2013 年 53 巻 9 号 p. 1617-1624
2013 年 53 巻 9 号 p. 1617-1624
In this study, the effects of adding metallic Fe particles to coke in an effort to reduce the CO2 emissions associated with sintering were investigated. The changes to the structure and permeability of the sintering bed were analyzed in a laboratory-scale sintering simulator. The results were then used to compare agglomerates material containing metallic Fe particles with a 100% coke bed. The pressure drop of the packed bed of the mixture of model mini-pellets (ACPs) and coke particles increases until the bed reaches the temperature at which the adhering layer of ACP starts to melt, and then starts to gradually decrease. The pressure drop for metallic Fe particles, however, begins to decrease at an earlier stage, and then increases until its peak value. This difference can be attributed to differences in the products formed from oxidation.
The sintering experiments were made by making changes to the CaO composition of the sintering bed: 5% to 15% was substituted with metallic Fe particles. A high CaO content resulted in a lower pressure drop of the bed from the start to 200 s sintering apparently due to the difference in the melting behavior of the materials at the initial stage of sintering.
Iron ore sinter is the most widely-used burden for blast furnace ironmaking in the world. In the sintering process, besides the physical and chemical characteristics of the produced sinter, which effect the blast furnace operation, the productivity of the sinter is important. Therefore, a number of studies have been done on the manufacturing of sinter itself. The permeability of the sintering bed is one of the most important factors in the sintering process as it has a direct effect on productivity.
The steel industry accounts for about 7% of the global anthropogenic CO2 emissions and it accounts for as much as 14% of the total emissions in Japan where the blast furnace-converter process is a major part of steel production. Further, iron ore sintering process accounts for about 3% of the total CO2 emissions in Japan. Although a large amount of sensible heat is released by the outlet gas and sinter product, this heat has not been able to be efficiently utilized. Therefore, it is essential to decrease the amount of fossil fuel used in the sintering process. One of the possibilities is utilization of alternative agglomeration agents instead of coke and to promote the efficiency of the reactions in the sintering process. There have been several attempts to use alternative agglomeration agents in the sintering process. The use of biomass char, for example, which is regarded as carbon neutral material, has been shown to lead to a significant decrease in sintering time as well as a decrease in the amount of NOx and SOx formed.1) It also, however, resulted in significant decreases in the yield and strength of the sinter product.2) Previous studies on the utilization of partial reduced iron ore3) and used steel can chip,4) which provided heat without CO2 formation, also resulted in considerable decreases in productivity due to the significant decrease in the permeability of the sintering bed. This decrease in the bed permeability occurred mainly at the bottom of the sintering bed. From observations made of the structure of the sintered bed, it appears that formed oxide melt dropped down toward the bottom of the bed and some voids became filled with melt. As yet, no clear explanation for the decrease in productivity has been put forward, and no solution to the problem has been presented.
If coke breeze is simply replaced by metallic Fe particles, the iron oxide content of the raw material will be increased. In the sintering operation, the control of the CaO composition is important because the composition of the blast furnace slag should be constant. In the case of the conventional sintering process using coke breeze, the CaO component in the bed is known to have a significant effect on the bed structure and pressure drop. Therefore, it is necessary to study the effects of the CaO composition when using metallic iron as the agglomeration agent.
The aim of this study is to clarify the changes in the permeability of the sintering bed when using metallic Fe particles. A sintering simulator which enables changes in bed-temperature, bed permeability, and composition of outlet gas during sintering to be measured was made in the same manner as explained in a previous study.5) Mini pellets were used as model granules of raw material with coke and metallic Fe particles. The effect of different agglomeration agents on the structural change of the sintering bed was analyzed.
