2015 年 55 巻 8 号 p. 1565-1572
2015 年 55 巻 8 号 p. 1565-1572
To efficiently recycle valuable metals such as chromium and nickel in stainless steel dust, a research was made to separate metal nugget included chromium and nickel from self-reduced products of coal composite stainless steel dust briquette, here is defined as a CCSB. Metal nuggets and slag were formed in self-reduction process of CCSB. After reduction, a large amount of C3S and a little C2S existed in slag due to its high basicity. However, C2S content was increased by solidification of oxide melt in slag, and then, it was much increased by decomposition reaction of C3S as reduced products were kept up at low temperature. The transformation of β C2S→γ C2S was occurred in slag during cooling as slag was shattered due to volume expansion, and then separation of metal nugget was achieved. The effect of basicity on separation of metal nugget was considered. Thermodynamics equilibrium calculations and non-equilibrium cooling calculations were carried out on chemical reactions in CCSB, and separation mechanism of metal nugget and the effects of holding time and temperature on separation were investigated. The optimum holding temperature and time were 1100°C and 15 min, respectively. Recovery ratio of iron, chromium and nickel were 92.5%, 92.0%, and 93.1, respectively. Metal nuggets were separated from self-reduced product of CCSB without using any auxiliary materials. This separation method can indicate one innovative process for stainless steel dust comprehensive utilization.
Stainless steel dust has been generated in stainless steel production using electric furnace and AOD/VOD furnace, which contains a large amount of valuable metal such as iron, chromium and nickel.1,2) Furthermore, stainless steel dust is classified as a hazardous industrial waste due to bearing large amounts of chromium.3) Therefore, it is of great significance to recycle valuable metals in stainless steel dust with high efficiency.
Up to now, many processes have been developed for recycling chromium and nickel in stainless steel dust, for example, INMETCO, Fastmet/Fastmelt and OXYCUP et al. In INMETCO process,4,5) recovery ratio of iron, chromium and nickel are about 95%, 86% and 95%, respectively and chromium content in melt metal is about 15.2% after final reduction. In this process, stainless steel dust is not used alone, iron containing materials such as scale and mud are used together. Fastmet/Fastmelt process is very similar to INMETCO, but chromium recovery rate is low.6) OXYCUP process produces a hot metal included chromium by using coke, resulting in a high coke ratio, a high amount of slag and a high Si content in hot metal.7) Above mentioned processes have all recycled chromium and nickel by using carbon composite stainless steel dust agglomerates included binder, and melt metal included chromium and nickel have been obtained by melting separation. Direct reduction of carbon composite Sukinda chromite mines agglomerates was studied at high temperature to recover Fe, Cr and Ni by G. U. Kapure et al. Separated metal and slag nuggets were obtained in RHF at cycle time of 25 min, the recovery ratio of Fe, Cr and Ni were about 90%, 40% and 90%, respectively, and Cr content of metal nuggets was about 8%.8) Furthermore, a novel process was proposed to produce ferronickel alloy nugget at a relatively low temperature from carbon composite nickel laterite agglomerates by semi-molten reduction in the reactor like RHF.9) Xue zhengliang et al. introduced a new process to produce iron nuggets by direct reduction of basic synthetic iron ore briquette by carbon. In this process, iron nuggets were separated from slag by using phase transformation of C2S.10)
On the one hand, CCSB manufactured through hot briquetting is a new type agglomerate containing valuable metals with excellent reduction performance. CCSB does not need any binders such as cement or bentonite because it is formed at temperatures where thermal plasticity of coal is appeared, with help of hot briquetting. It does not take long times to cure agglomerates, especially has a high heat conductivity and high reducibility due to an intimate contact between stainless steel dust and fixed carbon, self-reduction action of volatile matter and its lower porosity. Authors reported results of research on manufacture of CCSB, its self-reduction mechanism and formation of metal nuggets,11)however, no report has been yet made on separation of metal nuggets from self-reduction product of CCSB. A large amount of C3S and little C2S existed in slag self-reduction product of CCSB due to a high basicity.11) Moreover, when high basicity slag was cooled, C2S content was increased by solidification of oxide melt in slag.12,13) Polymorphs transformation of C2S was occurred during cooling as slag was shattered due to volume expansion.10,12) This reveals that the separation of metal nuggets may be achieved from reduced product of CCSB. Decomposition reaction of C3S is very important to separate the metal nuggets from self-reduction product of CCSB. Many researches were carried out on the decomposition of C3S, which were verified that the maximum decomposition temperature range for C3S was between about 1025–1175°C.12,13,14,15,16,17,18,19,20,21)
In the present study, coal composite stainless steel dust briquettes (CCSB) were manufactured through hot briquetting in order to efficiently recycle valuable metals such as chromium and nickel in stainless steel dust. Separation of metal nuggets from self-reduced product of CCSB without using any auxiliary material was focused on.
