of calcium, sodium, titanium, rare earth and other elements. Pyrochlore is the most important mineral for niobium extraction and production. Niobium can also be found in columbite, which is a niobate of iron, manganese, and magnesium. X-ray diffraction of the applied ore is shown in Fig. 2. From the figure, one can conclude that, (1) our applied ore is composed of hematite, magnetite, quartz, and some complex mineral including Ti, Ca, Na etc. (2) Hematite is the main ferrous oxide, and there is small amount of magnetite. (3) The co-exist ore containing niobium oxides and titanium oxides is the main Nb2O5-bearing ore. The co-exist ore and the compact structure are the main characteristics for niobium extraction and production. (4) The slag is mainly composed of silicate and quartz.
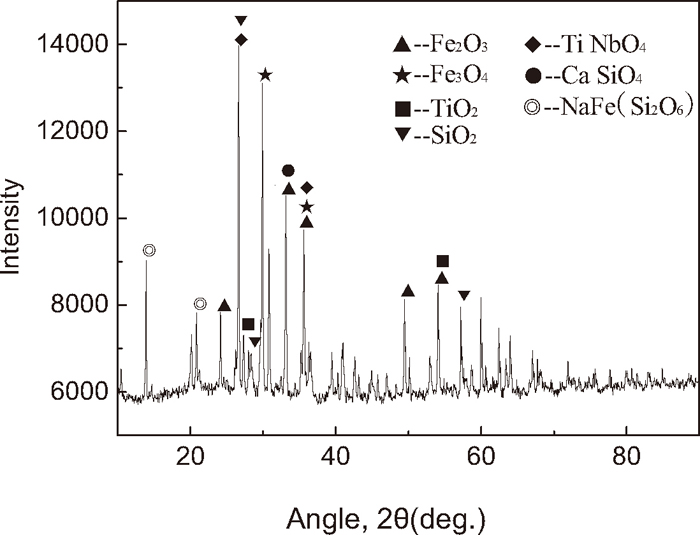
X-ray diffraction of Nb2O5-bearing concentration ore.
The size distribution of Nb2O5-bearing ore is shown in Fig. 3. From the figure, the powder of –50 μm is about 50%, –74 μm is about 76%, and –99 μm is about 90%. The size distribution of this applied ore is easy for pelletization. So the ore-coal composite pellets are used as the feed material for direct reduction experiments. The goal of direct reduction is that the ferrous oxides are reduced to metallic iron, but the niobium oxides are not reduced (based on the thermodynamic analysis).14) Then metallic iron can be separated with slag by magnetic separation. The niobium oxides present in slag. This slag is named as Nb2O5-enriched slag, in which the Nb2O5 content is increased. This Nb2O5-enriched slag is a good feed for producing ferroniobium, metallic niobium, and rare earth etc. The chemical compositions of coal and bentonite used in present work are listed in Table 2. Before the pelletization, the coal and bentonite are ground to –74 μm.
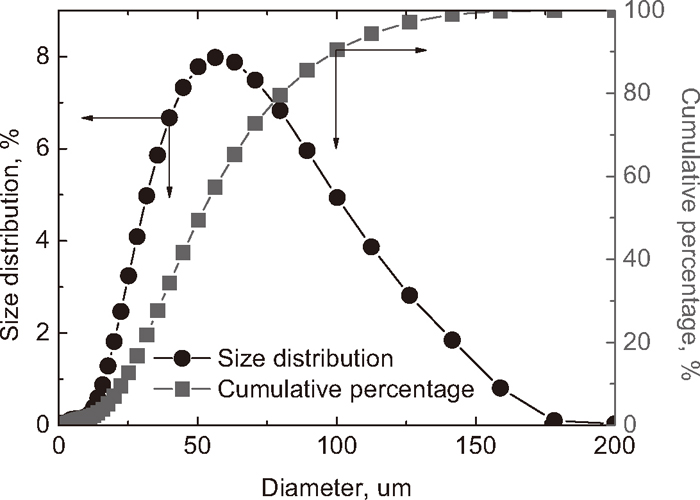
Size distribution of Nb2O5-bearing concentrate ore.
| Pulverized | Fixed Carbon | Total Carbon | Volatile Matters | Ash | H2O |
|---|---|---|---|---|---|
| Coal | 60.49 | 75.4 | 31.23 | 8.28 | 6.68 |
| Bentonite | SiO2 | Al2O3 | CaO | MgO | |
| 69.74 | 16.60 | 2.41 | 1.14 |
The key for selective reduction of ferrous oxides and niobium oxides in present work is not only to reduce iron oxides and no-reduce niobium oxides, but also the reduced metallic iron particles grow to big size which is benefit for the following step --- grinding and magnetic separation. Another key is that the pellet can not melt during the process of direct reduction. If melting, the process probably is difficult to be realized in industrial trial.
In order to get optimum operation parameters for the selective reduction of ferrous oxides and niobium oxides, series of direct reduction experiments were conducted. In these series of experiments, the effects of following variables on the reduction of ore-coal composite pellets were studied:
