2016 年 56 巻 9 号 p. 1519-1528
2016 年 56 巻 9 号 p. 1519-1528
In the electric arc furnace steelmaking process, the coherent jet was adopted to improve the stirring effect of supersonic oxygen jet and reaction rate in molten bath. However, so little research had been done for the coherent jet behavior. In this study, the coherent jet flow field with preheating oxygen in hot (1700 K) and cold (298 K) condition were studied. The axial velocity and total temperature of main oxygen jet were measured by combustion experiment. Flow field characteristics of coherent jet were simulated by Fluent software. The detail chemical kinetic mechanism including 53 species and 325 reversible reactions was adopted to achieve a more accurate result of shrouding flame and main oxygen jet. The results showed that the low-density region surrounding the main oxygen jet were formed by the combustion, and thus suppressed the entrainment of the ambient gas into the main jet. The higher oxygen temperature increased the axial velocity of coherent jet, with the potential core reducing. Moreover, the higher ambient temperature could prolong the potential core of coherent jet and conventional jet.
Oxygen lance is the main method to supply oxygen into the molten bath in steelmaking processes such as the Basic Oxygen Furnace (BOF) and the Electric Arc Furnace (EAF), and has a key function in dephosphorization and stirring the molten pool. In order to increase the stirring ability and reaction rate, the Laval nozzle is preferred to increasing the velocity of oxygen jet to be approximately 2.0 Mach number,1,2) since the high pressure energy is transformed into the kinetic energy. After the exit of Laval nozzle, the velocity of oxygen jet was keeping reducing due to entrainment of ambient gas, which formed potential core, supersonic core and subsonic zone. With increasing distance from the nozzle, the impact force of the oxygen jet decreased which would depress the mass transfer processes and the reaction rates in the furnace.3,4)
To prolonged the length of potential core and increase the kinetic energy of a supersonic oxygen jet, the coherent jet technology had been applied to steelmaking process,5,6) and has been used widely in electric arc furnace. The key of this technology is suppressing the ambient gas interact with main oxygen jet by the shrouding flame. Therefore, the oxygen jet could keep original diameter and velocity over long distance, and the stirring effect of molten bath has been increased. Moreover, with the greater penetration capacity, the supersonic oxygen jet could deliver great amounts of oxygen into the molten bath and depress the splash of liquid slag, comparing with the traditional supersonic oxygen lance.
The coherent jet technology was proposed by Mathur and Anderson9) et al. and then Mathur10) showed the fundamental results in industrial production. Sarma et al.11) analyzed the characteristics of supersonic jets and coherent jets at different ambient temperature by combustion experiment. Alam et al.12) reported numerical simulations of the supersonic jet with and without shrouding flame and validated against experimental data. The advantages and operating results of coherent jet oxygen lance is well acknowledged, but the detail of the flow field characteristics of coherent jet is not fully understood.13,14)
For improving the speed of computation, the one-step complete combustion between CH4 and O2 were used for most numerical simulation to research the protection effect of shrouding flame, in present study.11,14,15,16,27,31) However, a large amount of process products were formed such as CO, CH2 and OH, during the combustion process, which affected the flow and temperature fields of the shrouding flame. Therefore, to achieve a better result, the detailed chemical kinetic mechanisms (GRI-Mech 3.0) was used for the modeling of reactions with the EDC model in this study. Moreover, both the advantage and disadvantage of new method would be shown in the paper.
Numerical model of the supersonic jet at room temperature and steelmaking temperature was developed. Moreover, how the main oxygen temperature affected the potential core length of the jet was also been analyzed. The numerical simulation results showed good consistent with the experimental data. The behaviors of the supersonic coherent jet with various ambient temperature and main oxygen temperature were investigated to understand the flow field of supersonic oxygen jet with preheating oxygen under various ambient temperature.
The schematic view of coherent jet nozzle and combustion equipment, as shown in Figs. 1 and 2, were used to simulate the flow filed of coherent jet in steelmaking process. The measurement devices were fixed at the measuring points in advance. Then temperature in the furnace was raised by the burner inserted from side wall of the furnace and monitored by three groups of thermocouples (A group, B group and C group), and each group consisted of 4 thermocouples, as depicted in Fig. 1. However, it was difficult to obtain a homogeneous high-temperature distribution in the furnace. Therefore, the average temperature which monitored by thermocouples, was adopted as the ambient temperature for the purpose of this examination. The burner would be replaced by coherent lance after ambient temperature met the requirements. When the examination was tested at room temperature, the coherent jet was removed from furnace and monitored. In present study, the average temperatures of A, B and C groups were about 1711±12 K, 1702±18 K and 1689±10 K, respectively, at the beginning of the combustion experiments.
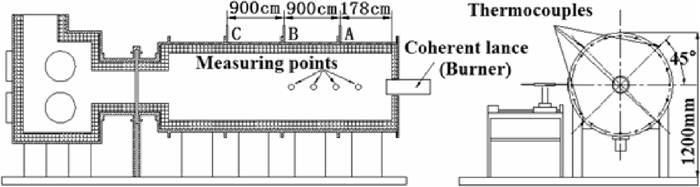
Schematic view of experimental equipment.
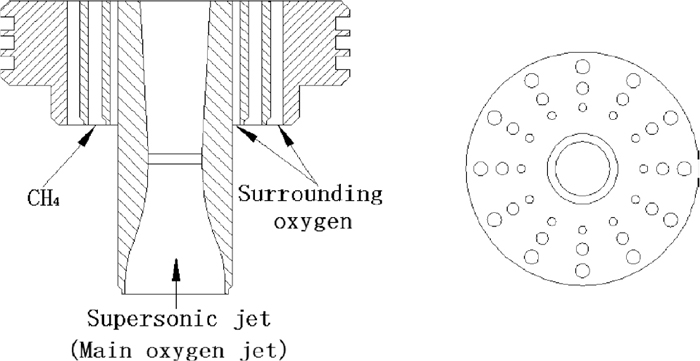
Cross sectional and front view of supersonic coherent jet nozzle.
