2016 Volume 56 Issue 1 Pages 14-23
2016 Volume 56 Issue 1 Pages 14-23
In Part 1, the thermo-physical properties of conventional mould slags used in the continuous casting of steel were reviewed. In Part 2, the properties of mould slags used in specialised continuous casting are collated and examined. The following types of slag have been studied (i) Fluoride-free (ii) Carbon-free (iii) non- Newtonian slags used to cast (iv) high Al steels (v) Ti-stabilised stainless steels (vi) thin-slabs at high speeds and (vii) round billets. The casting problems encountered with each type of slag are outlined and the characteristics of the developed slags described. The empirical rules developed for conventional casting apply to these specialised continuous casting covered here. So values of the viscosity, break temperature and fraction crystalline phase are determined by the mould dimensions, casting conditions and steel grade. Consequently, much of these data were produced in “New powder versus Conventional powder” and tend to have similar property values. However, some systematic studies have been carried out but there are no published values of the density, surface tension and thermal conductivity.
The use of non- Newtonian slags provides a new method of improving steel cleanliness and many of the casting problems encountered in the casting of high Al steels have been reduced using calcium aluminate –based slags; however, some further developments are still needed in both cases.
In Part 1 of this review the thermo-physical properties of conventional mould slags were reviewed and analysed. In Part 2 the properties of slags for specialised continuous casting are collated and reviewed. In Part 1 it was found that the values of the required physical properties of the mould slag were determined by the mould dimensions, the casting conditions and the steel grade being cast. Optimum lubrication and heat extraction of the newly-formed shell were obtained with these required values of the powder consumption (Qs) viscosity (η), break (or solidification) temperature (Tbr) and fraction crystalline phase (fcryst) of the slag film formed between the shell and the mould.1) The use of mould slags with these optimum values helps to minimise incidences of longitudinal cracking and sticker breakouts. However, other thermo-physical properties (e.g. interfacial tension (γmsl) or density (ρ)) must be optimised to minimise other defects and casting problems. The required values of the viscosity (η), break temperature (Tbr) and fraction of crystals (fcryst) in the slag film, in specialised casting follow the same empirical rules as those for conventional continuous casting. However, some properties may need adjusting to minimise specific casting problems (e.g. adjustment of η, γmsl to minimise slag entrapment or to cope with the Al2O3 accumulation during the casting of high Al, TRIP steels). The primary tasks of a mould slag in specialised casting are identical to those in conventional casting, namely:
(i) The liquid slag pool must seal off the steel and prevent oxidation.
(ii) The powder bed must provide thermal insulation to prevent the steel from freezing.
(iii) The liquid slag film must provide lubrication to the shell.
(iv) The solid slag film must provide the shell with the optimum heat extraction.
(v) The liquid slag pool must absorb non-metallic inclusions (e.g. Al2O3) from the steel.
Mould powders are a blend of (i) network formers (SiO2, Al2O3) (ii) network breakers (CaO, MgO) (iii) fluxes (e.g. Na2O, K2O, CaF2 and B2O3) (iv) carbon particles and sometimes (v) exothermic agents (e.g. Ca/Si or Fe/Si) which reduce the vertical heat flux in the bed (i.e. increase dpool). The amount and particle sizes of the free carbon are used to control the melting rate (QMR)2) for the given mould geometry (the transient demand for liquid slag, (i.e. QMR) decreases in the order of slabs>blooms>billets).1)
As mentioned above, the properties (Qs, η and Tbr) of the mould powder can be selected for the given mould dimensions, casting conditions and steel grades using the empirical rules outlined in Part 1 Section 1.4. The fractions of glassy and crystalline phase in the slag film (fgl and fcryst) are also important since fcryst controls the level of radiated heat flux between shell and mould, whereas, the glassy phase (i.e. fgl) aids lubrication. Thus fgl and fcryst must be optimised to get the best balance of heat transfer and lubrication. However, sometimes slag compositions are deliberately modified to deal with a specific problem (e.g. the viscosity may be increased to minimise slag entrapment) but such a step results in a decrease in powder consumption. The basicity, (%CaO/%SiO2) or (C/S) of the mould powder is often adjusted to obtain the correct balance of fgl and fcryst; basicities tend to range from 0.6 to 1.35; these values correspond to the basicities used to cast HC billets and MC slabs, respectively.
In recent years specialised mould fluxes have been developed to deal with certain casting conditions. The nature of the problems posed and the approach taken to meet them are described below.
