2017 Volume 57 Issue 4 Pages 689-696
2017 Volume 57 Issue 4 Pages 689-696
The influence of MgO, Na2O, and B2O3 on the viscosity of Cr2O3 bearing CaO–SiO2–Al2O3 slags was investigated using the rotating cylinder method. Structural information of slags with different MgO, Na2O, and B2O3 content was obtained via Raman spectroscopy. The viscosity of Cr2O3 bearing CaO–SiO2–Al2O3–MgO slags increases with Cr2O3 addition. The viscosity and activation energy (Eµ) of Cr2O3 bearing CaO–SiO2–Al2O3 slags decreases with increased MgO, Na2O, and B2O3 content. The effect of MgO and B2O3 on viscosity and activation energy (Eµ) of Cr2O3 bearing CaO–SiO2–Al2O3 slags was greater than that of Na2O. This could be due to the different ways in which these additives modify the slag network structure. Raman spectroscopy analysis revealed that MgO and Na2O work as network modifiers, and the degree of polymerization (DOP) of slags increases with the addition of MgO and Na2O. MgO is more effective at modifying the structure than Na2O, because Na2O may be more likely to produce network former [AlO4] units. In this study, Cr2O3 and B2O3 are found to behave as network formers and increase the DOP. This is characterized by the decrease in NBO/Si (the number of non-bridging oxygen per Si atom) value. B2O3 addition leads to the formation of low melting point eutectics and weaker polymerization strength, which contribute to the decrease in slag viscosity.
Cr is an important alloying element in many types of metallic materials, such as stainless steel. During smelting and refining of these materials, the poor fluidity of the molten Cr-bearing slag has a major limiting effect on process efficiency. The high viscosity of this Cr-bearing slag, caused by its high melting point, leads to poor separation of the metal melt, which then results in significant metal dross and high impurity content in the final metal products. Much previous work has been carried out on improving the fluidity of Cr-bearing slag. Behera et al.1) reported that the viscosity of aluminothermic ferro-chrome slags decreased with increased MgO content. CaF2 is another common addition to improve the fluidity of slags with high melting points (such as Cr2O3-bearing slag),2) however, increasingly strict environmental protection regulations are limiting the use of this material. B2O3 is considered to have analogous behavior to CaF2, and is becoming increasingly popular in the metallurgical industry. The lower melting point of B2O3 was reported to be more effective at lowering the break temperature and decreasing the viscosity of slags,3,4) even though B2O3 essentially behaves as an acid oxide (which is usually regarded as a network former and helps polymerize the network structure). Na2O was also found to be able to decrease the viscosity of some slags,5,6) however, its application in Cr-bearing slags has not yet been studied.
Recently, a consensus has been reached, that the macroscopic physical properties of a slag, such as viscous flow behavior, are determined by its structure. The decrease of slag viscosity is generally explained by the network modification behavior of basic oxides, which leads to the network structure becoming partly depolymerized.7,8,9,10,11) The viscosities of several types of Cr-bearing slag have been investigated, such as CaO–CaF2–Cr2O3 slags by Ostrovski et al.,12) CaO–SiO2–MgO–Cr2O3 slags by Minami et al.,13) and mold fluxes by Xu et al.14) The results of these studies showed that the viscosity of the slags increased as Cr2O3 content increased. These studies, however, mainly reported the effects of varying components, such as Cr2O3, MgO, and CaF2, on the viscosity of the slags. Detailed information on the structure of Cr2O3-bearing slags, along with the effects of different components, is extremely limited.
In order to find an effective flux to improve the fluidity, a detailed understanding of the effect of some components on the viscosity of Cr2O3-bearing slag is necessary, in particular the effect on the structure. In this study, the viscosity-structure relationship of Cr2O3-bearing CaO–SiO2–Al2O3–MgO, Na2O, B2O3 slags was investigated by using the rotating cylinder method in conjunction with Raman spectroscopy.
