2016 年 56 巻 9 号 p. 1563-1569
2016 年 56 巻 9 号 p. 1563-1569
Decreasing NOx emission in sintering process is a key issue in steel industry. NOx emission in sintering process is decreased by coke combustion under high temperature. It has been investigated that coating layer of CaO–Fe2O3 composition on coke surface (CF coating method) is effective for decreasing NOx. It has been considered that CaO–Fe2O3 coating layer promotes high temperature combustion and functions as catalyst for reduction of nitrogen oxide.
In this study, CaO coating of coke (Lime coating coke: LCC) has been studied as simple technology for decreasing NOx. As the results, LCC has been effective like a CF coating method and it has been understood that CF melt formation on coke surface is important for decreasing NOx. About coating CaO ratio, 10% was preferable. And decrease in mixing time of LCC with iron ores (Post-mixing) was also effective. By LCC post-mixing, 17.6% NOx decreased and sinter productivity increased.
For iron and steel making company, Japanese national law for decreasing NOx gas emission established in 1973 was driving force for decreasing the amount of NOx gas emission from sintering process. Sintering process is the maximum NOx gas emission in steel works. In addition, by increasing of sinter production, decreasing NOx gas emission has been required more.
Previous studies concluded that the large part of NOx gas emission from sintering process was based on nitrogen component in coke breeze,1) generally called “Fuel NOx”. And coke combustion test in coke breeze packed bed proved that coke combustion under higher temperature was effective for decreasing NOx emission, which decreased O2 concentration in gas film at surface of coke breeze.2)
For sintering operation, improvement of sinter packed bed permeability, such as quick lime blending, results in decreasing NOx because coke breeze combusts under higher temperature by increasing coke combustion rate.3) In terms of coke size, it has been known that the removal of fine coke such as under 5 mm is effective for decreasing NOx because it combusts easily under low temperature.4)
In addition, it was suggested that adherent layer of calcium ferrite on coke surface have the roll of promoter of NO reduction to N2 gas or promoter of combustion by melt formation.5,6,7) This method has not been applied because it could be difficult that the composition and thickness of adherent layer in large scale plant was controlled.
In this study, it was evaluated that instead of calcium ferrite, CaO adhering layer was also effective for decreasing NOx emission as simpler process about formation of adherent layer. This method is called LCC (Lime Coating Coke).
Coke sample with coating layer was prepared and sinter pot test was performed with it. The effect of adherent composition and amount on NOx emission was examined. Sinter pot size was 300 mm diameter and 600 mm height. Coke (or coke with adherent layer) combustion test in alumina ball packed bed was also done for considering chemical and physical reaction of coke surface. In addition, melting behavior between adherent layer and iron ore particles was observed with an electric furnace.
For the evaluation of NOx, ηNO (NOx conversion rate) which was molar ratio of NOx emission amount to N amount in raw materials was calculated. It was assumed that CO and CO2 in exhaust gas derived from ignition gas, coke breeze and lime stone, and that NOx in exhaust gas derived from coke breeze only.
| (1) |
ηNO: NOx conversion rate (%)
NOx: NOx content in exhaust gas (ppm)
CO: CO content in exhaust gas (%)
CO2: CO2 content in exhaust gas (%)
NCOKE: Nitrogen amount in raw materials from coke breeze (mol)
CLPG: Carbon amount in igniting gas (mol)
CCOKE: Carbon amount in raw materials from coke breeze (mol)
CLS: Carbon amount in raw materials from limestone (mol)
2.1. Preparation of Coke with Adherent LayerTable 1 shows coke size distribution for sinter pot test. Coke breeze A was under 5 mm size. Coke breeze B and C were prepared by screening coke breeze A with 0.5 mm and 1.0 mm mesh screen. Figure 1 shows coating procedure on coke breeze surface. Adherent materials and coke breeze were mixed with water for 180 seconds in agitating mixer. Water content in mixture was 8%. Two kinds of adherent materials were prepared. Coke with adherent layer of quick lime was called LCC. And coke with adherent layer of quick lime and under 0.25 mm Australian iron ore was called CFCC. In the preparation of CFCC, quick lime and fine Australian iron ore were the same weight. After mixing of coke and adhering materials, CaO in quick lime became Ca(OH)2.
| −0.25 mm | −0.5 mm | −1.0 mm | −3.0 mm | −5.0 mm | Mean size | |
|---|---|---|---|---|---|---|
| A | 12.9% | 8.2% | 27.0% | 27.9% | 24.0% | 1.78 mm |
| B | 0% | 0% | 34.2% | 35.4% | 30.4% | 2.18 mm |
| C | 0% | 0% | 0% | 53.8% | 46.2% | 2.92 mm |
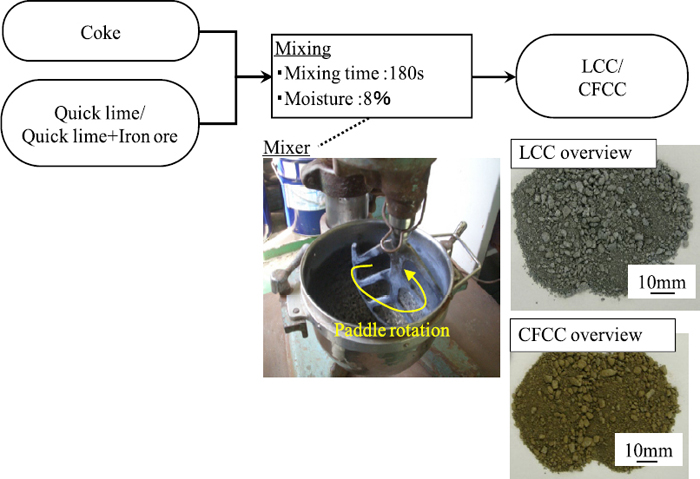
Preparation for LCC and CFCC. (Online version in color.)