2.1. Fluoride-free (F-free) SlagCaF2 is a key ingredient in conventional mould slags since it reduces the viscosity (η) and the melting (Tliq) and break temperatures (Tbr) all of which lead to an improvement in the lubrication supplied to the shell. The CaF2 also forms cuspidine (C3S2Fl) in the solid slag film which reduces the heat transfer between shell and mould. However, fluorides react with oxides at temperatures above 900 K (e.g. Eqs. (1) and (2)) which lead to gaseous Fluoride emissions;3,4) these emissions cause (i) acidification of the secondary cooling water (ii) corrosion of plant5,6) and (iii) a Health and Safety threat to plant personnel.3)
| (1) |
| (2) |
The threats to the environment and Health and Safety have led to a reduction in the use of fluorides in steelmaking processes. The removal of CaF2 poses no problem to the lubrication supplied since there are other fluxes (Na2O, Li2O B2O3,) available and suitable mould powders have been developed for billet casting where the heat transfer is less critical.7,8,9) However, the task is much more difficult for slab casting (especially for MC grades) where a suitable crystal phase to replace cuspidine in the slag film must be found in order to control the horizontal heat flux. Two approaches have been adopted, namely, (i) to replace CaF2 with other fluxes7,8,9) and (ii) to replace the crystallising phase (cuspidine (C3S2Fl)) with an alternative, crystalline phase.9,10,11,12,13,14,15,16) Several candidate, crystalline phases have been proposed e.g. perovskite (CT))9,10,11,12,13) Titanite (CST),14) Melilite (a solid solution between, akermanite (C2MS2) and gehlenite (C2AS));15) gehlenite8) and the phase, NC2S316) but, to date, none of these phases have proved a totally effective substitute for cuspidine.
The use of F-free slags has been shown to result in significant decreases in the SEN erosion rates.8)
2.2. Carbon-free (C-free) SlagsThe demand for liquid slag to lubricate the shell increases with increasing mould width (or decreasing R* (=2(w+ t)/w. t, where w and t are the width and thickness of the mould). Thus the melting rate of the powder must be controlled to meet this requirement. The melting rate, in practice, can be increased by (i) decreasing the free carbon content (% Cfree)2) (ii) increasing the carbon particle size (DC)2) and (iii) decreasing bulk density (ñbulk) of the powder (since the powder bed becomes more permeable which increases the qvert). Carbon is non-wetting to slag and thus C particles hinder the agglomeration of molten slag globules to form a liquid pool until the C is consumed by reaction with oxygen. However, the accumulation of C particles in the slag can cause C –pick up on the surface of LC and ULC steels and the creation of Carbon smears.17) Carbon particles are not essential to control the melting rate, they can be replaced by nitrides (e.g. Si3N4, BN) in the powders.17,18,19)
2.3. Non- Newtonian SlagsSlag entrapment occurs in the meniscus region as a result of turbulent metal flow. One tactic used to minimise entrapment is to increase the viscosity but this reduces the powder consumption (i.e. shell lubrication). An alternative action is to use non- Newtonian slags;20,21) most molten slags are Newtonian (i.e. viscosity values are unaffected by shear rate) but slags containing N are considered to be non-Newtonian since they form weak bonds which break at high shear rates. These bonds are formed by adding N atoms to the molten slag and these N− ions occupy sites in the Si–O tetrahedron and a weak bond is formed with a non-bridging, Ca2+ ion. This weak bond shears when the shear rate exceeds a critical value (see Fig. 2) and results in a decrease in viscosity. In the continuous casting mould the shear rate is low in the meniscus (“entrapment”) region but is much higher in the mouth of the mould/shell channel where slag infiltration occurs. Consequently, in a non- Newtonian slag the viscosity is high in the meniscus region (thereby reducing slag entrapment) and low in the mouth of the infiltration channel (leading to good lubrication).20,21)

A non- Newtonian slag (containing 0.2%N) was prepared by adding Si3N4 to a conventional slag (with C/S=0.9) at 1723 K and holding the slag at this temperature for 1 hour.20,21)
2.4. Mould Slags for Casting Steels with High Al ContentsHigh Al steels have excellent properties since Al enhances austenite stability. However, the Al in steel (denoted by underline) reacts with the more reducible oxides in the slag to form Al2O3 (Eqs. (3) and (4)). Of the various constituent, SiO2 is the most affected because of its high concentration in mould slags (Eq. (3)) but other oxides such as FeO, MnO, B2O3, TiO2 also react with Al (Eq. (4) where M=Fe, Mn etc.). In continuous casting, mould slags normally pick up 4–5% Al2O3 of which about half comes from steelmaking processes and half from the reactions shown in Eqs. (3) and (4).22) However, the amount of Al2O3 pick-up via Eqs. (3) and (4) increases with increasing Al content (ca. 15 and 25% for 0.5 and 1% Al, respectively). The mould slag struggles to dissolve these high levels of Al2O3 since (i) the driving force for dissolution (Csat-C) in the liquid slag pool decreases as its concentration © approaches Csat and (ii) Al2O3 particles tend to agglomerate through collisions with a consequent reduction in the (surface area/volume) ratio and (iii) the transport of the slag at the interface is slow because the slag viscosity increases with increasing Al2O3 content. The increased Al2O3 contents have marked effects on Tliq, Tsol and viscosity which, in turn, result in reductions in the powder consumption (Qs reqslag =0.55/ç0.5.Vc or Qs req powd =0.6/ç.Vc) and the lubrication supplied.