A fine mixture of hematite and calcium carbonate reagents was granulated on the surfaces of alumina balls of 2.0 mm in diameter by a disc pelletizer to prepare mini pellets 2.38–2.8 mm in size (called Alumina Cored Pellets, ACPs). The initial composition of the adhering layer of ACP can be regarded as the CaO–Fe2O3 binary system. Table 1 shows the mixing ratio of Fe2O3 and CaO used in the adhering layer of ACP. Metallic Fe (99.9%-Fe) and metallurgical coke (87 mass% - fixed carbon) particles 1.0–2.0 mm in size were used as the agglomeration agents. Heat generation of agglomeration agents was calculated based on the estimation that coke and metallic Fe were oxidized to CO2 and Fe2O3, respectively. The mixing ratio of the agglomeration agent to ACP was based on the heat equivalent to the case of 1.3 g-coke/26 g-ACP. When coke was completely replaced by metallic Fe, the mixing ratio of metallic Fe should be 6.1 g-metallic Fe/26 g-ACP. CaO content in the total sample bed can be estimated by the CaO and iron oxide contents in the adhering layer of ACP and oxidized metallic Fe used as agglomeration agent.
| Pellet name | Fe2O3 (%) | CaO (%) |
|---|---|---|
| ACP-15 | 85.4 | 14.6 |
| ACP-20 | 79.8 | 20.2 |
| ACP-25 | 73.9 | 26.1 |
| ACP-30 | 67.7 | 32.3 |
| ACP-35 | 61.2 | 38.8 |
A diagram of the sintering simulator is shown in Fig. 1. An alumina tube with an inside diameter of 35 mm was used as the reaction tube. The height of sample bed was set at 20 mm. The bed 50 mm in height packed with 2.0 mm alumina balls was placed n the sample bed to pre-heat the gas. An alumina ball bed 20 mm in height was placed under the sample bed to prevent the drop-down of the fine materials and slag melt. After setting the bed, they were heated up to 860°C under N2 stream in order to prevent the oxidation of agglomeration agent. Then, the N2 flow rate was increased to 4.5×10–1 Nm·s–1. When the bed-temperature reached 900°C, the gas was changed to a N2 - 21%O2 mixture. During the experiment, changes in the bed-temperature at 10 and 20 mm from the top of sample bed were recorded, and the concentration of CO, CO2 and O2 of the outlet gas, and the pressure drop through the bed was measured.

Schematic diagram of the sintering simulator.
After the experiment, the sample bed was mounted with resin to determine the structure and morphology of the cross section and specimens were analyzed by X-ray CT to provide 3D images of the bed structure.
Figure 2 shows the changes in CO, CO2 and O2 concentrations of outlet gas with time for the coke only case (Fe-0 ACP-15), the case of coke and metallic Fe mixture (50%-Fe, Fe-50 ACP-25) and the metallic Fe only case (Fe-100 ACP-35). ACP was prepared to keep CaO composition constant at 15% since increases in the amount of metallic Fe addition leads to increases in iron oxide content after oxidation. In the case of Fe-0 ACP-15, both CO and CO2 are generated in the combustion of coke, which took about 55 s to complete. The CO concentration in the outlet gas was about 0.5% in average during combustion. The O2 concentration increases rapidly in the first few seconds of the reaction due to the switch in the flowed gas from N2 to a mixture of N2–O2. The O2 concentration drops slightly for about 20 s, and then it increases rapidly, approaching 21%. In the case of Fe-50 ACP-25, while the O2 concentration increases as it does in Fe-0 ACP-15, no negative peak is observed. The CO2 emission is half that of Fe-0 ACP-15, yet the CO concentration curve of this case is quite similar to the case of Fe-0 ACP-15. In the case of Fe-100 ACP-35, O2 concentration increases continuously, with the reaction completed 55 s after the gas switch. With higher metallic Fe substitutions, the amount of O2 consumption is reduced under the present condition. In this study, the ratio of metallic Fe used as a substitute was set assuming that Fe would react with O2 completely, i.e. that it would be oxidized to form Fe2O3. The higher O2 concentration in the case of Fe-100 ACP-35 suggests that the Fe reaction was not completed during the experiment.