(1) The processing temperature (the temperature in the Muffle furnace) for reduction. Basically, higher temperature will result in melting of pellets, and lower temperature will result in low metallization degree and low rate of reduction.
(2) The reduction time. This is related to the reduction temperature, and proper time will result in high degree of metallization and big size of metallic iron, which is benefit for the following grinding and magnetic separation.
(3) The amount of carbon addition, in term of the gram-atomic of fixed carbon in the coal added to the gram-atomic of combined oxygen in iron oxides, which is denoted as C/O (mol C/mol O).
The experimental set-up in Muffle furnace is shown in Fig. 4. The temperature is measured by a thermocouple fixed near to the sample. As an example, the reduction experimental procedure consists of the following steps:
Experimental set-up in Muffle furnace.
(1) Pelletization. Mix raw materials with proper moisture content → pelletizing → drying → ore-coal composite pellets.
(2) Heat up the furnace to the pre-determined temperature.
(3) Place ore-coal composite pellets into a crucible, and put the loaded crucible into the muffle furnace at pre-determined temperature.
(4) Keep the furnace at the pre-determined temperature to reduce ore-coal pellets for pre-determined time.
(5) After pre-determined time, the crucible with the direct reduced iron (DRI) is taken out of the furnace. And the crucible is cooled under flowing argon to avoid re-oxidation of metallic iron during cooling.
(6) The degree of metallization (MD) is defined as the percentage of metallic iron in total iron. It’s calculated by “[(metallic iron) / (total iron)] × 100%”. The metallic iron and total iron are measured by chemical determination. The DRI pellets are examined by reflected light microscope and scanning electron microscope. And the formed phases in Nb2O5-enriched slag are identified and analyzed by high performance X-ray diffraction.
(7) A CXG-08SD(A) magnetic tube is used for magnetic separation. The range of the magnetic field density is 0–600 mT.
In order to investigate the effect of the reducing temperature on the reduction and melting of ore-coal composite pellets, a series of reduction experiments at different temperature were carried out, and the temperature is 1000°C, 1100°C, 1200°C, 1300°C, and 1400°C respectively. The reducing time and carbon addition are constant, and they are 25 min and C/O=1.0 respectively.
The appearances of pellets after reduction are shown in Fig. 5. From the figure, one can conclude that the pellets will seriously melt when the temperature is higher than 1200°C. According to the chemical composition of ore, the dominant oxides in slag should be SiO2, TiO2, and Nb2O5. Generally, the melting point of slag with these oxides should be very high, but our experimental result is contradictory with it, and the slag melt at 1300°C. In order to clarify this phenomenon, microscopic energy spectrum is used to analyze the composition of reduced pellet (Fig. 6).