As Fig. 2 depicted, CH4 and shrouding O2 were injected through the holes arranged in three concentric rings surrounding the Laval nozzle. The inner and outer rings of holes were used for the shrouding O2, and intermediate ring of holes was used for the CH4. The diameters of inner, intermediate and outer rings of holes were 4 mm, 6 mm and 7 mm, respectively.
The coherent jet for the experiment was a water-cooled lance, which inserted and fixed at the horizontal position in the furnace. The Laval nozzle was designed for the experimental conditions, and its diameters of throat and exit were 27.0 mm and 34.9 mm, 28.6 mm and 37.0 mm, 30.3 mm and 39.3 mm, when preheating oxygen temperature was 289 K, 373 K and 473 K, respectively. Both the temperature of surrounding oxygen and fuel injected into the lance were 298 K, and the flow rate of surrounding oxygen was twice bigger than that of CH4. The experimental conditions would be introduced later. In present study, the same parameters of supersonic jet nozzle would be used in both combustion experiment and numerical simulation, when the preheating temperature was same.
2.2. Measurement MethodIn this experiment, static pressure and total pressure of the jet were measured using a point tube. Then the velocity of coherent jet was obtained basing upon the compressibility of gas. At the exit of Laval nozzle, the oxygen with high-pressure energy was accelerated to roughly Ma 2.0. Since the supersonic jet interacted with ambient gas, its velocity attenuated gradually to 0 m/s. The relationship between Mach number, static pressure and total pressure of the jet was expressed as follows:17)
| (1) |
| (2) |
Present simulations used the following set of governing equations:18)
Continuity equation
| (3) |
Momentum conservation equation
| (4) |
Energy equation
| (5) |
In (3–5),
| (6) |
Turbulence kinetic energy equation for EDC model (k equation):
| (7) |
Turbulence dissipation equation for EDC model (ε equation):
| (8) |
In the equations, the Gk and Gb presented was the generation of turbulence kinetic energy due to the mean velocity gradient and the buoyancy, respectively. YM represented the contribution of the fluctuating dilatation in compressible turbulence to the overall dissipation rate. Sk and Sε were user-defined source terms. The σκ and σε were the turbulent Prandtl numbers for k and ε, respectively. The turbulent viscosity (μt) was achieved by combining k and ε as follows:
| (9) |
The turbulence/chemistry interactions were often modeled with two different approaches: the Eddy Dissipation/Finite Rate (ED/FR) model and the Eddy Dissipation Concept (EDC) model23). The previous work19,23,24,25,26) had found that the EDC hypothesis was expected to provide satisfactory results for combustion. Thus, the EDC model with overall and detailed chemical kinetic mechanisms (GRI-Mech 3.0) was presently used for the modeling of reactions. The GRI-Mech 3.0 was the full chemical mechanism, which consisted of 53 species and 325 reversible reactions.
The EDC combustion model handled chemistry representation and turbulence-chemistry interaction and assumed that reaction occurs in small turbulent structures, so called the fine scales. The combustion at the fine scales was assumed to occur as a constant pressure reactor. The evolution of species concentrations was then computed by integrating the chemistry within those fine scales. In EDC model, the species conservation equation for chemical species taken the following general form:
| (10) |
| (11) |
| (12) |
The schematic figure about boundary conditions of the computational domain was presented in Fig. 3. To get better results of numerical simulation, the geometrically simplified axisymmetric computational model (2D) was denied, and computational domain was constructed as a 3D geometrical model. The computational domain of the model included Laval nozzle, nozzles of surrounding co-flow and combustion zone. The combustion zone was 70 De downstream in the axial direction and 23 De in the radial direction, respectively.
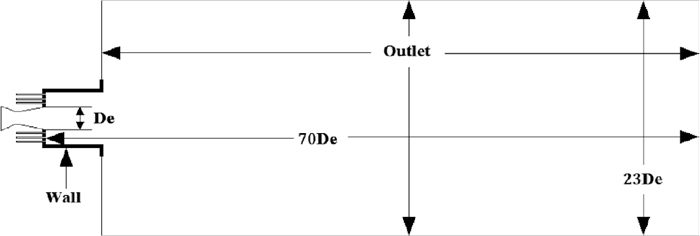
Boundary conditions of the computational domain.
In present study, the standard wall function was applied to this model. Initially, the computational domain started at rest with no CH4 or O2 blowing through nozzles. In the inlet position of Laval nozzle, pressure inlet boundary condition was adopted for oxygen, mass-flow inlet boundary condition was adopted for CH4 and surrounding O2 and pressure-outlet was adopted as the boundary condition in the outlet position of combustion zone. The Table 1 listed the detail values of boundary conditions.
| Name of boundary | Type of bounday conditions | Values |
|---|---|---|
| Main oxygen inlet | Stagnation pressure (Pa) | 792840 |
| Mach number | 2.0 | |
| Mass fractions (wt%) | O2=100 | |
| Oxygen temperature (K) | 298/373/473 | |
| CH4 inlet | Mass flow rate (kg/s) | 0.0794 |
| Mass fractions (wt%) | CH4=100 | |
| CH4 temperature (K) | 298 | |
| Surrounding oxygen inlet | Mass flow rate (kg/s) | 0.0317 |
| Mass fractions (wt%) | O2=100 | |
| Surrounding oxygen temperature (K) | 298 | |
| Outlet | Static pressure (Pa) | 101325 |
| Mass fractions (wt%) | O2=21, N2=79 | |
| Ambient temperature | Initial temperature (K) | 298/1700 |
Code based on the eddy-dissipaton concept method and the implicit method was used to discretize and solved the model equations. The calculations were conducted in steady solution mode and The SIMPLE algorithm method was utilized to solve pressure velocity coupling. A structured non-uniform grid system was used to mesh the whole domain. The second-order upwind scheme was employed for discretizing the equations in order to improve the accuracy of the simulations. The energy and species equations were subsequently solved and convergence was checked. Convergence was obtained when the residuals were less than 10−6 for the energy and 10−5 for all the other variables. The downstream outlet temperature and velocity were monitored and their variations were allowed to be within 5 K and 1 m/s, respectively, for convergence of their solution.
