2016 年 56 巻 9 号 p. 1634-1637
2016 年 56 巻 9 号 p. 1634-1637
A novel catalytic combustion-type CO gas sensor was devised using a catalyst composed of 11 wt% Pt supported on the apatite-like Mn-doped oxide La9.0Si5.8Mn0.2O27−δ. Its CO gas sensing performance was also investigated. Since the 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ catalyst oxidized CO at a temperature over 60°C, the sensor also responded to CO at temperatures above 60°C, showing superior CO detection at 130°C with high sensitivity and a rapid response time of 10–20 s.
Carbon monoxide (CO) is a highly toxic gas that causes serious health damage to the human body when inhaled, even at low concentrations. Since CO gas cannot be detected by human senses because of its colorless and odorless nature, a detection tool should always be used to prevent serious accidents caused by the CO exposure. Therefore, installing compact and inexpensive CO sensors at every site where CO gas might be emitted is of great importance.
Until now, the various types of compact CO gas sensors that have been developed are based on semiconductors,1,2) potentiostats,3) and catalytic combustion.4,5) Although potentiostat-type CO gas sensors are now widely used owing to their highly selective CO detection, they cannot operate for long periods because of the eventual evaporation of the liquid electrolyte used in the device. Semiconductor-type CO gas sensors, whose gas detection mechanism is based on a resistance change caused by gas adsorption on the semiconductor surface, exhibit stable sensor performance with low cost; however, this type of sensor has a potential problem with gas selectivity because coexisting gases also adsorb onto the semiconductor surface, which produces an unexpected sensor signal. In contrast, catalytic combustion-type CO gas sensors can show stable sensing performances for long periods because of their simple detection system, which is based on a Pt coil and a CO oxidation catalyst. The detection mechanism employed by a combustion-type CO gas sensor is as follows: when CO gas is oxidized on the catalyst, a quantity of combustion heat, which is directly related to the amount of CO initial present, is generated. Then, the temperature of the catalyst coated Pt coil is raised by the generated combustion heat, causing the electrical resistance of the Pt coil to increase. Since the resistance change should be proportional to the temperature change, the sensor signal (resistance change) is also exactly proportional to the amount of CO gas oxidized by the catalyst, i.e., the quantity of CO gas present in the surrounding atmosphere. Therefore, it is expected that commercially applicable CO gas sensors oxidize only CO at their operating temperature. However, conventional catalytic combustion-type sensors have a problem with selectivity because the traditional catalysts applied (Pt/Al2O3 or Pd/Al2O3) operate at temperature up to several hundred degrees Celsius. At such elevated temperatures, other gases, such as volatile organic compounds (VOCs), also combust, so that the sensors also respond to other gases. To overcome this problem, it is essential that novel catalysts that operate at moderate temperatures and oxidize only CO and no other gases.
Recently, we have fabricated a low-temperature operative catalytic combustion-type CO gas sensor6) by employing 10 wt% Pt/Ce0.68Zr0.17Sn0.15O2.0 as the CO oxidizing catalyst. Since this catalyst completely oxidized CO at 65°C, quantitative CO detection was successfully achieved at 70°C with a 50% response time (the time required for the electrical resistance of the device to reach 50% of the steady value eventually reached a given CO gas level) of 130 s. Although the desired low-temperature operation was successfully realized as the result of the enhanced co-catalytic effect arising from oxygen migration in the Ce0.67Zr0.18Sn0.15O2.0 lattice, more rapid gas detection is required for practical applications.
In this paper, we focus on apatite-type La10Si6O27, which has been reported to exhibit oxygen migration via oxide anion conduction in the lattice,7) similar to that observed for cerium−zirconium based solid solutions. Moreover, at temperatures below 300°C, the O2− ion conductivity in the La10Si6O27 solid is higher than those in the CeO2–ZrO2-based solids8) a higher co-catalytic effect is thus expected for a Pt-loaded La10Si6O27 catalyst when compared with the previously reported sensors. Furthermore, we doped Mn into the La10Si6O27 support to accelerate the O2− ion conduction by generating oxide anion vacancies caused by the introduction of lower valent Mn2+ into the solid. We then devised a catalytic combustion-type CO gas sensor using the Pt-loaded La10Si5.8Mn0.2O27−δ solid solution catalyst and investigated its sensing performance.