Chemical composition of stainless steel dust used in the present study was given in Table 1. Fe, Cr and Ni contents of stainless steel dust was 33.18%, 11.81% and 2.10%, respectively. Fe in the stainless steel dust existed in the form of Fe2O3, Fe3O4, FeCr2O4 and NiFe2O4, most of which were Fe3O4 and FeCr2O4. Cr existed in the form of FeCr2O4 and CrO, little chromium of CrO morphology. Ni existed in the form of NiFe2O4 and NiO, most of which was the NiO.11) A large amount of CaO was included in gangue, some of which existed as calcium carbonate.11)
| TFe | FeO | Ni | Cr | Zn | SiO2 | CaO | Al2O3 | MgO | loss on ignition |
|---|---|---|---|---|---|---|---|---|---|
| 33.18 | 18.67 | 2.10 | 11.81 | 0.28 | 4.15 | 15.01 | 1.13 | 1.01 | 6.42 |
Proximate analysis of coal and chemical analysis of ash were given in Table 2. Coal, especially high in volatile matters (34.86%) is favorable for self-reduction of CCSB.11) Stainless steel dust and coal were used in powdered form having an average particle size of −74 μm, which were weighed accurately, and then mixed thoroughly. CCSB was manufactured through hot briquetting under a pressure of 35 MPa at 200°C.
| proximate analysis | chemical analysis of ash | |||||
|---|---|---|---|---|---|---|
| FC | Vdaf | Aad | CaO | SiO2 | Al2O3 | MgO |
| 57.87 | 34.86 | 7.24 | 5.19 | 70.09 | 22.79 | 1.01 |
Figure 1 shows a schematic illustration of experimental apparatus. Two same experimental apparatuses were used in the present study. One of them was for reduction of CCSB and the other was for keeping up reduced product at low temperature.

Schematic diagram of experiment apparatus for self-reduction of CCSB and product treatment.
Operating temperatures were achieved with MoSi2 heating elements. The temperature was adjusted by two thermocouples and PID controller to ensure that the temperature error was less than 1°C before charging crucible with the samples. The inert atmosphere was maintained by blowing argon gas at a flow rate of 1.3×10−5 Nm3/s into corundum tube. After reduction, the crucible with reduction products was quickly taken out from the first furnace, subsequently was put into second furnace and kept up for certain time, and then cooled to room temperature in argon atmosphere.
2.3. Possible Reactions 2.3.1. Major Reduction ReactionsThe chemical formulas representing these possible main reactions are shown in Eqs. (1), (2), (3), (4), (5). The major self-reduction reactions taking place in CCSB are evaporation of volatiles from coal Eq. (1), the reduction of metal oxides Eqs. (4), (5), carbon solution loss reaction Eq. (2) and carbon-stream reaction Eq. (3), while formation reactions of iron and chromium carbides and liquid phase formation reactions. Self-seduction of CCSB is easily proceeded due to its excellent reduction performance.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
Not only metal oxides but materials forming slag such as CaO, CaCO3, SiO2, Al2O3 and MgO also exist in CCSB. The chemical reactions by Eqs. (6), (7), (8), (9), (10), (11), (12) are taken place as metal oxides are reduced at high temperatures. After reduction, the chemical reaction by Eq. (13) is taken place during cooling and decomposition of C3S as shown by Eq. (14) is taken place as reduced products is kept up at low temperature.