3.4. Effect of Chemical Kinetic Mechanism on SimulationThe Fig. 4 presented the flow characteristics of coherent jet by different combustion models with main oxygen temperature being 298 K at room ambient temperature. The average percentage of variation of the potential core length calculated with the full chemical mechanism (GRI-Mech 3.0) and one-step complete combustion model was about 41.9 pct, as depicted in Fig. 4(a). Moreover, Based on the measured values of combustion experiment, the results with GRI-Mech 3.0 showed a more accurate than that with one-step complete combustion. It seemed that the shrouding flame field was the reason which generated the variation of two kinds of combustion models.
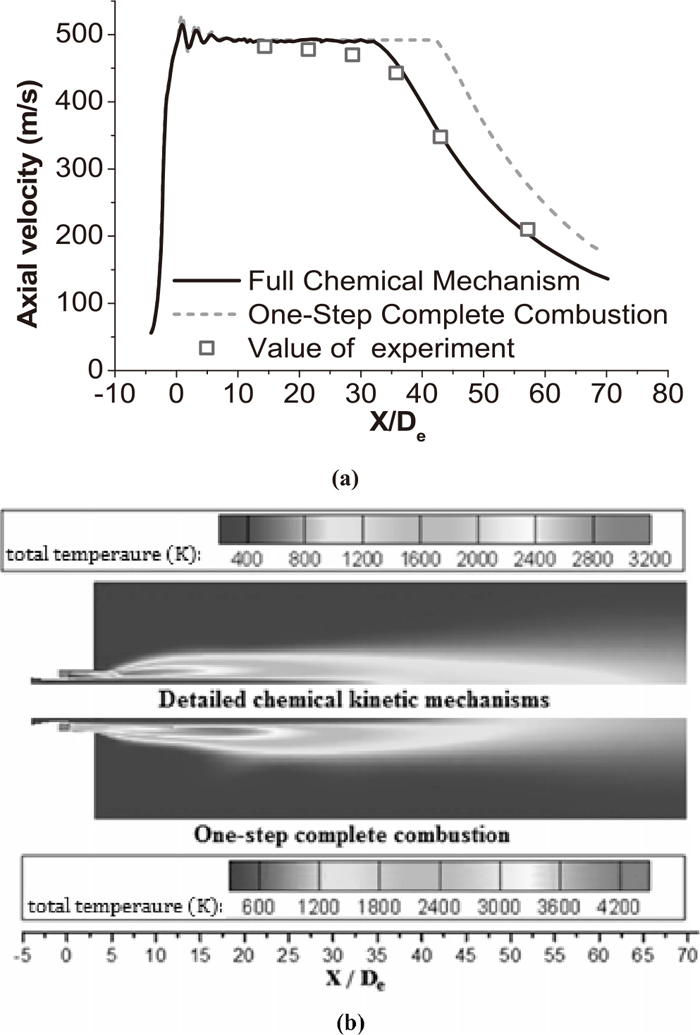
The flow characteristics of coherent jet by different combustion models with main oxygen temperature being 298 K at room ambient temperature. (a) Axial velocity distributions of supersonic jet with a shrouding flame at the jet centerline. (b) Total temperature distributions of the coherent jet on longitudinal section.
The high total temperature region with one-step complete combustion was much bigger than that with GRI-Mech 3.0, as shown in Fig. 4(b). During real combustion process, the process products were formed such as CO, CH2 and OH, and these products would absorb energy from flames which reduced the temperature flame.28) Since the process products had been ignored, the maximum of total temperature with the one-step complete combustion was much higher than the maximum of theoretical combustion temperature being 3300 K.28) The main oxygen jet would be protected better with a shrouding flame with a bigger high total temperature region making the potential core length longer.
Apparently, the detailed mechanisms predict more accurate results of the axial velocity and temperature profiles, as the previous literatures reported in.29,30) However, the computational time required with the GRI-Mech 3.0 was approximately 57 times that with one-step complete combustion. The present study used the full chemical mechanism in all subsequent calculations, since the total computing time with GRI-Mech 3.0 was about 27 hours for one case which was acceptable.
3.5. Grid Independency TestInitially, for verifying the sensitivity and feasibility of the numerical model, calculations for the coherent jet were done using the following three kinds of grid levels: coarse grid (254000 cells), medium grid (423000 cells), and fine grid (536000 cells). As Fig. 5 presented, the axial velocity distribution of coherent jet with a shrouding flame at the jet centerline using various grid levels was presented.
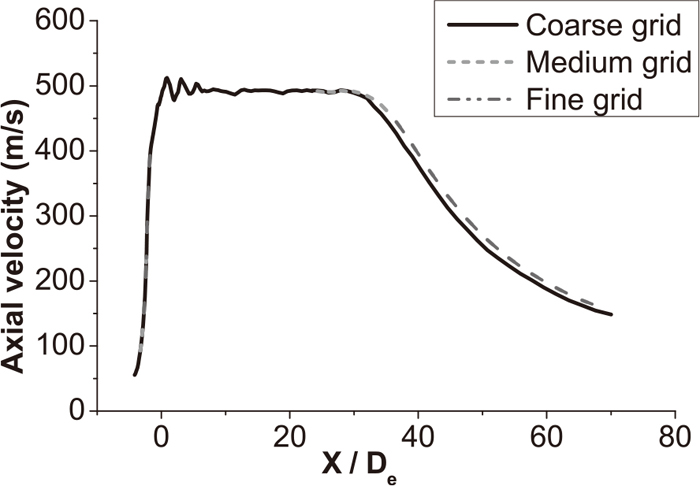
Axial velocity of coherent jet with three grid levels.