The La10Si5.8Mn0.2O27−δ solid solution was synthesized via a liquid-phase reaction. A stoichiometric mixture of La(NO3)3·6H2O (5.44 g), ethanol (1.9 cm3), acetic acid (3.4 cm3), and Mn(NO3)2·6H2O (0.065 g) was stirred for 1 h at room temperature. After that Si(C2H5)4 (1.65 mL) was added to the mixture and stirring was continued for 1 h at room temperature. The solvent was then removed at 80°C and the resulting powder was heated at 350°C for 12 h in air. The resultant powder was calcined at 1000°C for 2 h in air to provide the La10Si5.8Mn0.2O27−δ support. The catalyst, composed of 10 wt% Pt/La10Si5.8Mn0.2O27−δ, was obtained by impregnating the La10Si5.8Mn0.2O27−δ support with colloidal platinum stabilized with a poly (vinylpyrrolidone) (PVP) ethanol solution (Tanaka Kikinzoku Kogyo). The catalyst was subsequently dried at 80°C for 6 h and then calcined at 400°C for 4 h in air.
The sample was characterized by X-ray fluorescence (Rigaku, ZSX100e) and X-ray powder diffraction (XRD) (Rigaku, SmartLab) analyses. The CO oxidation activity of the 10 wt% Pt/La10Si5.8Mn0.2O27−δ catalyst was investigated using a conventional fixed-bed flow reactor by passing 1000 ppm CO diluted with air over the catalyst bed at a space velocity of 20000 L kg−1 h−1.
The sensor element was fabricated using a coil formed from 30-μm-diameter Pt wire. The 10 wt% Pt/La10Si5.8Mn0.2O27−δ catalyst was slurried in ethylene glycol and then, loaded onto the Pt coil. The coil was subsequently heated to approximately 300°C by applying a DC voltage of 2 V to evaporate the ethylene glycol.
The CO-sensing performance of the sensor was investigated over the temperature range of 60 to 150°C using an electrometer (Advantest, R8240) in atmospheres whose CO gas concentrations varied from 0 to 500 ppm. The CO concentration was regulated by diluting 1000 ppm CO (air balance) gas with synthetic air. Regardless of the CO gas concentration, the total gas flow rate passing over the sensor element was kept constant at 40 mL min−1. The sensor signal in response to exposure to CO gas was defined as (Rgas − Rair)/Rair, where Rgas and Rair are the electrical resistances of the sensor in the test gases in the presence and absense of CO, respectively. The sensor response time was defined as the time required for the electrical resistance of the device to reach 50% or 90% of the steady value eventually obtained at a given CO gas level.
The XRD pattern of the prepared sample is shown in Fig. 1. Only peaks assigned to the apatite-type structure were observed. Furthermore, the diffraction peak angles assigned to the apatite-type oxide were slightly shifted to higher angles when compared to those of La10Si6O27, indicating that the larger manganese ions (ionic radius of Mn2+: 0.080 nm; Mn4+: 0.053 nm9)) had partially substituted the Si (ionic radius of Si4+: 0.040 nm9)) sites in the apatite-type structure. Although peaks corresponding to Pt were not identified in the XRD profile, we have confirmed the presence of Pt in the sample by X-ray fluorescence analysis. Therefore, it appears that Pt is highly dispersed on the solid apatite-type particles. Moreover, the sample composition was estimated to be 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ from the results of the X-ray fluorescence analysis. From these results, the successful synthesis of the objective solid whose composition was almost the same as that expected from the amount of Pt used and mixing ration of the cationic reagents was confirmed.
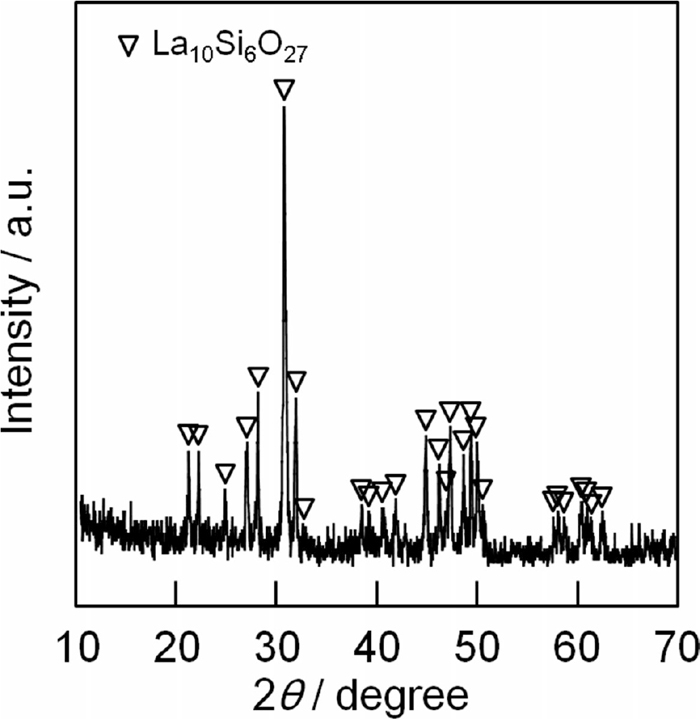
XRD pattern for the 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ catalyst.