Sinter raw materials of fine iron ore, lime stone and dolomite etc except coke breeze were controlled based on sinter chemical composition (SiO2=5.10%, C/S=1.78, MgO=1.30%, Al2O3=1.78%). Coke breeze content to raw materials was 4.5(%-raw materials except return fine and coke).
Figures 2 and 3 show experimental procedure. Mixing and granulating time was total 300 seconds. Moisture content after granulation was 7.5 wt%. Sintering conditions were 90 seconds igniting time, 600 mm bed height and 15.0 kPa suction. The evaluation of productivity, sinter strength and exhaust gas was performed.
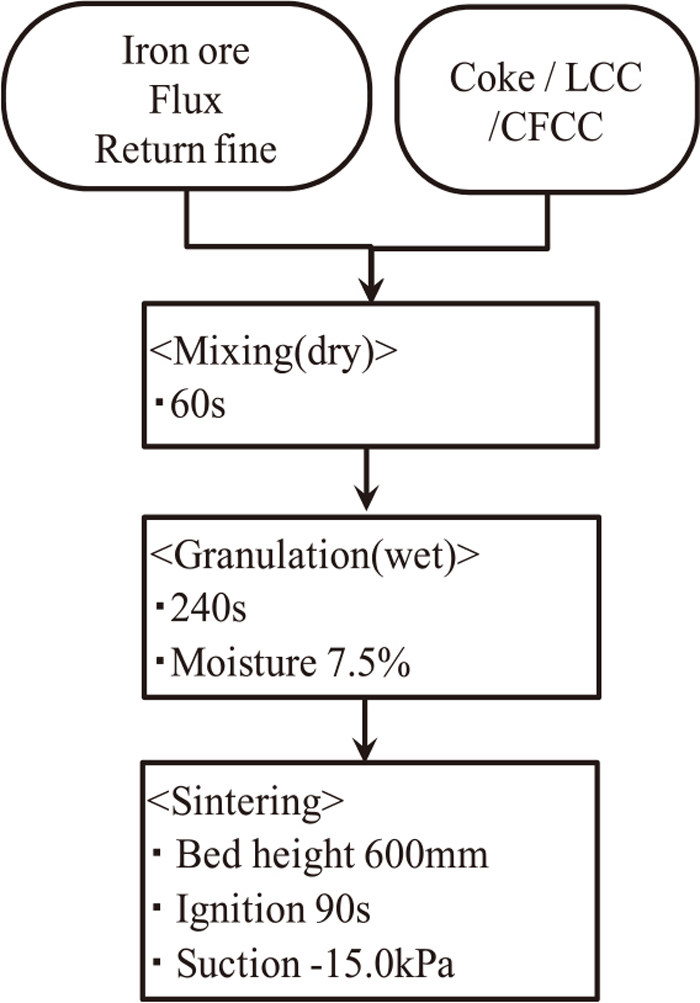
Flow of sintering test (S1–8).
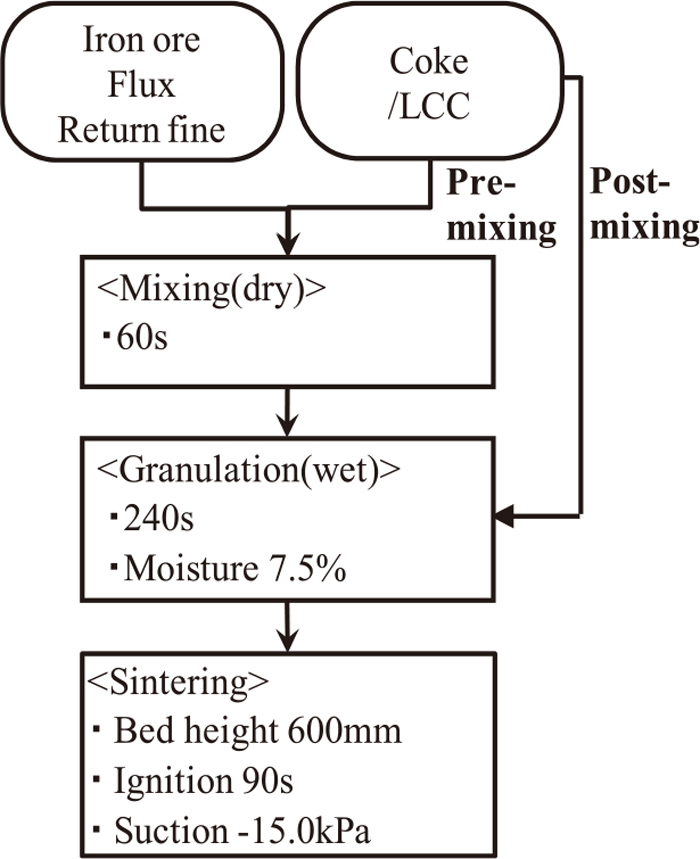
Flow of sintering test (S9–15).