| (3) |
| (4) |
The large amounts of Al2O3 formed can also be incorporated into the slag rim where they tend to form excessively large rims. The high Al2O3 content of the slag films tends to makes them fragile. Broken rims can be captured by the shell (leading to depressions) or alternatively, can block off the flow of slag into the mould/shell gap (resulting in sticker breakouts). Agglomerates of Al2O3 can build up in the mouth of the slag channel and hinder slag infiltration. It has also been reported that the high Al2O3 contents also cause erratic melting, fluctuating mould temperatures and false alarms on the sticker detection system. It has also been suggested that Al2O3 promotes the crystalline phase at the expense of the glassy phase, which, in turn, results in reduced lubrication (Fig. 1).15,23)

The CaO–Al2O3–SiO2 phase diagram showing (i) (●)=principal composition regions of both low basicity and calcium aluminate mould slags and ( )=high basicity, melilite slags showing primary crystallisation field of gehlenite (CAS) phase); arrows denote the effect of Al2O3 pick up when casting a high Al steel) [after Hanao15) O)).
)=high basicity, melilite slags showing primary crystallisation field of gehlenite (CAS) phase); arrows denote the effect of Al2O3 pick up when casting a high Al steel) [after Hanao15) O)).
One further problem is that melting temperatures of steels with high Al contents decrease with increasing Al content. Low steel temperatures lead, sequentially, to low vertical heat transfer (qvert) low melting rates and shallow slag pools. Thus in order to cast steels with very high Al contents (and low melting points) it may be necessary to provide the mould slag in liquid form to the mould since the steel provides insufficient heat to melt the mould slag. Several studies have been carried out on the development of liquid slag feeding.24,25)
Certain strategies have been adopted to aid the dissolution of large amounts of Al2O3 formed during the cast; these are:
(i) to keep the mould slag in a low melting region (see Fig. 1)
(ii) to add sacrificial FeO or MnO to the slag to reduce the changes in SiO2 content
(iii) to add exothermic agents to the powder to increase the volume of the molten pool which, in turn, reduces the Al2O3 concentration in the liquid slag.
(iv) by minimising Al2O3 formation by using a mould slag which is basically calcium aluminates (denoted CA) plus less-reducible fluxes (e.g. Li2O, Na2O, B2O3, CaF2).
Conventional mould slags used to cast high Al steels tend to have a low basicity (C/S). This is surprising since the high SiO2 content will promote the formation of Al2O3 by Eqs. (3) and (4). However, the low basicity helps to keep the slag in a low-melting region (Fig. 1) despite the steady pick-up of Al2O3 during the cast. The pick-up of Al2O3 results in further crystallisation of the slag and loss of glassy phase (which adversely affects lubrication).15) A selected mould slag composition for casting high Al steels has been derived by coupling a kinetic model to a thermodynamic database.26)
High basicity powders have advantages in that they (i) restrict the amount of available SiO2 for reaction via Eq. (3) and (ii) keep the O content of the steel low. However, the high basicities tend to result in low viscosities.15) Melilite (a solid solution ranging from akermanite (C2MS2) to gehlenite (C2AS) slags have been used for casting high Al steels since they retain more glassy phase when picking up Al2O3 (which aids lubrication) (Fig. 1) and also tend to use less CaF2 than those slags forming cuspidine as the primary phase.15)
A new family of mould powders has been designed and developed to minimise the amount of Al2O3 formed via Eqs. (3) and (4) when casting high Al steels. These powders are based on calcium aluminates (CA) and have a low SiO2 content (usually 5–10%). The CaO– Al2O3 system has a eutectic temperature (ca. 1690 K) at a composition corresponding to C12A7 (i.e. containing 48% CaO) and fluxes are added to reduce Tliq. The fluxes, MnO, B2O327) and TiO2 have all been found to be reduced and form Al2O3 via Eq. (4); this leaves only Li2O, Na2O, MgO, BaO and CaF2 to carry out the fluxing duties. Some development of the flux blend is still needed. However, calcium aluminate (CA)-based powders have proved useful in casting high Al steel grades.23,28,29)
2.5. Mould Slags for Casting Stainless SteelsThe principal issues in casting stainless steels are (i) the low liquidus temperature of austenitic stainless steels which results, sequentially, in lower qvert, lower melting rate, lower dpool and lower Qs and (ii) for Ti- stabilised steel grades, reactions occur between Ti in the steel and SiO2 in the slag (Eqs. (5) and (6)); the latter result in the formation of skin laminations (of CaTiO3 or TiN). In mould slags with high basicity, the TiO2 in the slag reacts to form perovskite (CaTiO3 or CT) which has a high melting point.15) Another problem is that TiN or Ti(CN) can be formed in the mould31,32,33) and since TiN has a very low solubility (<0.5%) in mould slag, these TiN particles tend to agglomerate forming “lumps” and “clogs”.32) Furthermore, these agglomerates can hinder the infiltration of liquid slag and thereby, reduce powder consumption and in extreme conditions, cause sticker breakouts.33) It has been proposed that skin laminations can be avoided by using a mould powder with a (C/S) ratio less than 1.0 in order to minimise the formation of perovskite (CT).30)
| (5) |
| (6) |
High- speed- and thin slab casting (with Vc up to 8 m min−1) results in (i) short residence times in the mould (ii) high heat fluxes (qhor) and (iii) turbulent metal flows (which are frequently decelerated using EMBR). These measures, in turn, result in (i) thin shells which are prone to bulging at the mould exit34) (ii) shells of uneven thickness which are prone to longitudinal cracking, (especially for MC steel grades) and (iii) a significant decrease in qvert, (due to the use of EMBR) which, in turn, leads to a shallow slag pools and low powder consumption (Qs). Thus the threats in high speed casting are: (i) poor powder consumption (ii) problems associated with mould level variation (due to bulging)) and (iii) a propensity to cracking due to the high heat extraction rates (qhor). Ideally, the mould powder should provide a slag film which results in a low qhor in the meniscus region (to minimise cracking) and a higher qhor in the lower mould to minimise bulging.