Figure 2 presents the CO oxidation activity of the 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ catalyst. The present catalyst began to oxidize CO at 30°C and the complete oxidation was achieved at 60°C. Similar to that of the 10 wt% Pt/Ce0.68Zr0.17Sn0.15O2.0 catalyst, which was developed by us,6) such high CO oxidation activity is thought to be the result of oxide anion migration in the La9.0Si5.8Mn0.2O27−δ support. As described in the introduction section, the oxide anion vacancies are generated as the result of charge compensation for the Mn2+ that is doped into Si4+ sites in the network. Oxide anions can easily migrate through such oxide anion vacancies in the crystal lattice; as a result, oxygen that migrates to the surface can be used for CO oxidation. From the CO oxidation activity results, we can expect that sensors employing the 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ catalyst will detect CO gas concentrations at temperatures above 60°C.
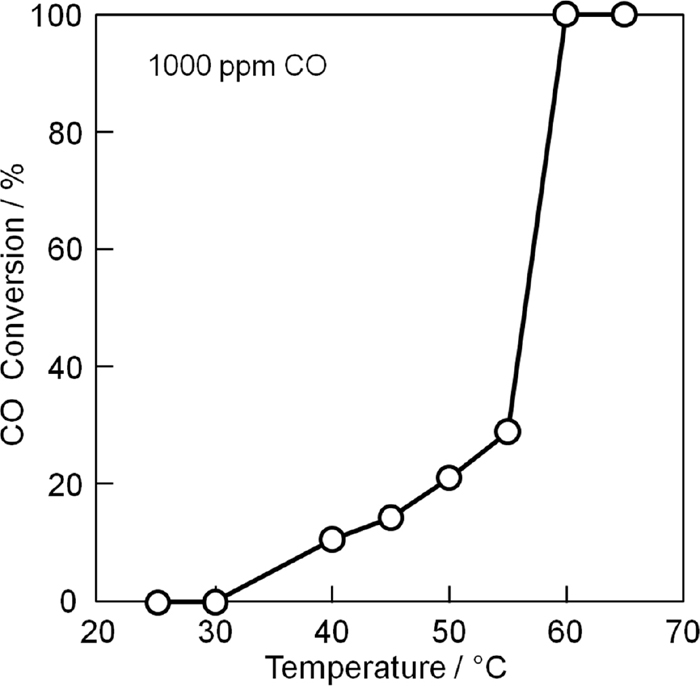
Temperature dependence of the ratio of CO conversion for the 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ catalyst.
To determine the optimum operating temperature of the sensor, we investigated the sensor output using 500 ppm CO at various temperatures ranging from 60–150°C (Fig. 3). The sensor outputs at 90°C and 110°C were almost the same, but slightly higher sensor outputs were obtained at 60°C, 130°C, and 150°C. Conversely, when operated at 60°C the sensor showed considerable electrical noise as a result of the high electrical resistance of the La9.0Si5.8Mn0.2O27−δ support. Since moderate temperature operation is essential for the selective detection of CO gas free from interference arising from other gases such VOCs, we decided that the optimum operating temperature of the present sensor with the 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ catalyst was 130°C.
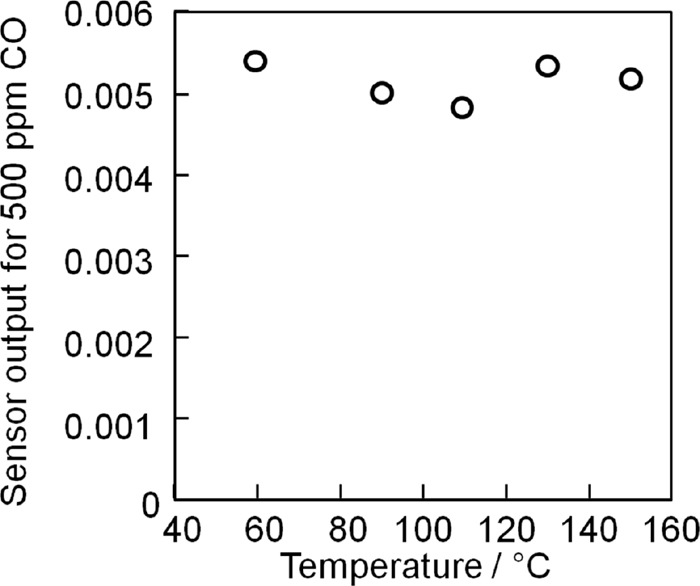
Temperature dependence of the sensor signals at 500 ppm CO for the novel sensor incorporating the 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ catalyst.