The average percentage of variation of the axial velocity profile calculated with the coarse and medium grid level was about 2.5 pct. The average percentage of variation was calculated by averaging the differences at several locations in the axial direction. Between the medium and the fine grid levels, the variation was negligible (less than 1 pct). Hence, it could be said that the solution was not sensitive to the grid. The computational time required for the fine grid level was approximately thrice that for the medium grid level. Hence, the results obtained with the medium grid were used for analysis and discussion in the present study.
Figures 6 and 7 show the axial velocity distribution of the supersonic oxygen jet with and without a shrouding flame at different ambient temperature and main oxygen temperature. For both cases, the axial velocity of jet shows repeated fluctuations at the exit of the Laval nozzle. During the design process of Laval nozzle, nozzle exit pressure is 101328 Pa and main oxygen temperature is 298 K in the present study. However, both nozzle exit pressure and oxygen temperature have changed with a shrouding flame and preheat process. Therefore, shocks are formed by the incorrect expansion of the supersonic jet. After repeated fluctuations of supersonic oxygen jet, the axial velocity of jet reaches steady state and potential core forms.
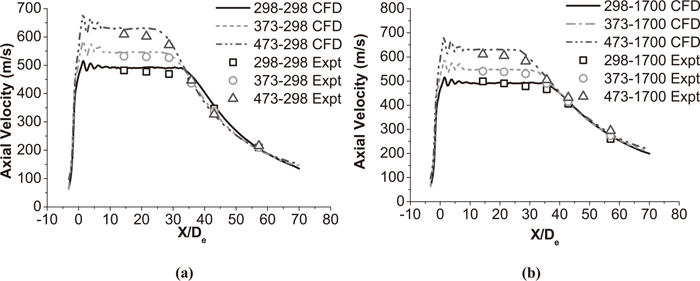
Axial velocity distributions of supersonic jet with a shrouding flame at the jet centerline. (a) The coherent jet under room ambient temperature. (b) The coherent jet under high ambient temperature.
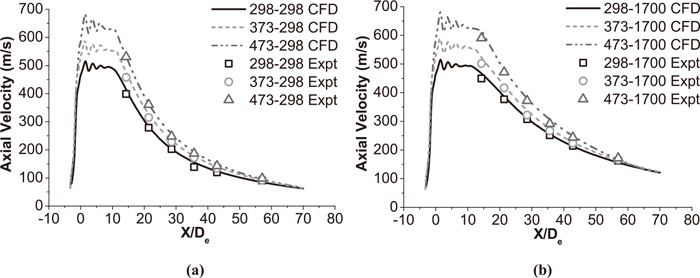
Axial velocity distributions of supersonic jet without a shrouding flame at the jet centerline. (a) The coherent jet under room ambient temperature. (b) The coherent jet under high ambient temperature.
In this paper, the supersonic jet with the shrouding flame will be addressed as a coherent jet, the supersonic jet without the shrouding flame will be addressed as a conventional jet and the main oxygen temperature will be addressed as the oxygen temperature. Based on average values of six kinds of working conditions in Fig. 6, within 5 nozzle exit diameters from the nozzle exit plane, the velocities of coherent jets are 491±8 m/s, 545±12 m/s and 628±14 m/s at the jet centerline, when the oxygen temperature are 298 K, 373 K and 473 K, respectively. As mentioned before, the parameters of coherent jet nozzle is same in various experimental conditions. And the oxygen temperature being 298 K is a decisive factor in coherent jet nozzle design. That means the coherent jet gets more obvious phenomenon of under-expanded with a higher preheating temperature of oxygen. As a result, the coherent jet achieves a greater fluctuation with a higher preheating oxygen temperature. Moreover, the coherent jet gets more kinetic energy with a higher oxygen temperature which makes the velocity of coherent jet greater at the nozzle exit plane. Based on the velocity distributions of conventional jets under six kinds of working conditions in Fig. 7, this flow pattern is more-or-less preserved.
1 nozzle exit diameter will be addressed as 1 De hereafter. The potential core of coherent jet is supposed to be end, when the axial velocity of supersonic oxygen jet continues to decline more than 5 De at the jet centerline, and the first point of this kind of downtrend is addressed as the end point of potential core. The length between the end point of potential core and the tip of main oxygen is defined as the potential core length of coherent jet. As shown in Fig. 6(a), under the ambient temperature being 298 K, the potential core length of coherent jet with 298 K, 373 K and 473 K oxygen temperature is 31 De, 28 De and 25 De, respectively. And the potential core length of coherent jet with 298 K, 373 K and 473 K oxygen temperature is 35 De, 32 De and 28 De, respectively, under the ambient temperature being 1700 K in Fig. 6(b). As previous studies,10) the higher ambient temperature can prolong potential core of coherent jet, but the higher oxygen temperature makes potential core of coherent jet shorter. The potential core length of the coherent jet at high temperature condition with 298 K, 373 K and 473 K oxygen temperature is 1.23, 1.14 and 1.12 times larger than that of the coherent jet at room ambient temperature, respectively. For coherent jet, it seems that the uptrend of potential core length would be slow with the oxygen temperature increasing, when the ambient temperature is increasing, at the tested condition.