Appearances of pellets after reduction at different temperatures (25 min).

Microscopic energy spectrum analysis for reduced pellet (1200°C, 25 min, C/O=1.0).
From Fig. 6, it can be seen that there are three main phases in the reduced pellets:
(1) White phase, which is metallic iron phase and represented by point A. Metallic iron grains have grow to be crystal stock, which are big enough for magnetic separation.
(2) Light grey phase, which is the compound mineral of titanium oxides and niobium oxides, represented by point B. The melting point of this phase is very high, and the mineral keeps its original shape with obvious angles and lines.
(3) Deep grey phase, which is the basal body---melting slag. There are large amounts of SiO2 and small amount of Na2O and FeO. The chemical composition of this slag is shown in Table 3. From the table, SiO2/(SiO2+Na2O) and SiO2/(SiO2+FeO) are 90.3 mol% and 79.5 mol% respectively. The liquidus lines of SiO2–Na2O and SiO2–FeO binary phase diagram are shown in Fig. 7. It can be seen that, for this kind of slag, some liquid presents at experimental temperature (1000°C to 1400°C). Higher temperature, more liquid phase. Therefore, the Na2O and FeO are the main reasons for lower melting point of slag in reduced pellets. Therefore, in order to realize the process of direct reduction at solid state, the reducing temperature should not be higher than 1200°C in the laboratory condition.
| Percentage | SiO2 | Na2O | FeO |
|---|---|---|---|
| Mol% | 73.21 | 7.87 | 18.92 |
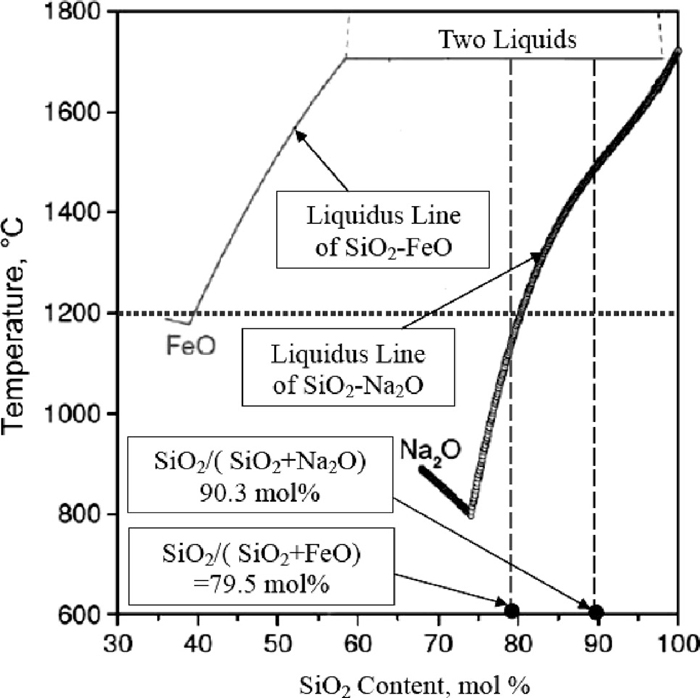
Liquidus lines of binary phase diagrams for SiO2–Na2O and SiO2–FeO.
The degrees of metallization of ore-coal composite pellets at different reducing temperature and for different reducing time are shown in Fig. 8. One can conclude that, (1) reducing temperature is more important than reducing time. (2) When the reducing time is 25 min, the degrees of metallization at 1000°C, 1100°C, and 1200°C are 13%, 66%, and 90% respectively. The carbothermic direct reduction of iron oxide, FeO + C = Fe + CO, is a strong endothermic reaction (ΔHθ298=154 kJ/mol).15) Therefore, higher temperature can increase the rate of reduction reaction and the degree of metallization.

Effects of reducing temperature and reducing time on degree of metallization.
In order to obtain Nb2O5-enriched slag by magnetic separation following direct reduction, not only the high degree of metallization of pellet is necessary, but also the size of metallic iron particles in reduced pellet is another significantly important influencing factor. The big size of metallic iron in reduced pellet is benefit for grinding ore and magnetic separation. Therefore, the microstructure and the size of metallic iron in reduced pellets at different temperature are observed and analyzed by reflected light microscope, which is shown in Fig. 9.

Micrographics of pellets after reduction at different temperature.
From Fig. 9, it can be seen that, (1) at 1000°C, the degree of metallization is lower. In Fig. 9(a), the crystal grains of metallic iron are very small and obscure, look like iron whisker. (2) At 1100°C, the degree of metallization is higher than that at 1000°C. In Fig. 9(b), the crystal grains of metallic iron grow and become bigger, and the metallic iron phase is obvious (white). (3) At 1200°C, the degree of metallization is highest. In Fig. 9(c), the crystal grains of metallic iron continue to grow and become bigger and bigger, and these big metallic iron particles are benefit for grinding ore and magnetic separation.
Based on the above analysis, higher temperature (higher than 1200°C) will result in melting of pellet and the direct reduction in solid state can not be realized. But lower temperature (lower than 1200°C) will result in low degree of metallization and small size of metallic crystal grains, which is not benefit for the following grinding ore and magnetic separation. Therefore, in the lab scale, the optimum reducing temperature for Nb2O5-bearing ore-coal composite pellets’ selective direct reduction is 1200°C.
3.3. Effect of C/O on Degree of MetallizationThe amount of carbon addition is also an important influencing factor on the degree of metallization of ore-coal composite pellets. Generally C/O is higher, the reducing rate is faster, and the degree of metallization is higher.16) In present work, the effect of C/O on degree of metallization of Nb2O5-bearing ore-coal pellets at 1200°C (optimum reducing temperature based on above discussion) is shown in Fig. 10. From the figure, it can be seen that, (1) when C/O is 0.7 and 0.8, the degrees of metallization of pellet are lower, only about 80% for 20 min, due to the insufficient reducing agent. (2) There is no obvious difference in degree of metallization between C/O=0.9 and C/O=1.0, and they are about 90% for 20 min. (3) Basically, there is no obvious increase in degree of metallization after 20 min. Therefore, the optimum C/O is 0.9, and the optimum reducing time is 20 min in our lab scale investigation.