Table 2 shows the test cases of sinter pot test. In case of 1 to 8, varying parameters were composition and amount of adherent material. CaO content to coke breeze was 4 to 20(%-coke). LCC or CFCC was mixed and granulated with sinter raw materials except coke breeze for 300 seconds as shown in Fig. 2. In case of 1 to 3, quick lime was blended (1.0 to 2.0%-raw materials except return fine and coke) for the evaluation of quantitative effect of quick lime on NOx.
| Coke type (size) | CaO for coating (CaO/coke) | Quick lime ratio for iron ore granulation | Mixing time of bonding agent | |
|---|---|---|---|---|
| S1 | Coke(A) | 0% | 1.0% | 300 s |
| S2 | Coke(A) | 0% | 1.5% | 300 s |
| S3 | Coke(A) | 0% | 2.0% | 300 s |
| S4 | LCC(A) | 4% | 1.0% | 300 s |
| S5 | LCC(A) | 10% | 1.0% | 300 s |
| S6 | LCC(A) | 20% | 1.0% | 300 s |
| S7 | CFCC(A) | 10% | 1.0% | 300 s |
| S8 | CFCC(A) | 20% | 1.0% | 300 s |
| S9 | Coke(A) | 0% | 1.0% | 300 s |
| S10 | Coke(A) | 0% | 1.0% | 20 s |
| S11 | LCC(A) | 10% | 1.0% | 300 s |
| S12 | LCC(A) | 10% | 1.0% | 150 s |
| S13 | LCC(A) | 10% | 1.0% | 120 s |
| S14 | LCC(A) | 10% | 1.0% | 60 s |
| S15 | LCC(A) | 10% | 1.0% | 20 s |
| S16 | Coke(A) | 0% | 1.0% | 300 s |
| S17 | Coke(B) | 0% | 1.0% | 300 s |
| S18 | Coke(C) | 0% | 1.0% | 300 s |
| S19 | Coke(A) | 0% | 1.0% | 300 s |
| S20 | Coke(B) | 0% | 1.0% | 300 s |
| S21 | LCC(A) | 20% | 1.0% | 20 s |
| S22 | LCC(B) | 20% | 1.0% | 20 s |
In case of 9 to 15, varying parameters were mixing and granulating time of LCC or CFCC with sinter raw materials as shown in Fig. 3 and the influence of the mixing and granulation time on collapse of adherent layer was evaluated. In these conditions, the composition of adhering layer was analyzed by EPMA method.
In case of 16 to 22, varying parameters were coke type and coke breeze size distribution. The effects of adherent materials and coke breeze size were examined respectively from these conditions.
2.3. Combustion TestCombustion test with alumina ball packed bed of coke (or coke with adherent layer) was performed for analysis of ηNO without reaction between adherent material and fine iron ore. Table 3 shows experimental cases, the composition of adherent materials and the amount of them were varied. Coke breeze size was 1.4 to 2.0 mm and Australian fine iron ore size was under 0.25 mm. Figure 4 shows the apparatus for coke combustion test. Blending ratio of Al2O3 ball (2.0 mmϕ), coke breeze and bentonite was 96:3:1. Betonite had role of binder between alumina ball and coke breeze. Granulation was performed with drum mixer and water content was 0.05%. After granulation, these materials were charged and formed packed bed (100 mm diameter and 400 mm bed height). Combustion test conditions were 90 seconds igniting time and 4.0 kPa suction. During combustion, chemical composition of exhaust gas was measured.
| QL (%) | Ore (%) | Coke (%) | |
|---|---|---|---|
| C1 | 0 | 0 | 100 |
| C2 | 5 | 0 | |
| C3 | 10 | 0 | |
| C4 | 20 | 0 | |
| C5 | 0 | 10 | |
| C6 | 0 | 20 | |
| C7 | 5 | 1.3 | |
| C8 | 5 | 3.3 | |
| C9 | 5 | 5.0 | |
| C10 | 5 | 7.5 | |
| C11 | 5 | 20 | |
| C12 | 10 | 10 |
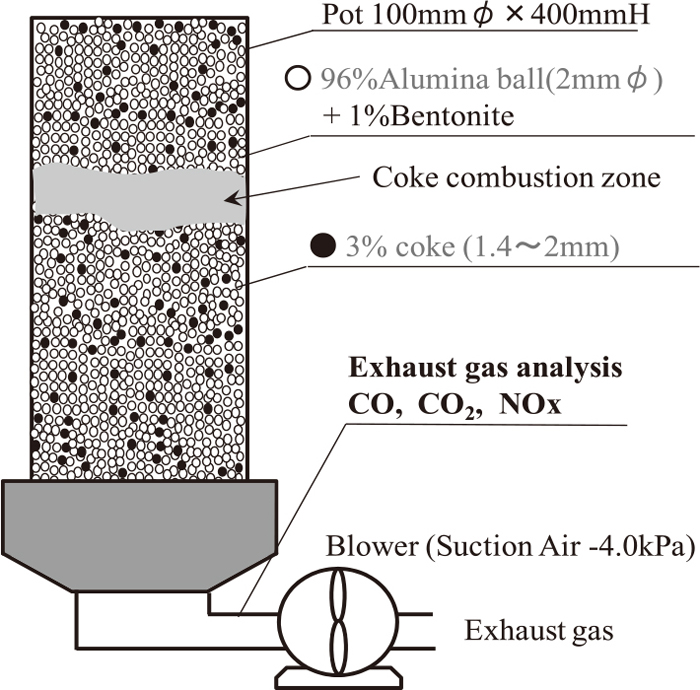
Apparatus for coke combustion test.