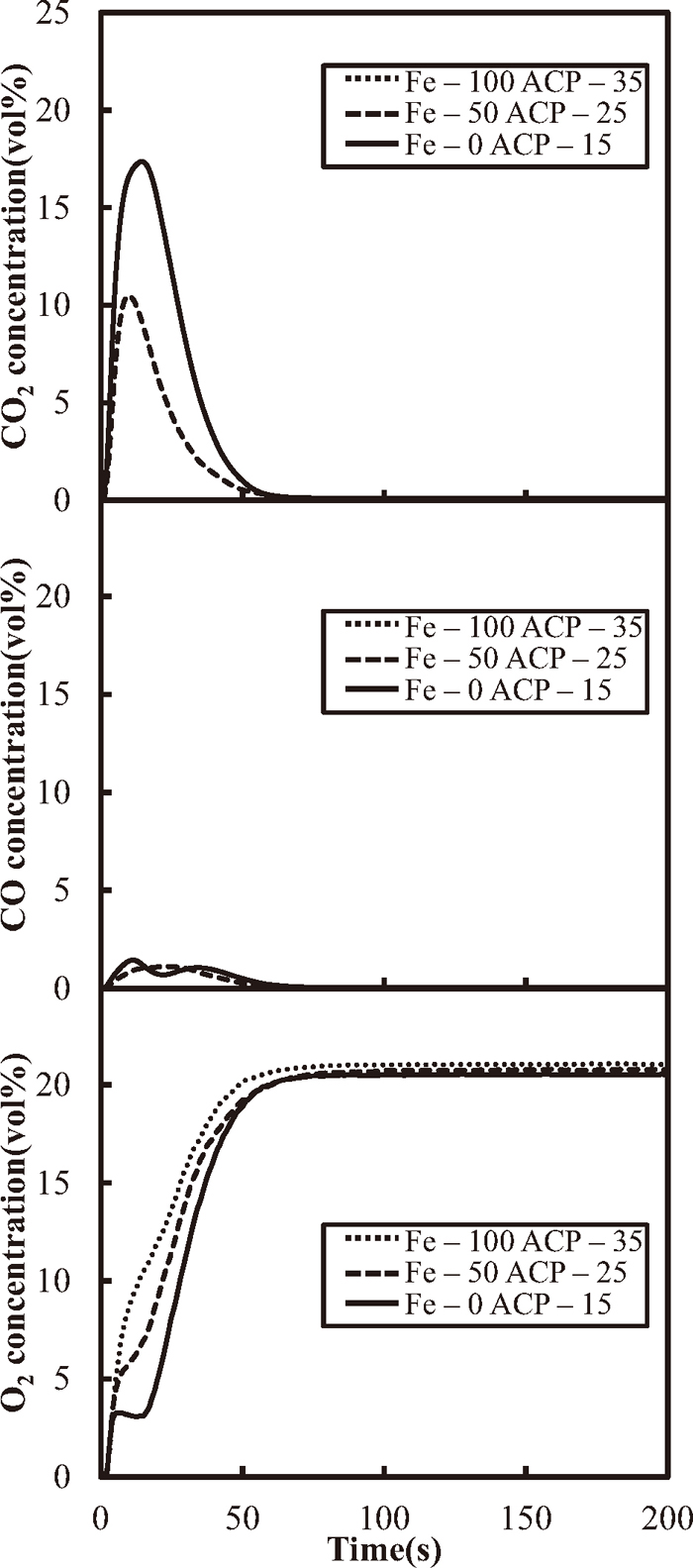
Changes in concentration of O2, CO2 and CO of outlet gas with time obtained coke (Fe-0 ACP-15), the mixture of coke and metallic Fe (Fe-50 ACP-25) and metallic Fe (Fe-100 ACP-35).
Figure 3 shows the change in the temperature of the sample bed-center and the lower border between the sample and the lower alumina beds with time. While the maximum bed-center temperature in all cases was about 1300°C, the temperatures fluctuated significantly for 50 s after starting in the case of Fe-50 ACP-25. This appears to be because the formed melt touched the thermocouple, and had a short-circuit effect. The lower border temperature showed similar behavior in each case until 30 s. After that, the temperature reached 1350°C in the case of Fe-0 ACP-15, and for Fe-50 ACP-25 and Fe-100 ACP-35, the peak temperature was about 1470°C at about 50 s.