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
Basicity of slag was changed with C/OCoal ratio of CCSB. Here, C/OCoal ratio was defined as a removable oxygen combined with metal oxides to fixed carbon mass ratio in CCSB. Table 3 shows relationship between basicity and C/OCoal ratio. Basicity was decreased with an increase in C/OCoal ratio of CCSB. Figure 2 shows morphologies of reduced product at different C/OCoal ratio in the range of 0.7–1.0. Reduction temperature and time was 1450°C and 25 min, respectively. Because the effect of volatile matters on self-reduction is very large, CCSB was plenty reduced in low C/OCoal ratio. Reduced products were shattered in C/OCoal ratio of 0.9 and 1.0, while were not shattered in C/OCoal ratio of 0.7 and 0.8. However, it was impossible to separate metal and slag in reduced products because metal nuggets were not formed in C/OCoal ratio of 0.9 and 1.0. The higher C/OCoal ratio the lower the slag basicity in reduced products, the lower slag basicity the much formation of C2S, and reduced product could be easily shattered. The related mechanism will be explained in Chapter 3.2 and 3.3. As C/OCoal ratio is lower, more favor for forming the metal nuggets and the C/OCoal ratio is higher, more favorable for separating metal and slag.
| C/OCoal ratio | 0.7 | 0.8 | 0.9 | 1.0 |
|---|---|---|---|---|
| Basicity | 3.00 | 2.93 | 2.87 | 2.80 |

Morphologies of reduced product for the CCSB at different C/OCoal ratios.
From a view point of reduction and metal nugget formation the optimum C/OCoal ratio was 0.8.11) However, basicity of slag was high as 2.94 in C/OCoal ratio of 0.8. In order to decrease basicity in reduced products, iron ore of 3% and 5% was added in the mixture of stainless steel dust and coal, respectively in CCSB manufacturing. Chemical composition of iron ore were listed in Table 4. In two cases, the C/OCoal ratios was given as 0.8, and basicity were about 2.80 and 2.74, respectively.
| T.Fe | FeO | SiO2 | CaO | Al2O3 | MgO |
|---|---|---|---|---|---|
| 64.5 | 28.03 | 7.00 | 0.85 | 0.99 | 0.50 |
After CCSB with different amount of iron ore addition amount were reduced for 25 min. at 1450°C, reduced products were taken out and cooled to ambient temperature in argon atmosphere. In two cases, reduced products were shattered, and when iron ore of 3% was added, morphology of reduced product was showed in Fig. 3. Reduced product were obviously shattered and metal nuggets were also formed. This fact implies that it is possible to separate metal nuggets and slag from reduced product with basicity below about 2.8. Neverthless, it is not a rational way to add auxiliary materials in CCSB.
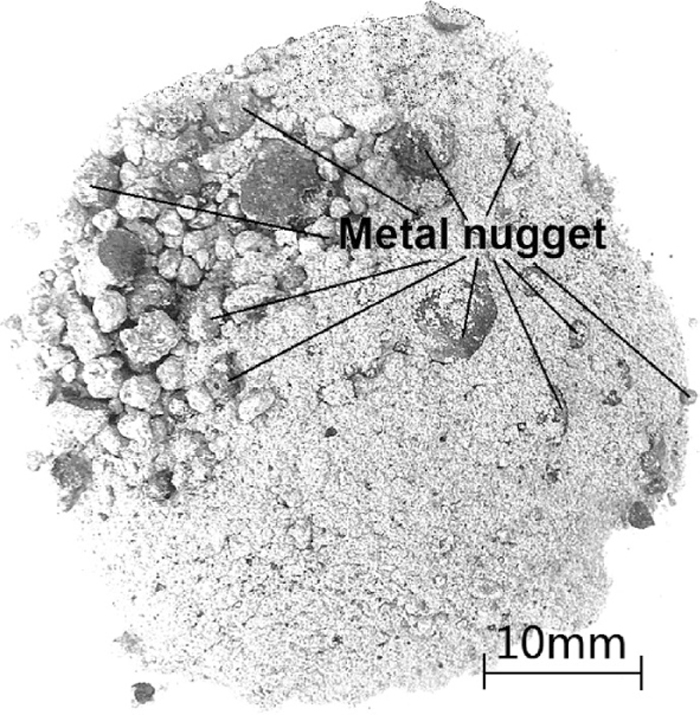
Morphology of reduction product of CCSB with 0.8 C/OCoal and 3% iron ore addition. Basicity of slag was 2.80.