Melting behavior between adherent materials and fine iron ore was observed by using infrared image furnace as shown in Fig. 5. Temperature in the furnace was increased by rate of 15°C/s up to 1400°C. One particle of coke breeze (2.0 mm) or LCC (CaO content was 10% to coke breeze) was set in Al2O3 crucible (8 mmϕ) and this particle was surrounded with fine iron ore (0.5 to 1.0 mm).

Observation system of coating layer melt formation.
Table 4 shows sintering pot test results of productivity and sinter strength. LCC or CFCC pre-mixing cases (S4 to S8) had the superiority for sinter productivity, product yield and sinter strength compared with coke breeze pre-mixing case (S1). Especially, S5 to S8 in which CaO content as adherent materials was more than 10% to coke breeze reached the same productivity as S3 of 2% quick lime content in sinter raw material except coke breeze, and indicated higher product yield and higher sinter strength than S3.
| Coke type | T (s) | FFS (mm/min) | η (%) | P (t/d/m2) | SI (%) | |
|---|---|---|---|---|---|---|
| S1 | Coke(A) | 300 | 26.4 | 67.3 | 33.6 | 74.6 |
| S2 | Coke(A) | 300 | 27.9 | 68.8 | 35.9 | 74.6 |
| S3 | Coke(A) | 300 | 29.8 | 67.2 | 37.3 | 72.8 |
| S4 | LCC(A) | 300 | 26.0 | 65.9 | 32.7 | 75.8 |
| S5 | LCC(A) | 300 | 28.0 | 70.4 | 37.0 | 75.9 |
| S6 | LCC(A) | 300 | 28.6 | 71.2 | 37.8 | 75.7 |
| S7 | CFCC(A) | 300 | 28.0 | 69.6 | 36.4 | 74.9 |
| S8 | CFCC(A) | 300 | 28.6 | 70.3 | 37.6 | 74.9 |
| S9 | Coke(A) | 300 | 25.8 | 70.0 | 34.1 | 74.7 |
| S10 | Coke(A) | 20 | 28.2 | 72.5 | 38.9 | 76.6 |
| S11 | LCC(A) | 300 | 27.3 | 73.2 | 37.6 | 76.0 |
| S12 | LCC(A) | 150 | 27.7 | 74.7 | 39.4 | 76.9 |
| S13 | LCC(A) | 120 | 27.9 | 76.5 | 40.6 | 77.2 |
| S14 | LCC(A) | 60 | 28.3 | 74.9 | 40.1 | 77.1 |
| S15 | LCC(A) | 20 | 29.6 | 73.4 | 40.9 | 74.7 |
| S16 | Coke(A) | 300 | 24.7 | 69.2 | 33.0 | 75.6 |
| S17 | Coke(B) | 300 | 25.7 | 70.3 | 35.2 | 73.4 |
| S18 | Coke(C) | 300 | 25.1 | 64.2 | 30.9 | 68.8 |
| S19 | Coke(A) | 300 | 29.3 | 65.7 | 36.4 | 74.0 |
| S20 | Coke(B) | 300 | 30.5 | 64.4 | 36.9 | 72.2 |
| S21 | LCC(A) | 20 | 30.9 | 72.4 | 41.2 | 75.2 |
| S22 | LCC(B) | 20 | 32.4 | 68.8 | 40.7 | 70.7 |
LCC post-mixing cases (S11 to 15) were higher product yield, sintering speed (FFS), productivity and sinter strength compared with coke pre-mixing case (S9). This superiority was accelerated in case of short mixing time of LCC with sinter raw materials except coke breeze.
In coke breeze B case (S20), product yield and sinter strength were lower than in coke breeze A case (S19). The same tendency was confirmed in case of LCC. So, it was confirmed that LCC which was produced from coke breeze containing fine coke breeze was better for sinter productivity and sinter strength.
3.2. Chemical Composition of Exhaust GasTable 5 shows chemical composition of sinter exhaust gas at sinter pot test. In LCC or CFCC pre-mixing cases (S4 to S8), ηNO and O2 were lower than in coke breeze pre-mixing case (S1). In LCC post-mixing cases (S11 to 15), ηNO and O2 were much lower than in coke pre-mixing case (S9). In coke breeze B case (S20), ηNO was lower and O2 was higher than in coke breeze A case (S19).