The mould slags used in high casting speed casting tend to have low viscosities since the casting speeds are high (Qslag req = 0.55/η0.5Vc35,36)) and slag viscosities tend to conform with this rule.37) Mould slags tend to have high Tbr values to ensure low qhor in the upper mould. However, since dpool and Qs tend to be low, there have been attempts to increase the melting rate by lowering the %C (but care must be taken to avoid the freezing of the steel meniscus when reducing %C (since C is a fuel)) or by increasing the Carbon particle size.2)
2.7. Mould Slags for Casting Round BilletsThe shell needs good uniform support when casting round billets and partially-crystalline, slag films (with their associated surface roughness) do not provide these conditions; thus a glassy slag film is required under these circumstances. However, it is also necessary to control the heat transfer (qhor). A glassy slag with high MnO or FeO content (to absorb the radiation conduction) and a high solidification temperature (to provide a thick slag film) are used to cast round billets.15,38) Melilite slags have been successfully used to cast round billets because they retain glassy phases.15)
We have seen in Part1 that SiO44− tetrahedra constitute the building blocks in silicate structure and that these tetrahedra join together in various forms (e.g. rings, chains etc.). The principal factors affecting properties, such as the viscosity, are the degree of polymerisation (represented by the parameter, Q) and to a lesser extent, cation effects (which are frequently represented by the field strength (z/r2)). For alumina-silicates the AlO45− ions are readily incorporated into the SiO44− network but require a cation for charge-balance (e.g. form NaAlO44−). The addition of Al2O3 follows the Lowenstein Al avoidance Rule (in which the formation of Al–O–Si bonds are preferred to Al–O–Al and Si–O–Si bonds) with only minor deviations from the rule being reported.
3.2. Non-Newtonian SlagsIn non- Newtonian slags, N ions replace O− ions in a Si–O tetrahedra and the N ion forms a weak bond to a non-bridging Ca2+ ion (Fig. 2(a)). This weak bond shears when the stress rate exceeds a critical value and results in a decrease in the viscosity of the slag.20,21)
3.3. Effect of TiO2 on StructureThe effects of TiO2 on the slag structure are not a big issue in conventional mould slags since TiO2 is mostly present only as an impurity. However, in slags for specialist casting the effect of TiO2 on slag structure is important since significant amounts of TiO2 are (i) formed by reaction of Ti in steel with SiO2, MnO etc. in the slag (e.g. Eq. (5)) and (ii) used in mould slags to provide (a) an alternative crystalline phase (perovskite, CaTiO3 or CT) to cuspidine (b) used to provide a less- reducible flux than B2O3, MnO etc. (or as a replacement for some of the SiO2 in the slag). It is well established that TiO2 additions result in a decrease in slag viscosity.11,30,32,39)
There are three possible ways TiO2 can modify the slag structure, namely (i) Ti4+ is incorporated into the SiO4 network (ii) Ti acts as a network breaker (by adopting coordination of 5- or 6-) and (iii) by forming TiO2-like structures (clusters).40) There is some dispute which is the dominant mechanism in the geological literature and recent publications are equally inconclusive. The results reported by Li et al.41) indicate the Ti prefers to form TiO2-like clusters and there is a small increase in polymerisation. In contrast, results reported by Zhang et al.40) support the formation of TiO6 octahedra (i.e., Ti acts as a network breaker). In the case where Ti4+ additions increase polymerisation, the reductions in viscosity have been attributed to the Ti–O bond being weaker than the Si–O bond (Si–O>Al–O>Ti–O>Ca–O).
3.4. Effect of B2O3B2O3 is [considered to be a network former in slags and is known to form complex boro-silicate structures. However, B2O3 additions frequently result in a decrease in viscosity.7,42,43,44) In pure B2O3 the structure is made up of 3- coordinated BO3 units arranged in the form of 2-dim rings. However, additions of CaO or Na2O result (shown in Eqs. (7) and (8)42)) in the formation of structural units with both 3- and 4- coordination (i.e. 3-dim BO4 units) respectively.42) It has been reported that for slags containing (35–51%) CaO, 30% Al2O3 (6–15%) SiO2 12%Na2O (0–9%) B2O3, the BO3 ring species were dominant.42) It has been pointed out43) that the viscosity is determined by the strength of the bond of the flow unit and the B–O bond is weaker than the Si–O bond leading to a reduced viscosity.