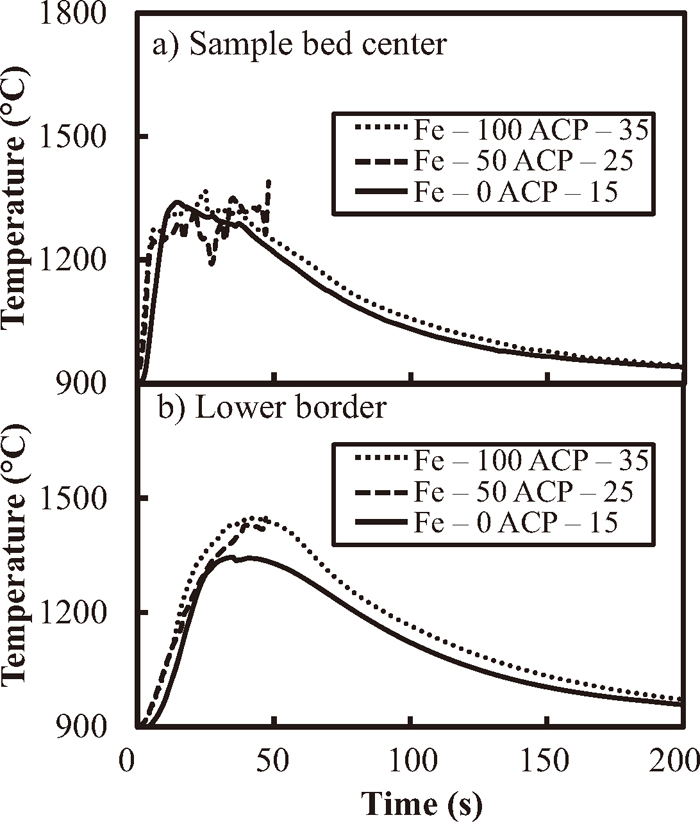
Changes in temperature at (a) the bed-center and (b) the lower border with time obtained for coke only (Fe-0 ACP-15), the mixture of coke and metallic Fe (Fe-50 ACP-25) and metallic Fe (Fe-100).
Figure 4 shows the relationship between the ratio of metallic Fe used as a substitute and the time the bed-temperature was over 1200°C. This is the temperature at which the melt starts to form since 1205°C, which is the eutectic temperature of CaO–Fe2O3 and CaO–2Fe2O3.6) No value is included for Fe-50 ACP-25 because the melt short-circuited the thermocouple, as mentioned above. A slight decrease in the time the sample bed-center was over 1200°C was observed, whereas it was longer in the lower border when using metallic Fe particles.
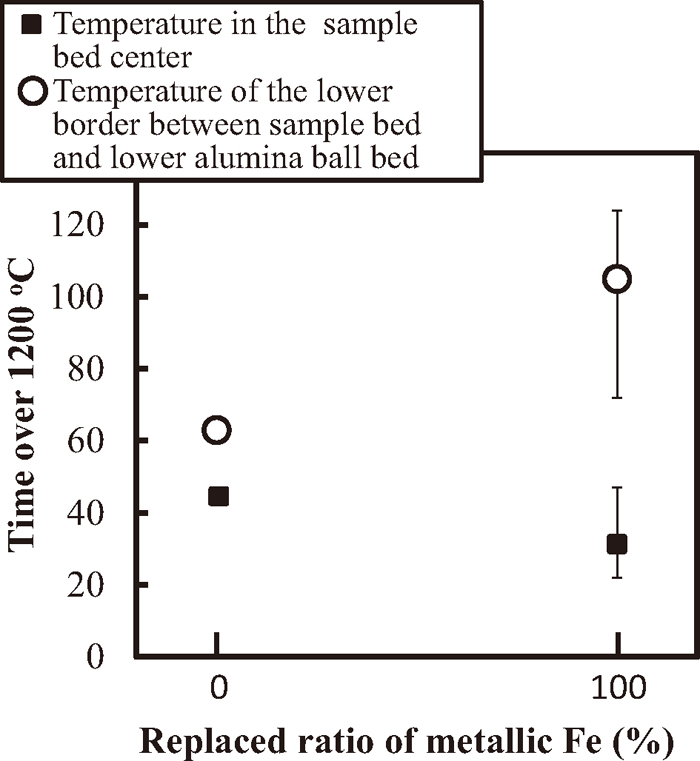
Relationship between the substitution ratio of metallic Fe and the amount of time the bed temperature was higher than 1200°C.