To separate metal nuggets from reduced product without using auxiliary materials, thermodynamic equilibrium calculations and non-equilibrium cooling calculations according to Gulliver-Scheil on slag phase reaction in CCSB were carried out by using Factsage 6.4. Hökfors Bodil carried out phase chemical equilibrium calculations and non-equilibrium cooling calculations according to Gulliver-Scheil in order to consider phase chemistry in process models for cement clinker and lime production.13) Slag consists of CaCO3, CaO, SiO2, Al2O3 and MgO in CCSB. Possible products were identified in inert atmosphere under a total pressure of 1.01325×105 Pa at different temperatures. Temperature was varied to intervals of 10°C in the range of 1000–1500°C. Figure 4 shows simulated equilibrium calculation results of five components system, CaCO3, CaO, SiO2, Al2O3 and MgO. C/OCoal ratio of CCSB was 0.8. CaCO3 in equilibrium phases did not exist above 1000°C, CaO could be completely disappeared by Eqs. (6), (7), (11) above 1300°C. MgO content was little changed and oxide melt is formed above 1390°C. The higher temperature the much the formation of C3S, and C2S could be formed at lower temperature. At equilibrium condition of 1450°C, C3S content was relatively high as 60.35% and C2S content was relatively low as 5.48% in slag. When C2S content is low in slag, slag cannot be shattered. This fact revealed that this condition was not favorable for separation of metal nuggets and slag. Neverthless, C2S content could be increased by solidification of oxide melt and decomposition reaction of C3S during cooling. Cooling of reduced products in realistic process are rapid and thus not in equilibrium. To calculate a relevant composition of the final product, non-equilibrium calculations on slag in CCSB were performed by Gulliver-Scheil method at different temperatures. Temperature was varied to interval of 10°C in the range of 1450–1150°C. Figure 5 shows non-equilibrium Gulliver-Scheil cooling calculation results on slag phase in reduced product. The input condition was identical to slag in CCSB (C/OCoal 0.8). During cooling, contents of C3S and MgO were hardly changed and C2S increased while oxide melt decreased. Moreover, melt oxide was completely crystallized at 1290°C. In other words, C2S content was increased from 5.48% to 14.06% as melt oxide is crystallized. This is favorable for separation of metal nuggets due to an increase of C2S. Figure 6 shows the relationship between major component contents and basicity of slag in CCSB at different C/OCoal ratios. Compositions of slag and its contents were calculated by non-equilibrium Gulliver-Scheil cooling at 1200°C. Basicity of slag was decreased with an increase in C/OCoal ratio of CCSB, MgO content was little changed, C3S content was decreased and C2S content was increased with an increase in C/OCoal ratio of CCSB. That was, C2S content was increased and C3S content was decreased with an decrease in basicity of slag.

Amounts of major slag phases calculated under chemical equilibrium at different temperatures. The input is slag consisting of the five components (CaCO3, CaO, SiO2, Al2O3 and MgO) and C/OCoal ratio of CCSB was 0.8.

Amounts of the major phases during Gulliver-Scheil cooling. The input is identical to the slag in CCSB (C/OCoal 0.8).

Major components content and basicity of slag in reduced product at different C/OCoal ratios. Calculated by non-equilibrium Gulliver-Scheil cooling from 1450°C to 1200°C.
Furthermore, C3S formed in slag could be slowly decomposed by Eq. (14), as a result, C2S could be much increased in slag. This fact implies that it is also possible to separate metal nuggets in high basicity of CCSB. Decomposition of C3S occurred when temperatures approached 900°C. Temperature range for the fastest decomposition of C3S was between 1025°C and 1175°C, both with and without doping.19,20,21) Decomposition kinetic of C3S(Alite) in clinker was studied by Xunrun Li under isothermal condition.15) The optimum model for decomposition of C3S was confirmed as Jander model and the fastest reaction temperature of decomposition of C3S was confirmed 1107.5°C. Supposing that decomposition rate of C3S in slag is close to that in clinker, it could be possible to separate to metal nuggets from reduced products by controlling holding temperature and holding time.
Figure 7 shows changes of C3S and C2S contents in slag with an increase of time at 1100°C. The decomposition model and model parameters were used that listed in the reference.15) As the decomposition model, Jander model describes the three-dimensional diffusion kinetics in solid-state reactions, defining as follows
| (15) |

Changes of C3S and C2S contents in slag with an increase of time at 1100°C calculation by Jander model,15) CCSB: C/OCoal ratio of 0.8 Point A and B are the C2S contents in slag after non-equilibrium Gulliver-Scheil cooling in C/OCoal ratios of 0.9 and 1.0.