| (7) |
| (8) |
In the CaO–Al2O3 system, Al–O bonds exhibit 4-, 5- and 6-fold coordination (i.e. forms AlO4, AlO5 and AlO6 structural units).45,46) The AlO4 units are the dominant species for XCaO >0.5 (where there are sufficient Ca2+ ions to charge balance the AlO45− ions); any excess Ca2+ cations are then free to depolymerise the AlO4 network and reduce the viscosity. Thus viscosity decreases with increasing CaO (or CaO/Al2O3 ratio). However, increasing Al2O3 has been reported to cause decreases in the number of AlO4 units with gradual increases in both AlO5 and AlO6 species.45,46,47) Since mould powders contain various fluxing agents, the charge balancing duties will be done by cations in the hierarchy Na+> Li+> Ca2+> Mg2+. Recently, it has been shown that that the addition of K2O to a CaO–Al2O3–SiO2 slag resulted in K+ ions taking over the charge-balancing duties performed by Ca2+ ions with the released Ca2+ ions gradually forming more NBOs.48) This was found to be accompanied by a slight increase in viscosity followed by a decrease in viscosity when (XK/XA) >0.9 (Fig. 3).48) This behaviour was attributed to the competition between the average bond strength of the NBOs (i.e. O–Ca and O–K) and the number of NBOs.48)

Values of log10 viscosity (dPas) as a function of the ratio (XK2O/XAl2O3) for slags containing a fixed Al2O3 content.48)
Calcium aluminate type mould slags usually contain some SiO2 (5–10%); the SiO2 results in the formation of some SiO4 networks. Any Al2O3 formed during the cast tends to get incorporated into the AlO4 networks rather than the SiO4 networks via Eq. (9).47) CaF2 additions reduce viscosity but do not break any Al–O bonds, so this viscosity decrease has been attributed to the liberation of silicate units (Q0Si) from the aluminate network.47) It was suggested that CaF2 additions had little effect on the overall polymerisation (i.e. the distribution of Qn species) or the number of BOAl units but does tend to increase the number of de-polymerised (Q0Al) units.47) TiO2 additions reduce the viscosities and have been reported to reduce the degree of polymerisation in the AlO4 network.41,44)
| (9) |
In continuous casting, the key physical properties (η, Tbr, fcryst) of mould slags are determined by the mould dimensions, casting conditions (e.g. Vc) and steel grade which can be predicted via empirical rules.1) It will be seen that much of the property data for specialist mould powders are used to compare the developed powder with those of the conventional mould powder. There have been few, if any, studies, designed to create a property database for the various types of mould slags. However, there are a few studies where the viscosities or liquidus temperatures have been determined in a systematic manner as a function of composition for a specific type of mould slag. In some cases, these data have been processed to give in the form of modified constants for the Riboud equation49) low (C/S)50,51) and CA-type slags for casting high Al steels.48) Although such calculated values are useful, the data would have wider applicability if they were incorporated into a more global model. The model to calculate viscosities of slags due to Zhang et al.52) covers the compositional range of the different types of mould slags covered here but does not contain B2O3 at the present time. However, there are virtually no property data for some properties like density, surface tension and thermal conductivity. Estimated values of these properties are based on measurements on slag systems which are very different from those covered in this review.
There are two methods widely used to determine the melting temperature (Tliq) of mould slags (high temp microscope and DTA) but, on occasions, there are significant differences in the Tliq values reported for the two methods. There is a need for a standardised database of properties and for the most accurate data to be incorporated into the database. Thus there is a need for a standardised method for Tliq measurements; the author prefers DTA where the values are corrected to a zero heating rate.
4.1. Fluoride-free SlagsThere are several types of F-free slags; physical-property data for these different types of slags are summarised in Table 1. Despite the advantages of lower F-emissions and longer SEN lifetimes, F-free fluxes have not been widely adopted by industry; plant trial data suggest that f-free fluxes can match the performance of fluxes containing F, especially in billet-casting, where heat-removal tends to be less critical than in slab-casting.