In Fig. 5, the changes in the pressure drop of the bed are shown for different amounts of metallic Fe. These pressure drops were calculated by subtracting the value of the upper alumina bed from the total value of measured data, since the former value was constant during the experiment. The pressure drop of the lower alumina bed is difficult to estimate because of the temperature change and the effect of the flow-down of the formed melt. Therefore, the pressure drop values are the sum of those of the sample bed and the lower alumina bed.

Changes in the pressure drop with time observed for various substitution ratios of metallic Fe.
In the case of Fe-0 ACP-15, the pressure drop increases after the reaction starts, and then decreases rapidly before slowing down significantly. In the case of Fe-100 ACP-35, on the contrary, the pressure drop starts to decrease just after the beginning of the experiment, and begins to increase at 20 s, peaking at 60 s and then decreasing gradually. The peak occurs at approximately the same time as the oxidation reaction finishes (see Fig. 2) and the bed-temperature starts to decrease (see Fig. 3). This suggests that the decrease in the pressure drop after 50 s was due only to the decrease in the bed temperature. In the case of Fe-50 ACP-25, a similar pressure drop change was noted to that of Fe-100 ACP-35, however, the increase in the pressure drop from 20 s was smaller than that of Fe-100 ACP-35. This is intermediate behavior in both Fe-100 ACP-35 and Fe-0 ACP-15. Further, Fe-25 ACP-20 and Fe-75 ACP-30 exhibit intermediate behavior between Fe-0 ACP-15 and Fe-50 ACP-25 and Fe-50 ACP-25 and Fe-100 ACP-35, respectively.
Figure 6 shows the difference in the pressure drop between 0 s and at 200 s for each case. The negative value of the vertical axis indicates that the pressure drop at 200 s is lower than the initial value. The difference was smaller when the ratio of metallic Fe in the bed was higher. The reason of this will be discussed later.

Effect of the substitution ratio of metallic Fe on the pressure difference between the initial value and at 200 s.
Figure 7 shows the vertical cross sections of the sample beds of Fe-100 ACP-35, Fe-50 ACP-25 and Fe-25 ACP-20 sintered for 10 and 25 s and that after sintering experiment. The white particles are the alumina balls used as the core of the ACP, and the thin gray layers around the balls are the adhering layer. The white and black particles are residual metallic Fe and coke particles, respectively. In the case of Fe-100 ACP-35 sintered for 10 s, several alumina particles agglutinated with the oxide melt of adhering materials and iron oxide formed by oxidation of metallic Fe. On the other hand, a number of metallic Fe particles still remained. At 25 s, a part of melt penetrated down to the lower part of the sample bed, and some clusters composed of several particles were formed. In the upper part of the sample bed, less of the melt remained, but was bound to alumina particles, with a certain network structure of particles formed. After sintering, the clusters were larger and the formed melt had further dropped down to the lower alumina beds beyond the border. In the case of Fe-50 ACP-25 sintered for 10 s, the coke particles had become small and the metallic Fe particles had begun to oxidize on the surface. The alumina particles had also begun to agglomerate. However, the voids in the lower part of the sample bed were not filled by melt like they were in the case of Fe-100 ACP-35. After sintering, the cross-sectional structure of the sample bed did not indicate any large differences from that at 25 s. In the case of Fe-25 ACP-20 after sintering, the voidage of the sample bed was higher and the cluster size was smaller than those of other conditions.