Where α is the volume fraction transformed, t is time, k is a rate constant.
The initial content of C3S and C2S in slag were that calculated by non-equilibrium Gulliver-Scheil cooling at 1200°C (C/OCoal ratio of 0.8).
As shown in Fig. 7, C3S content decreased and C2S content increased with increasing holding time, however, C3S and C2S content change were relatively slow. Furthermore, as mentioned above, reduced products were shattered in C/OCoal ratio of 0.9 and 1.0 during cooling because of higher C2S contents in slag (see Fig. 2).
In Fig. 7, point A and B indicated C2S contents in slag after non-equilibrium Gulliver-Scheil cooling in C/OCoal ratios of 0.9 and 1.0, respectively. Therefore, as reduced products are kept up at about 1100°C, C2S content in slag after about 5 and 20 min are close to that in C/OCoal ratio of 0.9 and 1.0. In the other words, this reveals that the metal nuggets could be separated from reduced products in C/OCoal ratio of 0.8.
3.3. Separation Mechanism of Metal Nuggets and SlagSlag in reduced product contains amount of C3S due to its high basicity. A large amount of CaO is included in CCSB, some of which exists as calcium carbonate. Reduction temperature of CCSB is very high as 1450°C, the reduction of CCSB is rapid heating process, calcium carbonate in CCSB could react with SiO2 directly and produce C2S and C3S by Eqs. (9), (10) before it is completely decomposed. These reactions could be thermodynamically and easily proceeded, not only that but reactions by Eqs. (6), (7), (8), (10) are also proceeded in CCSB simultaneously. Anyhow, formed C3S is too much, formed C2S is relatively little in reduced product. However, C2S plays a very important role to separation of metal nuggets from reduced products. C2S exists as five polymorphic states at ordinary pressures: α C2S (a hexagonal phase), α’H C2S (a hexagonal phase), α’L C2S (a hexagonal phase), β C2S (a monoclinic phase) and γ C2S (an orthorhombic phase).16) The temperature ranges of individual polymorphic phase stability are shown in Fig. 8. The arrangements of structures ions are closely similar in α, α’H, α’L and β polymorphs, but that in γ C2S is somewhat different. γ C2S is much less dense than the others, and this causes β C2S to crack and fall to a more voluminous powder during cooling. The transformation of β C2S→γ C2S depends on many factors, one of the most important factors is fineness of β crystals, β crystals smaller than 5μm are stabilizing this phase at low temperature. Crystals growth is enhancing the transformation to γ phase. The size of β crystals is governed by fineness of α’L particles. Coarse α’L crystals are transformed at 630–680°C to β phase which in turn gives at<500°C γ phase. Small crystals of α’L phase give small β particles which do not transform to γ phase. Growth of α’L crystals can be obtained by long heating of samples at the range of α’H stability, thus at 1160–1000°C. Moreover, as mentioned above, a little C2S is formed in reduced products (0.8 of C/OCoal ratio) at 1450°C, C2S content is increased by solidification oxide melt in slag. Althouth C2S is increased, it is not enough to separate metal nuggets. Nevertheless, C3S are decomposed to C2S and f-CaO when reduced products are kept up for certain time at 1100°C. As a result, C2S content is much increased in reduced products. Decomposition rate of C3S is the fastest at about 1100°C, at this temperature, α’L C2S crystals are grown, namely, big β C2S crystals can be obtained during cooling. This is favorable for transformation of β C2S→γ C2S, which implies that it is possible to separate iron nuggets included chromium and nickel and slag from reduced product efficiently.