Based on average values of three kinds of conventional jet in Fig. 7(a), under the ambient temperature being 298 K, the potential core length of conventional jet with 298 K, 373 K and 473 K oxygen temperature is 10 De, 11 De and 11 De, respectively. And the potential core length of conventional jet with 298 K, 373 K and 473 K oxygen temperature is 11 De, 12 De and 14 De, respectively, under the ambient temperature being 1700 K in Fig. 7(b). Compared with the values in Fig. 6, the higher ambient temperature also prolong potential core length of the conventional jet as coherent jet, but higher oxygen temperature achieves longer potential core which is opposite to coherent jet. The potential core length of the conventional jet at high temperature condition with 298 K, 373 K and 473 K oxygen temperature is 1.1, 1.2 and 1.3 times larger than that of the conventional jet at room ambient temperature, respectively. It seems that the uptrend of coherent jet would be accelerated with the oxygen temperature increasing, when the ambient temperature is increasing, at the tested condition. The higher oxygen temperature makes both coherent jet and conventional jet get a greater kinetic energy to maintaining their velocities, but it also increases the rate of a chemical reaction and turbulence kinetic energy of coherent jet which promote mass transfer between the main oxygen and the shrouding flame, which has been described in 4.3.
Based on average values of various condition of supersonic oxygen jets in Figs. 6 and 7, the average potential core length of the coherent jet with 298 K and 1700 K ambient temperature is 2.9 and 2.6 times larger than that of the conventional jet, respectively. Even though the potential core of coherent jet is reducing with oxygen temperature increasing, it still longer than potential core of conventional jet. Papamoschou and Roshko24) reported that if the ratio of the ambient density to the jet density decreases, the growth rate of the turbulent mixing layer reduces, as depicted in Fig. 8. The combustion flame creates a low-density region surrounding the main oxygen jet and thus reduces the growth rate of the turbulent mixing region. Therefore, as presented in Figs. 6 and 7, the coherent jet achieves a longer potential core than the conventional jet.
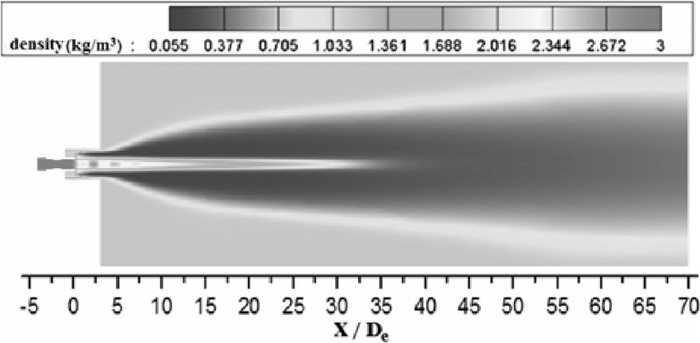
Density distribution of coherent jet at room temperature with oxygen temperature being 298 K on longitudinal section.
The results of numerical simulation are in good agreement with the experiment results of the conventional jet, and the average percentage of variation of the axial velocity distribution is about 1.0 pct. For the coherent jet, the average percentage of variation of the axial velocity distribution got by numerical simulation and combustion experiment is about 4.2 pct. During numerical simulation, the density of oxygen is defined as the density of ideal-gas, since the oxygen is compressible under high pressure. However, the oxygen has a deviation from the ideal gas under high temperature.29) With the temperature increasing, the deviation will be improved. As a result of that, the variation of the axial velocity distribution happens spontaneously and this may be why the variation of the axial velocity distribution occurs.
The difference between the numerical simulation and the combustion experiments also may have resulted from the uncertainties involved during the simulation process. The possible reasons of uncertainties are expressed as follows:
(a) The turbulence model used in the simulation. The previous researches31,32) used the modified k-ε model to simulating the supersonic gas jet behavior and combustion process at different condition. Jones and Whitelaw33) reported some discrepancies in the measured and predicted velocity and temperature contours in their calculation of turbulent combusting flow using the standard k-ε model.
(b) Discretization of partial differential equations. An effort was used to overcome this error by improve computational grids. However, during generating grids, it will always be affected by human factors and operation, since human decided how to divide up geometry model for appropriate grid.
(c) The heat transfer between wall and the cooling water at the region of supersonic coherent jet nozzle. The heating state of wall was affected by cooling water and coherent jet. As described earlier, discrete ordinate (DO) radiation model34) and weighted sum of gray gas model was used to model the radiation of the combustion process. However, the heat transfer between wall and the cooling water was ignored, and the wall was defined as constant temperature wall being 308 K.
The detailed discuss on numerical error generated by the k-ε turbulence model, generation method of adaptive mesh, heat transfer process, and thermo-physical properties of gas during the simulation is not research emphasis in this paper.
4.2. Analysis of Temperature DistributionFigure 9 shows the total temperature distribution of coherent at the jet centerline under room and high temperature with various oxygen temperature. The total temperature of the coherent jet does not change after exiting from the Laval nozzle at first, and then increases rapidly to a maximum value, and after that it asymptotically approaches ambient temperature. The total temperature represents the total energy of jets including thermal energy and kinetic energy. The velocity of jet decreases after passing through the shock wave speed, but its temperature and pressure rise. As a result, the total energy of jets is not lost, and the value of the total temperature is being constant at first. At the end of the potential core, the central jet mixes with the surrounding hot atmosphere formed by the combustion flame, and the static temperature of the jet increases. After this increase, heat and mass transfer occur between the jet and the ambient flow, and the total temperature of the jet asymptotically approaches the ambient temperature. As Fig. 9(a) presents, the total temperature of jet increases with a higher oxygen temperature, and achieves maximum value after being heated by flame. Because of a greater temperature gradient, it seems that the thermal convection between ambient gas and main oxygen jet is accelerated when oxygen temperature is high. Therefore, it transfers more heat energy to the ambient flow in the same time, which makes the total temperature reduce more dramatically at the room ambient temperature. As Fig. 9(b) presents, the jet also slowly approaches the ambient temperature. But it is still being heated after shrouding flame, because the temperature of ambient is higher than that of jet. The experiment data with high ambient temperature is not achieved in presented study. Moreover, the numerical simulation result shows a good agreement with the experimental data.