| Coke type | T (s) | CO (%) | CO2 (%) | O2 (%) | NOx (ppm) | ηNO (%) | |
|---|---|---|---|---|---|---|---|
| S1 | Coke(A) | 300 | 0.86 | 9.2 | 13.1 | 240 | 30.7 |
| S2 | Coke(A) | 300 | 0.84 | 9.2 | 13.0 | 236 | 29.9 |
| S3 | Coke(A) | 300 | 0.78 | 9.0 | 13.1 | 227 | 29.2 |
| S4 | LCC(A) | 300 | 0.96 | 9.5 | 13.2 | 233 | 28.7 |
| S5 | LCC(A) | 300 | 0.83 | 9.5 | 12.9 | 227 | 27.8 |
| S6 | LCC(A) | 300 | 0.82 | 9.4 | 12.9 | 225 | 27.9 |
| S7 | CFCC(A) | 300 | 0.83 | 9.4 | 12.9 | 231 | 28.4 |
| S8 | CFCC(A) | 300 | 0.81 | 9.3 | 12.7 | 226 | 28.2 |
| S9 | Coke(A) | 300 | 0.91 | 8.9 | 13.5 | 226 | 30.7 |
| S10 | Coke(A) | 20 | 1.29 | 10.5 | 12.0 | 283 | 32.0 |
| S11 | LCC(A) | 300 | 0.88 | 9.1 | 13.3 | 213 | 27.8 |
| S12 | LCC(A) | 150 | 0.92 | 9.5 | 12.9 | 213 | 26.6 |
| S13 | LCC(A) | 120 | 0.98 | 9.7 | 12.6 | 218 | 26.4 |
| S14 | LCC(A) | 60 | 1.01 | 9.4 | 12.9 | 209 | 26.0 |
| S15 | LCC(A) | 20 | 1.04 | 10.1 | 12.2 | 217 | 25.3 |
| S16 | Coke(A) | 300 | 0.8 | 9.0 | 13.6 | 227 | 29.8 |
| S17 | Coke(B) | 300 | 0.9 | 9.1 | 13.4 | 211 | 27.2 |
| S18 | Coke(C) | 300 | 0.5 | 7.9 | 14.6 | 164 | 25.3 |
| S19 | Coke(A) | 300 | 0.8 | 8.8 | 13.2 | 224 | 29.5 |
| S20 | Coke(B) | 300 | 0.8 | 8.7 | 13.1 | 204 | 27.2 |
| S21 | LCC(A) | 20 | 1.0 | 10.4 | 11.8 | 223 | 24.6 |
| S22 | LCC(B) | 20 | 0.8 | 9.8 | 12.3 | 200 | 23.7 |
Table 6 shows exhaust gas analysis and combustion time in coke combustion test. Combustion time was defined as time to 19.5% O2 content in exhaust gas. Figure 6 shows the influence of CaO content to coke breeze on ηNO. Adherent materials of fine iron ore or quick lime showed small effect for decreasing ηNO. Adherent materials of quick lime and fine iron ore showed much lower ηNO, and the optimum composition for decreasing ηNO was CaO·Fe2O3 or CaO·2Fe2O3. These results indicated that NOx emission was decreased when the amount of calcium ferrite melt was large. 10% CaO content to coke breeze had superiority for decreasing ηNO compared with 5% CaO content to coke breeze. This superiority was consistent with the sinter pot test result.
| CO (%) | CO2 (%) | O2 (%) | NOx (ppm) | Combustion time (min) | ηNO (%) | ||
|---|---|---|---|---|---|---|---|
| C1 | No coating | 0.92 | 6.2 | 13.8 | 234 | 18.5 | 28.9 |
| C2 | CaO(5) | 0.71 | 6.4 | 13.3 | 224 | 16.5 | 27.7 |
| C3 | CaO(10) | 1.07 | 5.6 | 14.5 | 202 | 20.1 | 27.1 |
| C4 | CaO(20) | 1.20 | 4.7 | 15.7 | 188 | 19.6 | 28.9 |
| C5 | Ore(10) | 1.15 | 5.0 | 15.3 | 186 | 19.4 | 27.2 |
| C6 | Ore(20) | 1.31 | 5.4 | 14.4 | 204 | 18.4 | 27.4 |
| C7 | CaO(5)–Ore(1.3) | 0.74 | 6.1 | 13.6 | 205 | 18.3 | 26.2 |
| C8 | CaO(5)–Ore(3.3) | 0.84 | 6.0 | 13.8 | 177 | 18.7 | 22.5 |
| C9 | CaO(5)–Ore(5) | 0.94 | 5.6 | 14.1 | 164 | 19.0 | 21.8 |
| C10 | CaO(5)–Ore(7.5) | 1.07 | 5.4 | 14.3 | 148 | 17.9 | 19.9 |
| C11 | CaO(5)–Ore(20) | 1.05 | 5.2 | 14.6 | 139 | 18.7 | 19.4 |
| C12 | CaO(10)–Ore(10) | 1.34 | 5.3 | 14.8 | 151 | 18.6 | 20.6 |
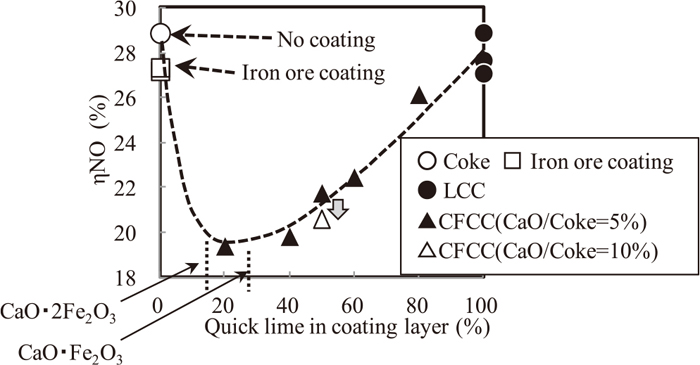
Relation between coating layer composition and ηNO.