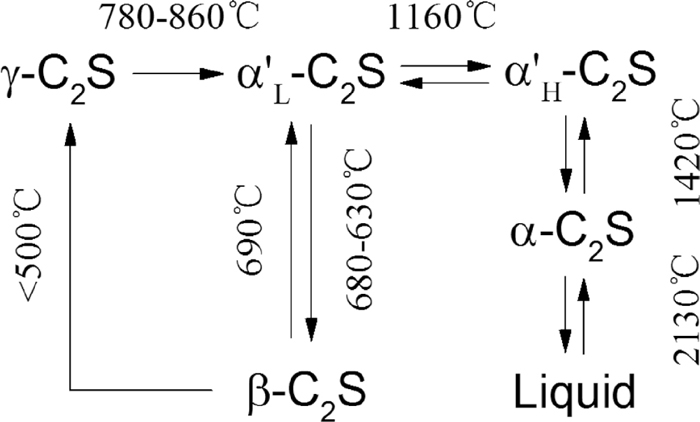
Transformation of different C2S phases.12)
As mentioned above, keeping up reduced products at low temperature for certain time could promote separation of metal nuggets and slag. In order to observe the effect of holding time on separation of metal nuggets and slag, reduced products of 1450°C were taken out from the first furnace, and then kept up at the second furnace of 1100°C for 5, 10, 15 and 20 min. Morphological changes of reduction products with increasing holding time at 1100°C were shown in Fig. 9. Slag in the directly cooled reduced products were not shattered, but the reduced products kept up for certain time were obviously shattered, And the longer the maintain time the much the amount of the shattered slag. In cases that kept up for 5 and 10 min, the relatively large pieces of slag existed in reduced products, however, in cases that kept up for 15 and 20 min, slag was almost completely shattered in reduced products. Metal nuggets and slag were separated by screening reduced products with 150 mesh griddle. Figure 10 shows changes of proportions of -150 mesh slag occupied in reduced products at different holding times. The amount of shattered slag were remarkably increased with increasing holding time, furthermore, proportion of shattered slag of -150 mesh in reduced products is about 0.28 at holding time of 15 min. Figure 11 shows proportions of -150 mesh slag occupied in reduced products at different temperatures for 20 min. The amount of slag of -150 mesh was the highest at about 1100°C and reduced products were hardly shattered at 1200°C. The reason could be estimated that result of fastest decomposition of C3S and growth of α’L C2S crystals at 1100°C as mentioned in Chapter 3.2 and 3.3.

Morphological changes of the reduced products with increasing holding time at 1100°C.
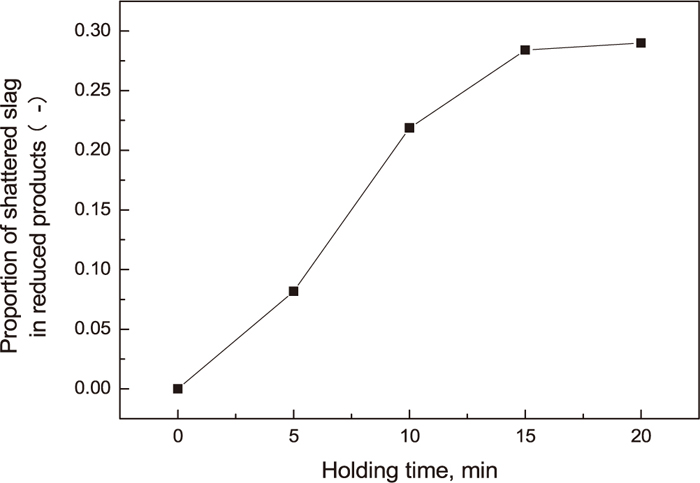
Proportions of slag with -150 mesh occupied in reduced products at different holding times at holding temperature 1100°C.

Proportions of slag with -150 mesh occupied in reduced products at different holding temperature for holding time 20 min.
Figure 12 shows morphologies of metal nuggets and shattered slag separated from reduced products at holding time of 15 min. Figure 13(a) shows X-ray diffraction pattern of separated slag from reduced product of CCSB with 3% iron ore addition by direct cooling. (C/OCoal ratio 0.8) The X-ray diffraction pattern of separated without iron ore addition slag in Fig. 13(b). C2S, C3S, MgO, C3A, CA existed in slag phase. This relatively accords with thermodynamics calculation results mentioned in Caption 3.2. Moreover, f-CaO existed in separated slag from reduced products of CCSB through holding treatment, but not in separated slag from reduced product of CCSB with iron ore addition by direct cooling. Because f-CaO was formed by the decomposition reaction of C3S when reduced products were kept up at low temperature. Furthermore, in this case, recovery ratio of iron, chromium and nickel were 92.5%, 92.0%, and 93.1%, respectively. Recovery rate of Fe, Cr, Ni were similar to each other because Fe, Cr, Ni were almost completely reduced and formed metal nuggets, and special small metal nuggets still existed in shattered slag. Images of SEM of metal nugget and separated slag was showed in Fig. 14. EDS analysis of metal nugget and separated slag phases was carried out, and results are presents in Table 4. The bright phases marked in Fig. 14(d) are special small metal nuggets, which could be easily separated by using the other ways. Anyhow, it is still needed to study for more increasing recovery ratio of valuable metals.