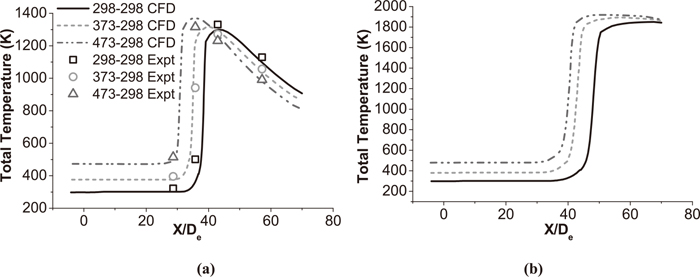
Total temperature distributions of the coherent jet at the jet centerline. (a) The coherent jet under room temperature. (b) The coherent jet under high temperature.
Figure 10 shows the total temperature distribution of conventional jet at the jet centerline under room and high temperature with various oxygen temperatures. The total temperature of the conventional jet does not change after exiting from the Laval nozzle at first, and then asymptotically approaches ambient temperature as the distribution of coherent jet in Fig. 9. For the conventional jet with oxygen temperature being 298 K, its total temperature increases as first and then reduces to the ambient temperature at ambient temperature being 298 K. While the velocity of jet is accelerated by the Laval nozzle, its velocity increases and temperature reduce. That develops a temperature gradient between ambient gas and conventional jet, so the ambient flow transfers heat energy to the conventional jet. As a result, the total temperature of jet increases and the ambient total temperature reduces nearby the jet, as depicted in Fig. 11.
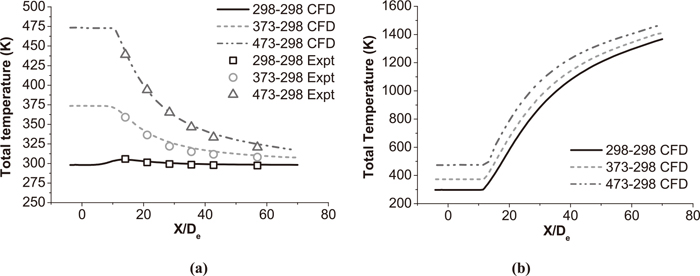
Total temperature distributions of the conventional jet at the jet centerline. (a) The conventional jet under room temperature. (b) The conventional jet under high temperature.
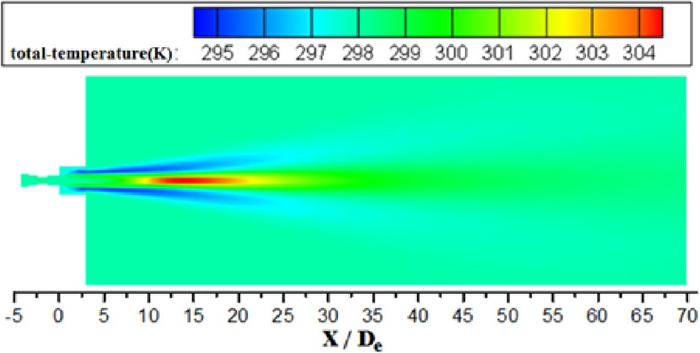
Total temperature distribution of the conventional jet on longitudinal section with oxygen temperature being 298 K under room ambient temperature.
Figure 12 shows the total temperature distribution of the coherent jet on longitudinal section under various conditions. The shape of high temperature zone, which is the red part in the figures, formed by the shrouding flame is different for the three cases. As expected, the high temperature zone is bigger in high ambient temperature. Since the high ambient temperature reduces the density of ambient gas, which reduces the turbulent mixing rate between the flame and the ambient gas. Therefore, the rate of decline for thermal energy of jet decreases which expands the high temperature zone. Fig. 12 illustrates two shrouding flames are achieved just after the exit from the nozzle because oxygen is supplied from both the central main oxygen and the surrounding oxygen, and CH4 is injected from the middle ring of holes, as presented in Fig. 3. Combustion occurs from both sides of the CH4 flow, and the two flames merge to form a single flame downstream of their initial reaction zone.
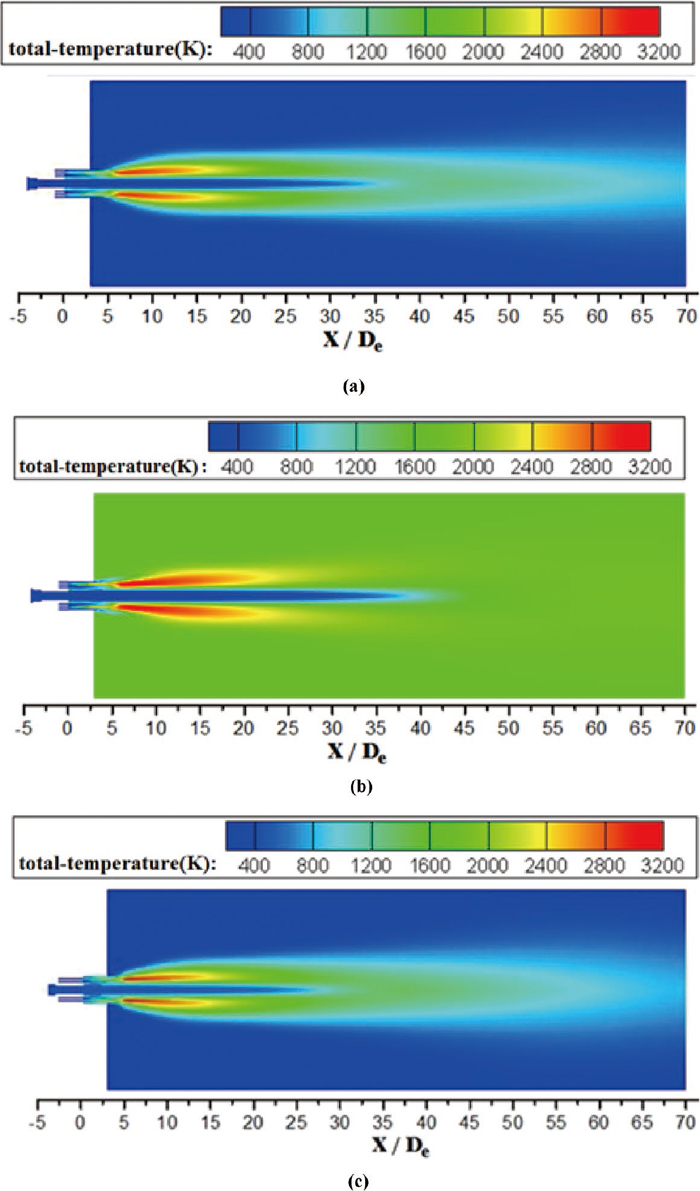
Total temperature distributions of the coherent jet on longitudinal section. (a) The oxygen temperature is 298 K with room ambient temperature. (b) The oxygen temperature is 298 K with high ambient temperature. (c) The oxygen temperature is 473 K with room ambient temperature.