Figure 7 shows the influence of adherent layer composition and amount on combustion time. In case of quick lime as adherent materials (LCC), combustion time was longer than other cases at over 10% CaO content to coke breeze. In case of mixture of quick lime and fine iron ore as adherent materials (CFCC), combustion time was the same as coke breeze without adherent materials.
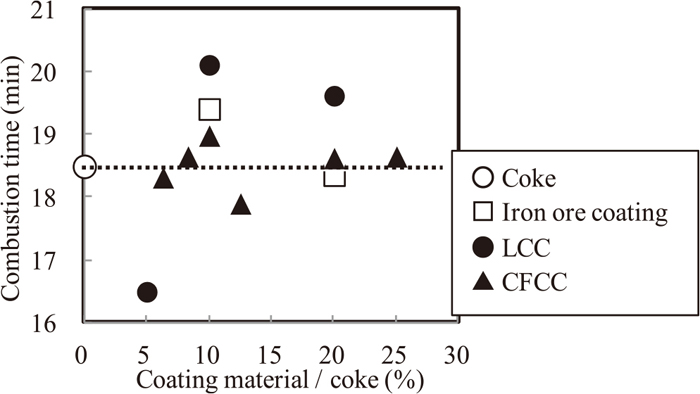
Relation between coating material ratio and combustion time.
Figure 8 shows observation photograph of fine iron ore and coke with adherent layer. In case of LCC, melt formed at 1200°C by chemical reaction between CaO and fine iron ore. Moreover, the diffusion of melt promoted reaction and coke surface appeared by removing adherent materials. On the other hand, in case of coke breeze without adherent materials, melt formation was little up to 1300°C.

Melting behavior of coating layer and iron ore. (Online version in color.)
Figure 9 shows the effect of blending quick lime ratio on ηNO and sinter productivity at sinter pot test. Coating quick lime to coke breeze was more effective for decreasing ηNO than blending quick lime in sinter raw material. ηNO decreased with increasing adherent quick lime ratio on coke breeze up to 10%. The effect was saturated at the ratio of quick lime over 10%. LCC was the same level for ηNO compared with CFCC. For sinter productivity, LCC and CFCC were higher than blending quick lime in sinter raw material when quick lime blending ratio to coke breeze was over 10%.
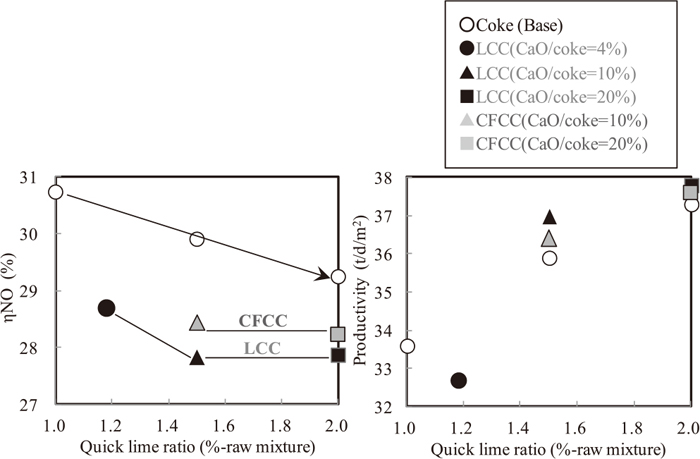
Relation between quick lime ratio, ηNO and productivity (S1–8).
In cases of LCC and CFCC, O2 content in exhaust gas was lower (Table 5) and coke combustion rate was also improved. This could be the reason of higher productivity. Lower quick lime blending ratio is better for sintering cost as it is expensive. So, the optimum CaO ratio to coke breeze is 10%
4.2. The Influence of LCC Mixing Time with Sinter Raw Materials on NOx Conversion Rate and Sinter ProductivityFigure 10 shows the influence of LCC mixing time with sinter raw materials except coke breeze on NOx emission and sinter productivity. With decreasing mixing time of LCC, ηNO decreased and productivity was improved. At the condition of 20 seconds mixing time, ηNO decreased by 5.2% corresponding to NOx decreasing rate of 17.6%. Meanwhile, ηNO increased by decreasing mixing time of coke breeze. Oyama et al examined that decreasing mixing time of coke breeze (up to 30 seconds) was effective for improving sinter productivity, and the result of LCC was the same as this in terms of sinter productivity.8) But decreasing productivity could be brought by mixing time of less than 20 seconds due to inhomogeneous mixing. In case of LCC, O2 content in exhaust gas was lower (Table 5) and this was the certain evidence of higher coke combustion amount per unit inlet air amount. This has the role of increasing sintering speed under the same inlet gas rate and results in higher productivity.