Effects of C/O on degree of metallization of pellets (1200°C).
The reduced pellets will be magnetic separated for metallic iron and Nb2O5-enriched slag, so the elements distribution in reduced pellets is necessary to be investigated. The optimum parameters based on above analysis are C/O=0.9, reducing temperature is 1200°C, and reducing time is 20 min. The elements distribution of reduced pellet at these optimum parameters is give in Fig. 11. From the figure, one can conclude that, (1) metallic iron and slag have gathered respectively, and they are obviously two phases, so they can be magnetically separated. (2) Nb and Ti are associated together and inserted in slag, they are non-magnetic substances, and stay in slag phase after magnetic separation. (3) The distributions of Na and Si are similar, that’s why the melting point of this slag is very low.

Elements distribution in reduced pellet (C/O=0.9, 1200°C, 20 min).
10 grams of some selected reduced pellets at different reducing parameters are the feed materials for magnetic separation tests. The pellets are ground to powder with 80% of –200 μm, and the size distribution of powder before magnetic separation is shown in Fig. 12. A magnetic tube is used for magnetic separation. The density of magnetic field in current work is kept at 90 mT. and the Nb2O5 content in slag after magnetic separation is shown in Fig. 13.

Size distribution of powder before magnetic separation.

Nb2O5 content in slag after magnetic separation, %.
From Fig. 13, one can conclude that, (1) when the carbon addition is lower, C/O=0.7 (No. 071220), the reducing agent is insufficient and the degree of metallization is lower, so the FeO content in slag is higher and result in the lower Nb2O5 content in slag. (2) Lower reducing temperature, 1100°C (No. 081120), result in lower degree of metallization, and the Nb2O5 content in slag is lower too. (3) C/O=0.9 is good enough for high degree of metallization and high Nb2O5 content in slag. So, higher C/O than 0.9 is not required, because the ash of coal can increase the slag volume and decrease the Nb2O5 content in slag. In Fig. 14, The X-ray diffraction of slag after magnetic separation exhibits that some of niobium oxide is reduced to niobium carbide (NbC–Nb2C), and some of niobium is still in the form of pyrochlore (Ti.4Fe.3Nb.3O2). Therefore, most of niobium is enriched in slag after magnetic separation, which is the goal of our present work.

X-ray diffraction of slag after magnetic separation.
Based on above analysis and discussion, the sample 091220 (C/O=0.9, 1200°C, 20 min) is the best one in our test range, and its Nb2O5 content in slag is highest, about 7.71%, which is a good feed for producing ferroniobium or metallic niobium in electric furnace. The Nb2O5 content in original Nb2O5-bearing ore is only 4.32%. Therefore, the Nb2O5 content in this Nb2O5-enriched slag is 1.8 times of the original ore. The recovery of Nb is about 85%, which is higher than that of Sinter-BF-BOF route in China (about 72%).
In this study, the direct reduction followed by magnetic separation for Nb2O5-bearing ore is investigated. The main findings could be summarized as follows:
(1) For the selective direct reduction of Nb2O5-bearing ore-coal composite pellets, the optimum C/O is 0.9, the optimum reducing temperature is 1200°C, and the optimum reducing time is 20 min in our lab scale investigation. At the conditions of these optimum parameters, the degree of metallization of ore-coal composite pellet is more than 90%.
(2) In direct reduced pellets, metallic iron and slag have gathered respectively, and they can be magnetically separated. Nb and Ti are associated together and inserted in slag, they are non-magnetic substances, and stay in slag phase after magnetic separation. The distributions of Na and Si are similar, that’s why the melting point of this slag is very low.
(3) Based on the process of direct reduction followed by magnetic separation, 7.71% of Nb2O5-enriched slag is obtained, which is a good feed for producing ferroniobium or metallic niobium in electric furnace. The Nb2O5 content in this Nb2O5-enriched slag is 1.8 times of the original ore, and the recovery of Nb is about 85%. These experimental results can give some theoretical references for industrial application in future.
The authors wish to gratefully acknowledge the contributions of associates and colleagues in Northeastern University, China and Baotou Iron and Steel (Group) co., LTD, China. Also, the financial support by the Seed fund issued by Ministry of Education, China (N120402007) and the Department of Liaoning Science and Technology Program (NSFLN: 2011010429-401) are very much appreciated.