For coherent jet with high oxygen temperature, the high temperature zone is smaller, but the high temperature zone moves forward to the Laval nozzle. It seems that the combustion occurs sooner with a higher oxygen temperature. Under the ambient temperature being 289 K, the distance, between the tip of oxygen nozzle and the location where the total temperature of the coherent jet increases and reaches 1200 K, is 39.0 De and 31.5 De at the jet centerline with the oxygen temperature being 298 K and being 473 K, respectively. After preheat process, the high temperature oxygen occurs a dramatic incorrect expansion at exit of nozzle. At the same time, the high temperature oxygen makes the combustion occur sooner, which drives the total temperature of the coherent jet and incorrect expansion more dramatic. That increases mixing between the flame and the main oxygen and reduces the potential core of coherent jet, which means kinetic energy of coherent jet converts into heat energy of flame. Therefore, the high oxygen temperature can prolong the medium temperature region of the shrouding flame.
4.3. Analysis of Turbulent Kinetic Energy DistributionIn fluid dynamics, turbulence kinetic energy is the mean kinetic energy per unit mass associated with eddies in turbulent flow. Physically, the turbulence kinetic energy is characterised by measured root-mean-square velocity fluctuations. The turbulence kinetic energy also presents the kinetic energy transfer between main oxygen supersonic jet and shrouding flame. Figure 13 shows the turbulence kinetic energy distribution of the coherent oxygen. For the oxygen temperature being 298 K, the maximum turbulence kinetic energy value in the turbulent mixing layer is approximately equal, as shown in Figs. 13(a) and 13(b). Considering the ambient density is lower than main oxygen due to high temperature, the flame tends to expend into the ambient gas, which delays the kinetic energy transfer between main oxygen supersonic jet and shrouding flame and prolongs potential core. For oxygen preheating process, the kinetic energy of coherent jet transfers to flame due to the incorrect expansion, as described previously. As depicted in Figs. 13(a) and 13(c), the maximum turbulence kinetic energy value of the coherent jet with oxygen temperature being 473 K in the turbulent mixing layer is approximately 1.5 times larger than coherent jet with oxygen temperature being 298 K. Since the larger turbulence kinetic energy value, the turbulent mixing layer merges with the jet centerline in advance, at approximately 27 nozzle exit diameters.
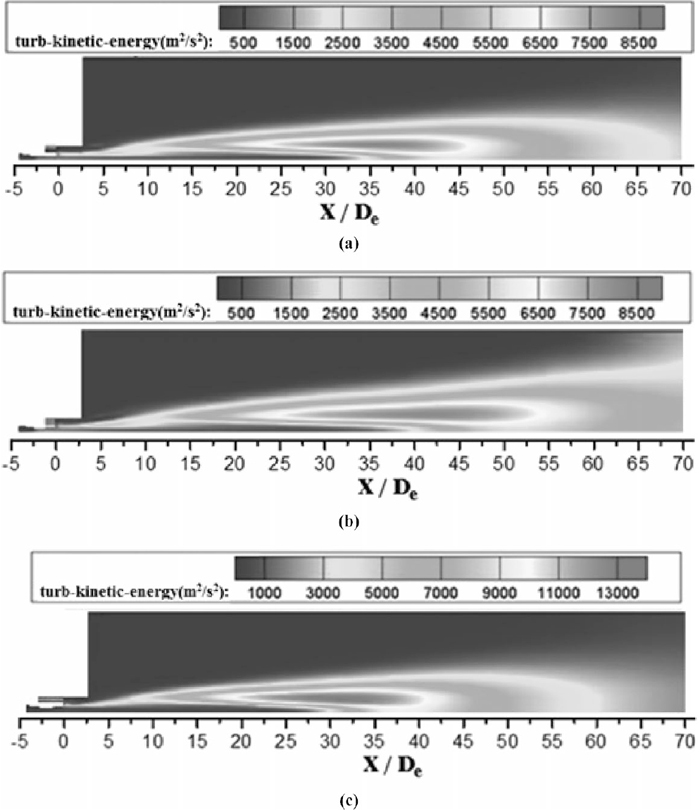
Turbulence kinetic energy distributions of the coherent jet on longitudinal section. (a) The oxygen temperature is 298 K with room ambient temperature. (b) The oxygen temperature is 298 K with high ambient temperature. (c) The oxygen temperature is 473 K with room ambient temperature.