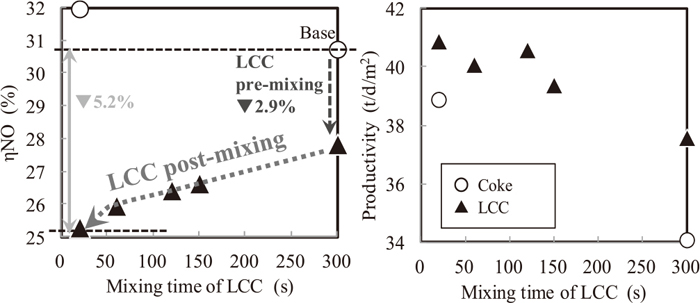
Relation between LCC mixing time, ηNO and productivity (S9–15).
Figure 11 shows EPMA results. In case of post-mixing of coke breeze (mixing and granulating time of coke breeze with sinter raw material was 20 seconds), little adherent materials was confirmed on coke particle. In case of pre-mixing of coke breeze (mixing and granulating time of coke breeze with sinter raw material was 300 seconds), Fe component was confirmed. On the other hand, in case of post-mixing of LCC, higher Ca and lower Fe content were confirmed on coke particle compared with pre-mixing of LCC. In pre-mixing of LCC, CaO adherent material was removed from coke breeze surface. The lower ηNO at post-mixing of LCC was caused by keeping higher CaO content on coke breeze surface.
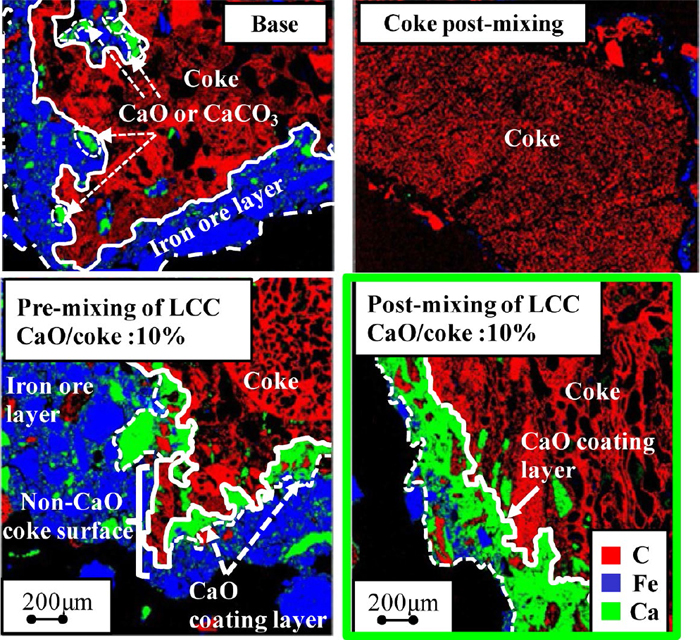
EPMA results of raw mixture. (Online version in color.)
Figure 12 shows the influence of coke breeze size on maximum temperature in sinter packed bed and on ηNO. The maximum temperature was mean value at the position of 300 mm height and 110 mm height from the bottom of sinter packed bed. With decreasing −1.0 mm ratio of coke breeze, the maximum temperature increased and ηNO decreased as well as previous research. Fine coke breeze as −1 mm particle is considered to be difficult to reach higher temperature at coke particle due to small combustion heat. So, +1 mm coke breeze increases maximum temperature and results in decreasing ηNO.
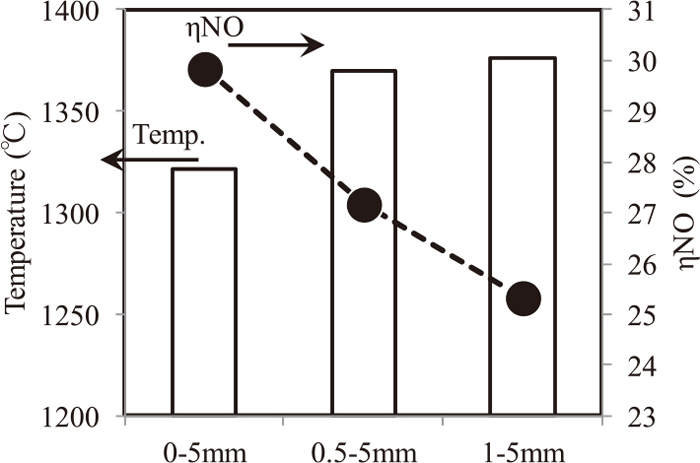
Results of temperature in sintering bed and ηNO with different size of coke breeze (S16–18).
Figure 13 shows ηNO in case of LCC with coke breeze of 0.5 to 5 mm. ηNO decreased in case of LCC with 0.5 mm to 5 mm coke compared with the case of coke breeze of 0.5 to 5 mm and with the case of LCC (coke size 0–5 mm). This result indicates that both adherent CaO and removing −0.5 mm coke have the role of decreasing NOx emission independently.