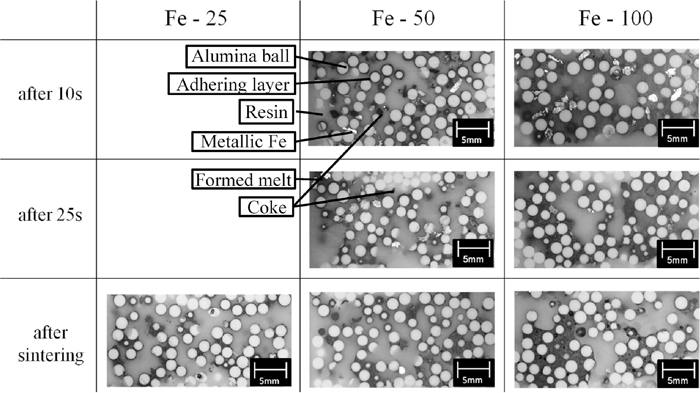
Vertical cross-sections of the sample beds of Fe-25 ACP-20, Fe-50 ACP-25 and Fe-100 sintered after 10 and 20 s and after sintering was completed.
These results suggest that the reasons for the high temperature at the lower border of bed in the case of Fe-100 ACP-35 is that the iron oxide formed by the oxidization of metallic Fe in upper part of sample bed melts and flows down to the lower part. While this was occurring, the Fe in the melt changed from Fe2+ to Fe3+ due to oxidation, which provided a certain amount of heat to the lower part of sample bed.
The reasons for the higher pressure drop observed in the case of Fe-100 ACP-35 remain unclear at this stage. The 3D analyses of the sintered bed produced by X-ray CT for the cases of Fe-0 ACP-15 and Fe-100 ACP-35, with their vertical and horizontal planes, are shown in Figs. 8 and 9. The white parts are the oxide melt, the gray circles are alumina balls and the other areas are voids. In case of Fe-0 ACP-15, a permeable structure is formed. In the case of Fe-100 ACP-35, on the other hand, melt filled most of the spaces in the lower part of bed, as shown in the cross sections of d) and e) shown in Fig. 9. It would appear that the gas flow path was very narrow and the pressure drop was followed by an orifice mechanism rather than a packed bed one.

CT images of sample bed of Fe-0 ACP-15 after sintering. (a: Vertical cross section, b, c, d, e and f: Horizontal level images at distances of 5 mm).

CT images of sample bed of Fe – 100 after sintering. (a: Vertical cross section, b, c, d, e and f: Horizontal level images at distances of 5 mm).
The change in the O2 concentration of the outlet gas for 15 mass%-CaO (Fe-100 ACP-35) and 5 mass%-CaO (Fe-100 ACP 15) is shown in Fig. 10. Though little difference was noted in the O2 concentration, the lower concentration in the case of 15 mass%-CaO (Fe-100 ACP-35) suggests that higher CaO promotes the oxidation of metallic Fe in the formed melt.
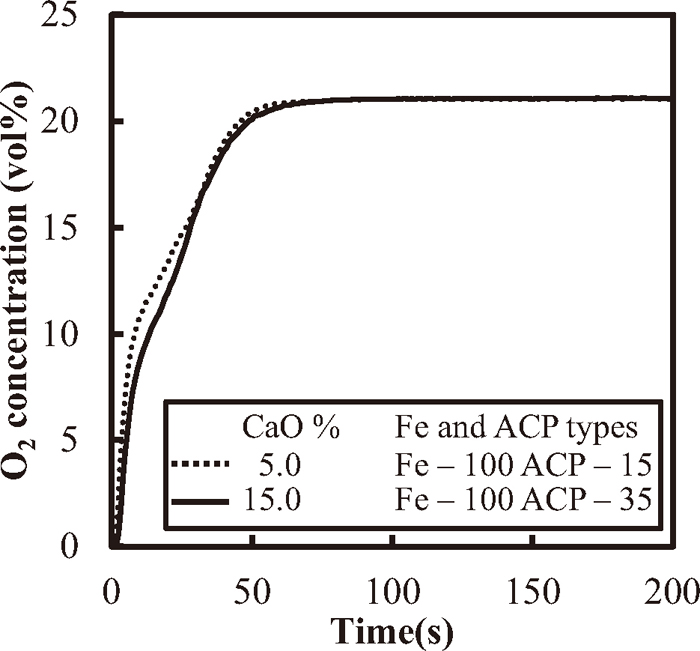
Change in O2 concentration in the outlet gas observed for Fe-100 for different CaO concentrations in the adhering layers of ACP.