The maximum of turbulence kinetic energy is approximately equal with various conditions, as shown in Fig. 14. For the conventional jet, under the high ambient temperature, the thermal energy of ambient gas converts into the kinetic energy of the periphery of conventional jet, which reduces the kinetic energy gradient and suppresses the high turbulence kinetic energy region in the turbulent mixing layer. As a result, the red parts are in the range of 7 to 13 De, 7 to 9 De and 7 to 15 De in Figs. 14(a), 14(b) and 14(c), respectively. With a preheat process, the high turbulence kinetic energy region is prolong, which accelerates the kinetic energy transfer between the ambient gas and the periphery of conventional jet. However, kinetic energy of the periphery of conventional jet also increases after being heat. Hence, the potential core is still prolonged with the bigger values of viscosity and turbulence kinetic energy.
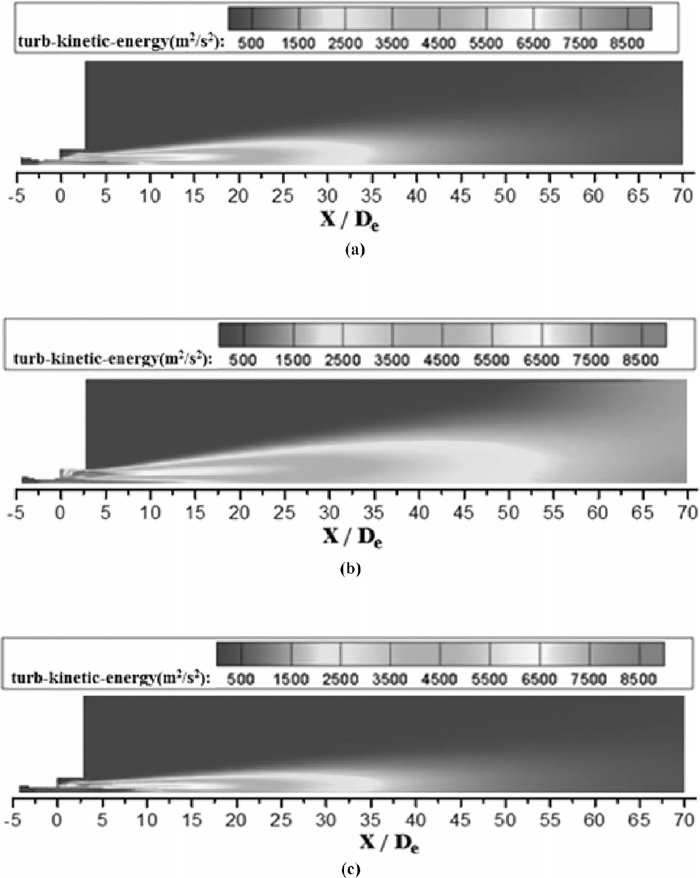
Turbulence kinetic energy distributions of the conventional jet on longitudinal section. (a) The oxygen temperature is 298 K with room ambient temperature. (b) The oxygen temperature is 298 K with high ambient temperature. (c) The oxygen temperature is 473 K with room ambient temperature.
As presented in Figs. 13 and 14, the mixing turbulence layer merges sooner without a shrouding flame. The shrouding flame reduces the density of the ambient gas surrounding the main oxygen supersonic jet, which in turn reduces the viscosity and turbulence kinetic energy in the turbulence mixing layer. As a result, the reduced turbulence kinetic energy then delays the mixing layer merges, which in turn increases the potential core length of the coherent jet.
Flow Field Characteristics of the coherent and conventional jet with and without shrouding flame under various oxygen temperature and ambient temperature were performed. The present study showed that the shrouding flame decreases the entrainment of the ambient gas to the central oxygen supersonic jet, which results in a low expansion rate for the coherent supersonic jet. Obviously, the higher ambient temperature can prolong the potential core of coherent jet and conventional jet. However, the potential core of coherent jet reduces with oxygen temperature increasing, which is opposite to conventional jet. The higher oxygen temperature makes both coherent jet and conventional jet get a greater kinetic energy to maintaining their velocities, but it also increases the rate of a chemical reaction in shrouding flame which promote the turbulence kinetic energy and mass transfer between the main oxygen and the shrouding flame, which reduces the potential core of coherent jet.
In present study, the EDC model with overall and detailed chemical kinetic mechanisms (GRI-Mech 3.0) was presently used for the modeling of reactions, which consisted of 53 species and 325 reversible reactions. Therefore, the numerical simulation achieves better results than the model with the one-step complete combustion. As a result, the numerical simulation results showed a good agreement with the experimental data.
The CFD model only was validated against experimental velocity distribution data and total temperature distribution data under room ambient temperature. No experimental data are available in the literature for the total temperature of jet with ambient temperature being 1700 K or mass fraction of different species to compare the numerical simulation results against the experimental data. Hence, more experimental study is required to establish a more rigorous CFD model of the coherent jet and conventional jet.
The present study can provide some useful insights into coherent jet technology. Compared with the oxygen temperature being 298 K, the coherent jet with a higher oxygen temperature gets a bigger velocity when the X/De is same. However, the preheating oxygen temperature forms a greater incorrect expansion of the supersonic jet at nozzle exit. As a result, it increases mass transfer between the main oxygen and the shrouding flame and reduces the potential core of coherent jet. Therefore, the preheating technology is not appropriate for same structures of coherent jet with oxygen temperature being 298 K. It seems that a longer distance between main oxygen and shrouding flame may resolve this problem. Additional work is required to incorporate the structures of coherent jet nozzle under high oxygen temperature.
The authors would like to express their thanks for the support by the National Nature Science Foundation of China (NSFC 51334001) and the National Key Technology R&D Program of the 12th Five-Year Plan (12FYP 2012BAC27B01).