| Replacement Phase | Property | Method | Values | Comments |
|---|---|---|---|---|
| Fluxes | η1573 | RCyl | η1573: Effect of B2O3 *[7, 53] [54, 55] of Na2O [9] | *For billets |
| Tliq | HS micro | Tliq *[8]; Effect of B2O3a [56] | a CaF2 replaced by B2O3 bCaF2 replaced by Na2O | |
| Tbr | RCyl | Effect of B2O3 on Tbr *[7, 53] | ||
| qhor | Simulation | [55] | ||
| Phases | XRD | C2AS + M3AS3 [8]; C11BS4 [56] C2AS [9] | ||
| Plant trial | Comparable performance to Conv [7, 53, 8, 9] | |||
| Secondary cooling water less acidic [57] | ||||
| CT | Structure | Effect ot TiO2, B2O3 [11, 43, 58] | ||
| η1573 | RCyl | η1573: Effects (C/S); T; M; N; B; L; Mn [13]; T,B [11, 43] | ||
| Tliq | HT Micro | Tlq: Effects of (C/S); T; M; N; B; L; Mn [13] | ||
| Tbr | RCyl | Tbr: Effects of B,T [11, 43] | ||
| Tg | DTA | Tg: [44] | ||
| qhor | Simulation | Effect of (C/S); T; M; N; B; L; Mn [13] | ||
| Qt, qhor | Caster | Qt: for FF= 0.71 -0.77; qhor ↑when %Na2O>8*, %MnO>4* | *=Promotes glass | |
| Phases | XRD | CT+CS+C2AS [44] | ||
| Cryst kin | SHTT | Incubation time↑ : additions of B ↑, T↓ [44] ↓Na2O [59] | plain C& low alloy steel | |
| Plant trials | Satisfactory surface quality [13]-with fewer cracks | |||
| CST | η1573 | RCyl | Effect of crystallisation η1573 &Tbr remaining liquid [60] | CST as a replacement for C3S2Fl [61] |
| Tliq | DTA | Tliq=1569−1585 K [61] Effect of crystn on remaining liquid [60] | ||
| Tbr, Tcrys | DTA | Tbr=1929−1220 K; | ||
| TTT-crystn | DTA | For (C/S)=ca. 0.8 and XTiO2<0.17 [61]; Na2O on incubation time [59] | ||
| TiO2 additions→↓ Activation energy for glass (or liq)→ cryst [62] | ||||
| Melilite | η1573 | Osc Plate | η1573 (Melilite)= ca 2 η1573 (cuspidine) with same (C/S) [15] | |
| Tbr | Tbr= ca 1500 K [15] | |||
| NC2S3 | η1573 | Osc Plate | η1573 =ca 10 for NC2S3: L, M and N addition:→↓ η [16] | Mould powder η1573=3.2 & Tbr=1373 K, tested on pilot plant with 0.47%C steel- no cracks |
| Tliq | Tliq=1560 K for NC2S3 [16] | |||
| Tbr | Osc Plate | Tbr=ca 1370 K for NC2S3 [16] | ||
| Additions of M, L and N →↑ Tbr [16] |
The mineral constituents of both standard and C-free (CF) powders tend to be very similar so the thermo-physical properties and qhor values (from both simulation experiments and plant trials) also tend to be very similar. Reported property values are summarised in Table 2.
| Powder Type | Property | Method | Values | Comments |
|---|---|---|---|---|
| C- free | η1573 | RCyl | η1573 for Conv. and CF: [17] | Powders sometimes colour-coded too |
| Tliq | HS micro | Tliq=1280 K for Conv. and CF: [17] | ||
| Tbr | RCyl | Tbr for Conv. and CF: [17] | ||
| k298 | THW | Powder: k298= ca 0.1 Wm−1K−1; 3 methods [17] | ||
| qhor | Plant trial | qhor (CF) = qhor (Conv). [17] | ||
| Qt; dpool | Plant trial | Conv vs CF: Qt; =0.49; 0.57; dpool=15; 13.5 mm [18] |
The property data reported for Non- Newtonian slags are summarised in Table 3. Comparisons of the viscosity (as a function of shear rate) and powder consumption (Qs) for Non- Newtonian and conventional mould slags are given in Figs. 4(a) and 4(b). The contact angle of the slag on solid steel was found to be consistently lower than for the non- Newtonian slag than for the Conventional slag (Fig. 5). Plant trials indicated that non- Newtonian slags resulted in steels with lower inclusion contents and also resulted in higher values of the powder consumption and horizontal heat flux; the latter may prove a problem when casting MC steel grades, and was attributed to the lower contact angle providing better contact.
| Slag Type | Property | Method | Values | Comments |
|---|---|---|---|---|
| Conv. | η1573 dPas | RCyl | η1573; Conv=4.8; NN: {10} η1573=7; {110} η1573=5; | { } =shear rate, s-1: |
| vs | θ | SD on steel | (θconv- θNN) at 1473 K: =+8°; 1573 K= +10°; 1673 K = +18°; | |
| NN | qhor | qhor (NN)> qhor (Conv) due to better wetting | qhor increases as θ decreases | |
| Qs, kgm−2 | Mini-caster | Vc=1.6 m min−1; ConvQs =0.38; NN Qs = 0.50; Qs (Conv)< Qs | ||
| Nincl, 100 g−1 | Caster | (NN) Nincl (Conv)>Nincl (NN); qtot (Conv)= 1.2 qtot (NN) | qtot from water temps |
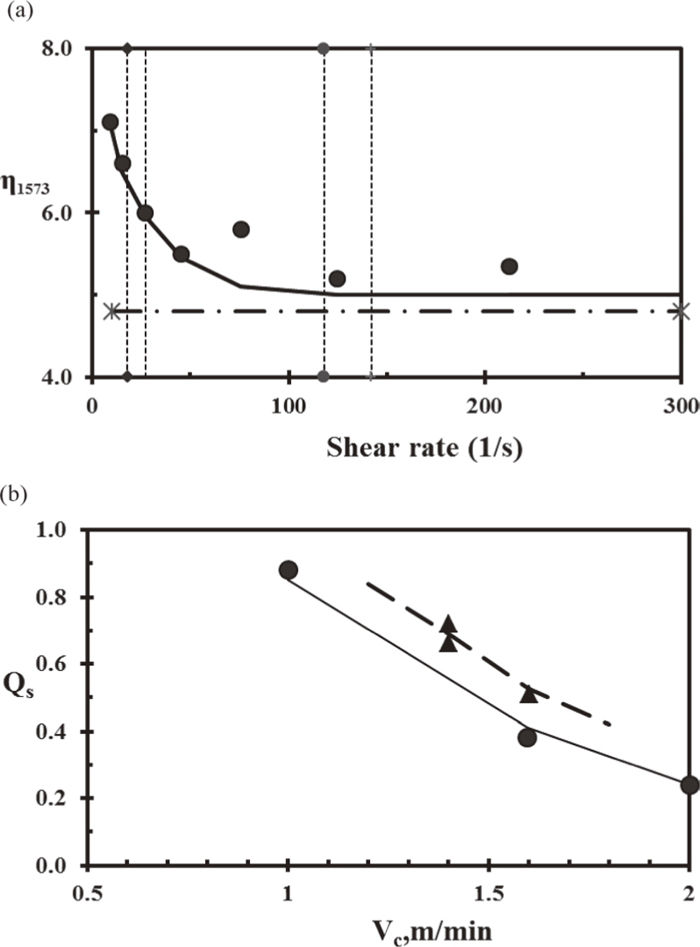