The changes in the pressure drop of the bed observed for Fe-100 with different CaO composition in the adhering layers are shown in Fig. 11. When the CaO composition is the highest, at 15 mass%-CaO (Fe-100 ACP-35), the pressure drop increased incrementally during the initial stage before decreasing rapidly. In the other cases, the pressure drop increases during the initial stage, similar to the case of Fe-0 ACP-15 shown in Fig. 5. Pressure drops show minimal value of about 20 s, and they increase again and then decrease gradually after the second peak.

Changes in the pressure drop of the Fe-100 beds with different CaO composition in the adhering layers of ACP.
In order to quantitatively discuss the effect of CaO composition on the pressure drop change, there are three characteristic values indicated in the pressure drop curves shown in Fig. 12 which need to be explained. These are the pressure drop difference between the initial value and minimal value at 20 s (Difference-A), that between the minimal value at 20 s and second maximal values at 75 s (Difference-B) and that between the initial value and one at 200 s (Difference-C). Negative values of these differences indicate that bed permeability is improved.

Definitions of pressure drop differences A, B and C from the pressure drop curve.
Figure 13 shows the effect of the CaO content in the sample bed on the pressure drop difference-A. With 7.5 mass%-CaO (Fe-100 ACP-20), the pressure drop difference-A was less than that for 5.0 (Fe-100 ACP-15) and 10.0 mass%-CaO (Fe-100 ACP-25). Then, it decreases with increasing CaO content from 10 (Fe-100 ACP-25) to 15 mass%-CaO (Fe-100 ACP-35). In the sintering bed, the surface of the metallic Fe particles is oxidized first, forming a FeO layer which becomes thicker as oxidation progresses. In order to determine the formation behavior of oxide melt in the bed, the reaction between the FeO that forms on the surface of metallic Fe particles in the early stage of the reaction, and the fines of CaO–Fe2O3 system of the ACP which adhere to them must be considered. The melt formation behavior provided by the phase diagram of the FeO–Fe2O3–CaO system, calculated by Fact Sage (see Fig. 14) indicates some possible changes in the composition of the melts formed when using ACP. If the minimum liquidus temperature is low, the melt forms at a lower temperature, allowing the structural change of the bed to proceeds more easily.

The effect of the CaO content in the sample bed on pressure drop difference-A.

Composition of the minimum liquidus temperature for adhering layers of different ACP on the phase diagram of FeO–Fe2O3–CaO system.
Changes in the minimum liquidus temperature were shown to have a significant effect on the pressure drop difference-A. The bed temperature at 20 s observed for different case of CaO content are plotted against the average CaO mass% of the sample bed in Fig. 15, together with the liquidus temperatures estimated from Fig. 14 corresponding to the composition of adhering layers of ACP and those estimated by the binary phase diagram of CaO–Fe2O3 system.6) In the case of 7.5 (Fe-100 ACP-20), 12.5 (Fe-100 ACP-30) and 15 mass%-CaO (Fe-100 ACP-35), the bed temperature at 20 s sintering is higher than the liquidus temperatures estimated using Fig. 14. It suggests that the melt formed in the early stage at the contact point between the FeO formed on the metallic Fe particles and the adhering layer of the ACP. The liquidus temperature of the 12.5 (Fe-100 ACP-30) and 15 mass%-CaO (Fe-100 ACP-35) are considerably lower than the bed-temperatures, suggesting that as the melt formation proceeds, the structural change of the sintering bed accelerates. The pressure drop difference-A is larger for the 12.5 (Fe-100 ACP-30) and 15 mass%-CaO (Fe-100 ACP-35) cases, as shown in Fig. 13. In the case of 5 (Fe-100 ACP-15) and 10 (Fe-100 ACP-25) mass%-CaO, however, the bed temperatures are lower than the corresponding liquidus temperatures. It is worth noting that they are also lower than the liquidus temperature estimated by the binary phase diagram of CaO–Fe2O3 system, which indicates that the adhering layers of ACP cannot melt by themselves. Hence, very little structural change to the bed occurs and the pressure drop difference-A is lower than in both cases that when the CaO content was high in the early stage of sintering.