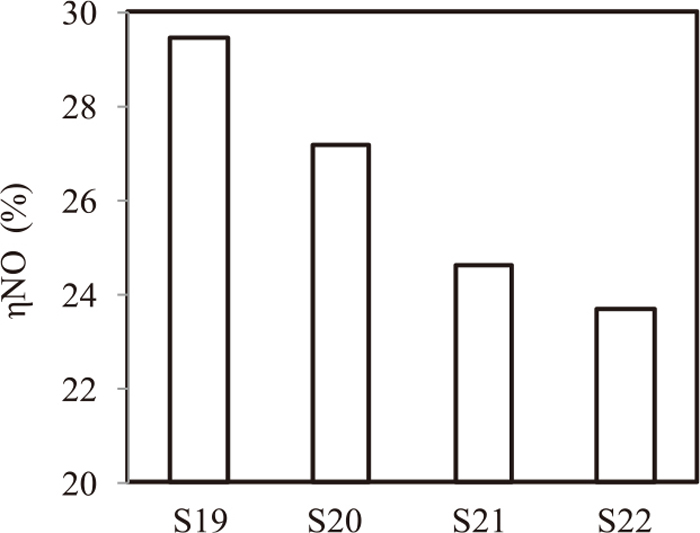
Effect of fine coke removal & LCC (S19: Base, S20: fine removal, S21: LCC, S22: fine removal & LCC).
Carbon balance was calculated for evaluation of LCC combustion behavior. Combustion ratio ηC was defined as the ratio of carbon amount in exhaust gas to in raw mixture. CO and CO2 gas were generated by the combustion of ignition gas and coke breeze and the de-carbonation of lime stone.
| (2) |
| (3) |
| (4) |
| (5) |
ηC: combustion ratio (−)
output C: total carbon amount in exhaust gas (mol)
input C: total carbon amount in raw mixture (mol)
CCOKE: carbon amount from coke breeze (mol)
CLPG: carbon amount from ignition gas (mol)
CLS: carbon amount from lime stone (mol)
CO: CO content in exhaust gas (%)
CO2: CO2 content in exhaust gas (%)
Q: exhaust gas volume (Nm3/min)
T: sintering time (min)
Table 7 shows the result of carbon balance. Combustion ratio was improved in case of post-mixing coke and LCC compared with pre-mixing of coke. Adherent layer of LCC melt easily around 1200°C, so combustion ratio could be improved.
| S9 | S10 | S15 | |
|---|---|---|---|
| Coke type | Coke | Coke | LCC |
| Mixing time of bonding agent | 300 s | 20 s | 20 s |
| CLPG (mol) | 14.1 | 14.1 | 14.1 |
| CCOKE (mol) | 159.7 | 161.3 | 159.8 |
| CLS (mol) | 65.7 | 66.3 | 60.2 |
| T (min) | 23.3 | 21.3 | 20.3 |
| Q (Nm3/min) | 1.93 | 1.98 | 2.13 |
| Output C (mol) | 195.6 | 221.9 | 215.4 |
| ηC (−) | 0.82 | 0.92 | 0.92 |
Kasai et al considered that coke with calcium ferrite layer combusted selectively under high temperature and decreased NOx emission.5) Their mechanism could be reasonable from this research. There is the difference in adherent layer composition between calcium ferrite coating method and LCC, but adherent layer on coke surface melts easily in both cases during sintering. LCC is effective for decreasing NOx because lime coating layer melts with iron ore in sintering bed and this method is simple. Kasai et al performed sintering pot test for evaluation of CF coating coke and confirmed that the reactivity of CF coating coke was decreased. This could be because the amount of coating material was too much.5) Coating material should be high adhesive property and its amount should be minimized. So, quick lime as coating material is appropriate. Figure 14 shows LCC combustion behavior in sintering bed. No adherent layer on coke surface is effective for improvement of coke combustion reactivity. But coke combusts under low temperature and NOx emission become higher. Both decreasing NOx emission and improvement of coke reactivity are achieved by both deactivate combustion under low temperature (less than 1200°C) and active combustion under high temperature (more than 1200°C). As the decreasing NOx mechanism, there could be the catalytic function of calcium ferrite to promote NO reduction besides higher combustion temperature. The clarification of contributing rate of them is essential.
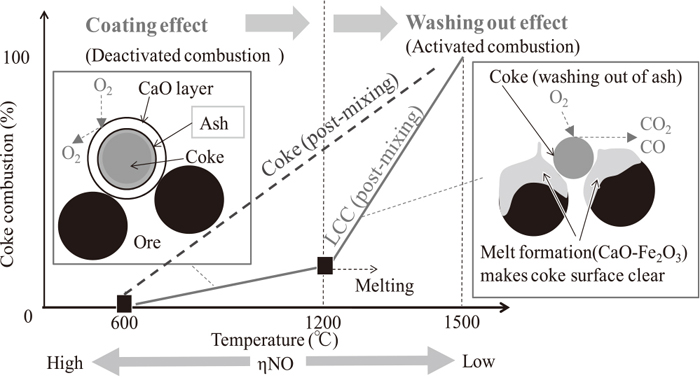
LCC combustion in sintering bed.
For decreasing NOx emission from sintering process, Lime coting coke (LCC) has been examined. As the results, coke breeze with adherent CaO on its surface is effective for decreasing NOx even at 10% CaO content to coke breeze. Sinter pot test indicated decreasing NOx emission by 18%. At blending LCC to sinter law materials, mixing time in drum mixer must be short because adherent layer is broken easily. The mechanism of decreasing NOx emission is considered due to calcium ferrite formation by chemical reaction between adherent CaO on coke breeze surface and fine iron ore. The effect of coating CaO and coke breeze size on decreasing NOx emission was evaluated independently. It is suggested that the effect on inhibiting NOx emission is increased by optimization of coke breeze size control.