(a) Comparison of viscosity (dPas) of non-Newtonian slag at 1573 K as a function of shear rate (●, solid line) and conventional slag (dash-dot); the vertical, dotted lines denote upper and lower bounds of the regions of high and low shear rate20,21) and (b) Powder consumption, Qs (kgm−2) as a function of casting speed; ●, solid line =conventional slag; ▲, dashed line= NN slag.20,21)

The property data for low (C/S), high (C/S) and calcium aluminate slags are given in Table 4. The effects of compositional changes on the slag properties resulting from the slag/metal reactions (Eqs. (3) and (4)) have been explored by several groups for each type of slag. The effect of increasing (A/S) ratio on the viscosity and melting temperature (Tliq) for a low (C/S) slag is shown in Figs. 6(a) and 6(b), respectively.51) It can be seen that Tliq increases with increasing (A/S) ratio while the behaviour of the viscosity is much more complex. Increasing the Al2O3 content at the expense of the SiO2 and MnO contents would be expected to (i) increase the number of cations on charge-balancing duties for Al3+ ions (ii) to reduce the number of Mn2+ ions available for charge-balancing and network breaking duties. Consequently, the number of cations available for network breaking will be decreased as a consequence of these effects and the slag viscosity will increase. However, a comparison of the viscosities of sillcates (ηs) and alumina-silicates (ηas) as a function of Q (a measure of degree of polymerisation) indicated that ηs>ηas when Q>2.8 due to the greater bond strength of the Si–O bond (cf. Al–O).63) The complex relationships shown in Fig. 6(a) are a manifestation of these competing effects.

Effect of (%Al2O3/%SiO2) or (A/S) ratio on (a) viscosity and (b) melting temperature of low basicity slags used in casting of High Al steels;51) slag compositions (%): all contain 10%F, 1.5%MgO; slag A=14C(43-21)S; (3-30)A; 10N; 2L(6-1)Mn; 0B slag B=10C(43-21)S (3-30)A; 8N; L; 5.5(6-1)Mn; 0B; slag C=10C; (43-21)S (3-30)A; 8N; 0 L; (6-1)Mn; 8B; slag D=9C(43-21)S (3-30)A; 8N; 4L; (6-1)Mn; 5B;.51)
A kinetic model coupled to a thermodynamic database have been used to determine the effect of Al2O3 pick –up by a low- basicity mould slag during the casting;26) a satisfactory slag composition was designed using this technique. This approach could prove very useful in the future for the design of mould powders.
4.5. Mould Slags for Casting Stainless SteelsThe problems encountered are associated with (i) the pick up of TiO2 due to slag/metal reactions (Eqs. (5) and (6)) and (ii) the low liquidus temperature associated with austenitic steels (such as low slag pool depth and poor powder consumption). The low powder consumption in Ti-stabilized steels is also due to the low solubility of TiN in slag33) and the clustering of TiN particles which congregate in the infiltration channel and block slag infiltration.32,69) The properties of slags used to cast stainless steels are given in Table 5.
| Slag | Property | Method | Values | Comments |
|---|---|---|---|---|
| η1573 | RCyl | η1573; Effect of TiO2 [12, 30, 31, 43] TiC* [70] | Chen [12] replaced some CaF2 by TiO2: | |
| Stainless steel slag | Tliq | Tliq [12] | η1573 *Effect of solid TiC | |
| Tbr | RCyl | Tbr [12, 30, 31, 43] | ||
| qhor | Simulat’n | [12] | ||
| γmsl | Drop wt | Increase in TiO2 causes decrease in γmsl: [71] | ||
| Phases | XRD | CT [12]; CT formation avoided by keeping slag (C/S) <1[30] <5%T →cuspidine; >5%→ cuspidine+CT [43] | ||
| TiO2 pick up | 6% when casting austenitic steel cf 3% for ferritic: TiN low solubility in slag –tends to cluster [33, 69] |
As mentioned in Section 2.6, the principal problems associated with high- speed- and thin slab casting (with Vc up to 8 m min−1) are: (i) poor powder consumption (ii) problems associated with mould level variation (due to bulging)) and (iii) a propensity to cracking due to the high heat extraction rates (qhor). Ideally, the mould powder should provide a slag film which results in a low qhor in the meniscus region (to minimise cracking) and a higher qhor in the lower mould to minimise bulging.