Figure 4 displays a representative response curve for the present sensor incorporating the 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ catalyst when the CO gas concentration was varied in a step-by-step manner from 0 to 500 ppm and vice versa at 130°C. The sensor signal ((Rgas − Rair)/Rair) smoothly and reproducibly changed with varying CO gas concentration with the sensitivity of 0.0053 for 500 ppm CO which is higher than that of our previously reported sensor (0.0047).6) Furthermore, the 50% and 90% response times were 10–20 s and 50–75 s, respectively, which were faster than those of our previously reported sensor that employed the 10 wt% Pt/Ce0.68Zr0.17Sn0.15O2.0 catalyst (30–40 s and 80–110 s, respectively at 130°C.6) To clarify the reason for higher sensitivity, we investigated the specific heat capacities of the 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ and 10 wt% Pt/Ce0.68Zr0.17Sn0.15O2.0 catalysts. The 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ catalyst has a specific heat capacity approximately two thirds of that of the 10 wt% Pt/Ce0.68Zr0.17Sn0.15O2.0 catalyst at 130°C. Because the smaller heat capacity realizes larger temperature change in the material, the sensor incorporating 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ catalyst showed a higher sensitivity (sensor signal (resistivity of the Pt coil) is exactly related to the temperature of the catalyst). In addition, faster response might be also caused by the larger temperature change. In this system, the initial sensor output change strongly affects to the 50% or 90% response time. The smaller specific heat capacity of the present 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ catalyst might cause larger temperature change of the material at the initial stage when the target gas flow compared with the 10 wt% Pt/Ce0.68Zr0.17Sn0.15O2.0 catalyst, and as a result, 50% or 90% response time might be shortened.
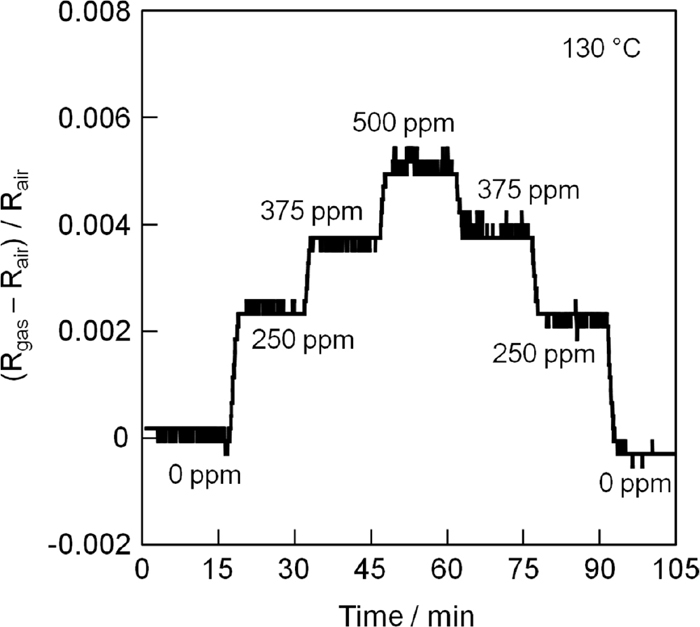
Representative sensor response curve obtained by varying CO concentration from 0 to 500 ppm and vice versa at 130°C.
Figure 5 depicts the steady sensor signals for various CO gas concentrations at 130°C. The present sensor produces a signal that varies in a linear fashion with changing CO gas concentration, similar to our previously reported sensor,6) suggesting that the present sensor can quantitatively detect CO gas concentration. The operating temperature of the present sensor was 130°C, which is considerably lower than the predating temperatures of the conventional sensors that use Pt/Al2O3 or Pd/Al2O3 catalysts (250–400°C). Since the present sensor realizes rapid and reproducible responses to CO gas concentration changes at a temperature of 130°C, it should be considered to be a potential candidate for use as a practical CO gas detection tool.

Sensor signals at various CO concentrations for the present sensor incorporating the 11 wt% Pt/La9.0Si5.8Mn0.2O27−δ catalyst at 130°C.
The present work was partially supported by the Steel Foundation for Environmental Protection Technology.