The relationship between the bed temperature at 20 s sintering, the liquidus temperatures and the CaO content of the sample beds.
The relationship between the pressure drop difference-B and the CaO content of the sample bed is shown in Fig. 16. The CaO content had less effect on pressure drop difference-B. This is because this increase in pressure drop is caused by the formation of large clusters formed at the lower part of the bed and is not strongly depend on the CaO content of the formed melt.

The effect of CaO content in the sample bed on pressure drop difference-B.
Figure 17 shows effect of CaO content of the sample bed on pressure drop difference-C. A similar tendency is noted between pressure drop difference-C and pressure drop difference-A shown in Fig. 13. The decreases in the pressure drop after its peak value observed at about 55 s sintering can be mainly attributed to the decrease in the bed-temperature. It should be noted that it occurs in much the same way in all cases. Pressure drop difference-C is mainly dependent on the pressure drop in the early stage (pressure drop difference-A), which was affected by the melt formation behavior. These results indicate that the CaO content has a significant effect on the melt formation in the early stage when metallic Fe particles are used as the agglomeration agent. When the CaO content is higher than 10 mass%-CaO (Fe-100 ACP-25), the increase in the CaO content leads to an increase in the amount of formed melt. This accelerates the structural change of the bed and improves bed permeability.

The effect of the CaO content in the sample bed on pressure drop difference-C.
The pressure drop difference-C obtained for Fe-0 ACP-15, Fe-100 ACP-15 and Fe-100 ACP-35 are shown in Fig. 18. A comparison of these results indicates that pressure drop difference-C Fe-0 ACP-15 is lower than when metallic Fe particles were used, suggesting that the improvement in permeability due to increasing CaO content was not sufficient to recover from the decrease in permeability due to the utilization of metallic Fe particles in this condition.

The pressure drop difference for Fe-0 ACP-15, Fe-100 ACP-15 and Fe-100 ACP-35.
In the present study, blocking phenomenon of gas flow path by the melt was observed when its amount was increased. It suggests that similar phenomena will occur in the actual sintering bed although such parts will lead to inhomogeneous sintering underneath. It seems to be consistent with the results of the sinter-pot tests obtained using partially-reduced iron as an agglomeration agent.3) In this case, a higher temperature profile in the lower sintering bed may promote the blocking of the gas flow path at the bottom of the bed.
The melt formed during the present series of experiments can be regarded as CaO–FeO–Fe2O3 system. However, the actual sinter is multicomponent system containing SiO2, Al2O3 and MgO. Increase in SiO2 content will significantly increase viscosity but will sometimes decrease it liquidus temperature. In this way, quantitative prediction of the effect of melt components is difficult. In addition, oxidation behaviors of metallic Fe and FeO will be strongly affected by the melt property. Therefore, further study on oxidation rate and melting behavior of Fe components are essential to effectively utilizing metallic Fe as an agglomeration agent.
A series of sintering experiments were carried out in a sintering simulator to determine the effects of using metallic Fe as an alternative agglomeration agent. Further, the effect of the CaO content of the sample bed on permeability is examined. The results can be summarized as follows:
(1) When metallic Fe particles were substituted for coke, the oxidation rate of metallic Fe was lower than that of coke.
(2) When using coke as an agglomeration agent, the pressure drop increases and starts to decrease rapidly after a few seconds, before slowing down. However, when using metallic Fe particles, it increases again, showing a second peak after the rapid decrease stage.
(3) The decrease in permeability noted when using metallic Fe particles as an agglomeration agent can be attributed to a blockage of the gas flow path in the lower part of the bed due to the increasing amount of formed melt.
(4) When metallic Fe particles are used, formation occurs more easily in the early stage of sintering when the CaO content is higher. This accelerates changes in the structure of the sintering bed and improves bed permeability.