The values of viscosity and Tbr37,38) are, in general, consistent with values derived from empirical rules but there is a tendency for Tbr values to be slightly higher than the calculated values, presumably to ensure that the slag film provides a satisfactory slag film. Pilot plant trials72,73) have shown that the interfacial thermal resistance, Rint, is lower than the values measured in simulation experiments which has been attributed to the effect of the ferrostatic pressure on the shell in the plant trials.72,73)
Attempts have been made to increase the melting rate and the slag pool depth by reducing the %C in the powder but frequently this is carried out by adding the carbon in the form of carbon black (with low particle size) which tends to negate any advantages with reducing the %C. The reduction of the %C can also lead to freezing of the steel meniscus (since carbon combustion leads to reduction in the thermal gradient of the powder bed).
The properties of slags used for high speed (thin-slab) casting are given in Table 6.
| Slags | Property | Method | Values | Comments |
|---|---|---|---|---|
| High speed (thin slab) casting slags | η1573 | RCyl | η1573: [37, 38]- fits with empirical rules | |
| Tliq | HT micro | Tliq [37] | ||
| Tbr | Tbr [37] | |||
| qhor | RCyl | qhor [37] | ||
| Qs | Plant trials | Qs: Thin slab: Qs=0.3/η0.5Vc. slab casting Qs= 0.55/ η0.5Vc [37] | ||
| Phases | ||||
| XRD | Cuspidine; combeite; nepheline [37] | |||
| Slag film =ca1 mm [72, 73] Rint: =4×10−4 m2KW−1: [73] |
Melilite slags have been used for casting round billets since surface roughness of the slag film is low and the film contains a significant amount of glassy phase. Furthermore, the relatively high basicity (i.e. (C/S) ratio) ensures that the oxygen potential is low.15,38) Since crystalline slags induce surface roughness, the radiated heat transfer through the glassy slag has to be reduced by adding MnO to absorb the IR radiation rather than through scattering of the IR radiation. The properties of melilite slags have been outlined above in Tables 1 and 4.15)
The slags covered in this review, are, for the most part, still in process of development and require further development. Consequently, it is not surprising that there is a paucity of property data for most of the slag types covered in this paper. Since the prime thrust is powder development, it is also not surprising that many of the property measurements relate to “New vs conventional powder type studies” and so property data tended to be scattered over a wide compositional range. However, some workers have made systematic studies on the effect of specific additions (e.g. B2O3, TiO2 or Al2O3) on the properties (usually viscosity and Tbr) and some workers have represented their viscosity data in the form of modified constants48,50,51) for the Riboud viscosity model.49) However, at the present time the reader will have to use a number of models to calculate the viscosities of different slag types. The slag viscosity model proposed by Zhang et al.,52) looks to be an attractive option (and covers slags based on calcium aluminates) but at the present time does not include B2O3 additions.
For some properties, such as the densities, surface tensions and thermal conductivities, there are no published data available for the slags covered in this review. Such data are needed to validate the predictions of any future models calculating these properties from chemical compositions of the slags.
With regard to the casting performance of the various slag types covered here. The non- Newtonian slags provide an interesting option to reduce slag entrapment in the cast steel. It would also appear that slags based on calcium aluminates offer the best hope for casting steels with high Al and Mn contents; however, there are still problems to be overcome (i.e. their extensive crystallisation with concomitant loss of lubrication23) and the fact that fluxing agents appear to be limited to Li2O, Na2O and CaF2). It would also appear probable that liquid slag feeding will have to be developed to cast steels with very high Al contents (low Tliq values).
(1) Some property data are available for the various types of mould slag covered in this review; these are mostly data for viscosities, liquidus and break temperatures and heat flux values in some cases.
(2) There are no data for densities, surface and interfacial tensions and thermal conductivities, some data are needed to test the predictions of models to calculate properties from chemical composition.
(3) The plant performances of calcium aluminate mould slags used to cast high Al steels appear to be promising but some problems remain to be solved.
(4) Liquid feeding of the mould flux will probably be needed to cast high Al steels.
(5) In high speed casting a slag film which retards heat transfer in the meniscus region but promotes heat transfer in the lower mould (to minimise bulging) remains the target.
(6) The use of a kinetic model coupled to a thermodynamic database for the slag during the casting of high Al steels heralds the arrival of a powerful tool for future studies.
As in Part 1; the shorthand for chemical formulae: A=Al2O3, B=B2O3, C=CaO, F=FeO, Fl=CaF2 etc. has been adopted.