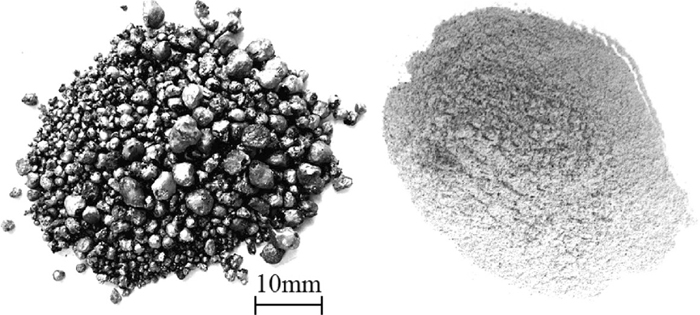
Metal nuggets and shattered slag separated from reduced products (holding temperature: 1100°C; holding time: 15 min).

X-ray diffraction patterns of separated slag. a) separated slag from reduced product of CCSB with 3% iron ore addition by direct cooling (C/OCoal ratio 0.8), b) separated slag from reduced products of CCSB through holding treatment (C/OCoal ratio 0.8, holding time :15 min, holding temperature 1100°C) C2S: Ca2SiO4; C3S: Ca3SiO5; C3A: Ca3Al2O6; CA: CaAl2O4; C3MA2: Ca3MgAl4O10.

Images of SEM of metal nugget and separated slag. (a, c): metal nugget; (b, d): separated slag Point A, B: (EDS analysis of slag) see Table 5.
| Metal | Fe | Cr | Ni | C | |
|---|---|---|---|---|---|
| Bright phase | 80.0 | 4.1 | 12.8 | 3.2 | |
| Grey phase | 61.5 | 32.7 | 1.4 | 4.4 | |
| Dark phase | 55.5 | 37.4 | 2.2 | 5.0 | |
| Slag (Fig. 14(b)) | Ca | Si | Al | Mg | O |
| A | 47.9 | 13.9 | 4.0 | 1.4 | 32.8 |
| B | 48.7 | 11.0 | 2.3 | 0.6 | 37.4 |
Coal composite stainless steel dust briquettes (CCSB) were manufactured through hot briquetting. Morphologies of reduced products were observed at different C/OCoal ratios and iron ore addition ratios, respectively. Thermodynamics equilibrium calculations and non-equilibrium cooling according to Gulliver-Scheil modeling on slag phase reactions in CCSB were carried out by using Factsage 6.4. The increase of C2S was considered with time in slag by using the decomposition kinetic model of C3S. Separation mechanism of metal nuggets and the effects of holding time and temperature were investigated on separation. XRD, SEM and EDS analysis were carried out on slag phase. The following conclusions were obtained:
(1) Reduced products were shattered by adding high silica content in iron ore. it was possible to separate metal nuggets and slag in basicity below about 2.8.
(2) From results of thermodynamic calculations, C3S content was relatively high at 60.35% and C2S content was relatively low as 5.48% in slag (C/OCoal ratio of 0.8) at 1450°C.
(3) From results of non-equilibrium Gulliver-Scheil cooling calculations, C2S content in slag (C/OCoal ratio of 0.8) was increased from 5.48% to 14.06% as melt oxide was crystallized. C2S content in slag was increased increasing C/OCoal ratio.
(4) Althouth a little C2S existed in slag after reduction, it was increased by solidification oxide melt in slag, and was much increased by decomposition reaction of C3S. Polymorphs transformation of C2S was occurred during cooling, and separation of metal nuggets was achieved.
(5) The optimum holding temperature and time were 1100°C and 15 min on separation of metal nuggets, respectively. Recovery ratio of iron, chromium and nickel were 92.5%, 92.0%, and 93.1, respectively.
Metal nuggets were separated from self-reduced product of CCSB without using any auxiliary materials. This separation method can indicate one innovative process for stainless steel dust comprehensive utilization.
The work was supported by Fundamental Research Funds for the Central Universities of China (N110202001).