The high melting point (and the associated experimental difficulties) of Cr2O3-bearing slag is the main reason that viscosity data is relatively scarce, compared with the more usual slag systems containing CaO, SiO2, Al2O3, and FeO. The purpose of this study was to understand the effect of MgO, Na2O, and B2O3 content on the viscosity and structure of Cr2O3-bearing slags in a fully liquid phase. A type of slag with a lower melting point is therefore necessary. The phase diagram of CaO-SiO2-Al2O3-based slags is shown in Fig. 1(a). When the CaO%/SiO2% ratio (C/S) is kept between 0.3 and 1.1 with moderate Al2O3 content, the liquidus temperature will be below 1573 K (1300°C). Liquid regions below 1573 K of the CaO–SiO2–Al2O3 slag system, with varying content of added MgO, Na2O, and B2O3 are shown in Figs. 1(b), 1(c), and 1(d), respectively. These suggest that by increasing the content of MgO, Na2O, and B2O3, the liquid regions are enlarged, indicating that these components can act as fluxes to decrease the melting point of the slag system described above. Point C in Fig. 1(a) (C/S = 0.9) was selected, with 1–5% Cr2O3 addition, to investigate the effect of Cr2O3 on viscosity. Points M, N, and B in Figs. 1(b), 1(c), and 1(d), respectively (C/S = 0.6) were selected as the representative slags for this research. In addition, 1% Cr2O3 as well as MgO, Na2O, or B2O3 at the target content level, was added. The slag compositions used in this research are shown in Table 1.

Phase diagrams of CaO–SiO2–Al2O3-based slags with (a) the liquidus temperature below 1623 K and liquid regions below 1573 K with different content of (b) MgO, (c) Na2O, and (d) B2O3.
| Sample | CaO/SiO2 | Al2O3 | Cr2O3 | MgO | Na2O | B2O3 |
|---|---|---|---|---|---|---|
| C1 | 0.9 | 13 | 0 | 3 | ||
| C2 | 0.9 | 13 | 1 | 3 | ||
| C3 | 0.9 | 13 | 3 | 3 | ||
| C4 | 0.9 | 13 | 5 | 3 | ||
| M1 | 0.6 | 15 | 1 | 0 | ||
| M2 | 0.6 | 15 | 1 | 4 | ||
| M3 | 0.6 | 15 | 1 | 8 | ||
| M4 | 0.6 | 15 | 1 | 12 | ||
| N1 | 0.6 | 15 | 1 | 0 | ||
| N2 | 0.6 | 15 | 1 | 4 | ||
| N3 | 0.6 | 15 | 1 | 8 | ||
| N4 | 0.6 | 15 | 1 | 12 | ||
| N5 | 0.6 | 15 | 1 | 16 | ||
| B1 | 0.6 | 15 | 1 | 0 | ||
| B2 | 0.6 | 15 | 1 | 4 | ||
| B3 | 0.6 | 15 | 1 | 8 | ||
| B4 | 0.6 | 15 | 1 | 12 | ||
| B5 | 0.6 | 15 | 1 | 16 |
All the slag samples were prepared using analytical reagents. CaO powder was heated at 1273 K for 6 h to decompose hydroxides and carbonates. SiO2, MgO, Al2O3, and Cr2O3 powders were baked at 1073 K in a muffle furnace for 4 h to remove crystallization water. B2O3 was baked at 473 K for 2 h and stored in a desiccator. These materials were then weighed to obtain the target mass ratio as shown in Table 1, and were subsequently mixed.
To prepare the slag samples, 140 g of mixed powders were pressed into a Mo crucible and pre-melted at 1793 K under a protective atmosphere of pure Ar for 2 h. On completion of the pre-melting process, the slags were quenched.
2.2. Viscosity MeasurementViscosity measurements were carried out using the rotating cylinder method, with a Brookfield digital viscometer (Brookfield Engineering Laboratories Inc, Middleboro, MA). The experimental apparatus is shown in Fig. 2(a), which illustrates the U-shape molybdenum silicide heating elements used in the electric resistance furnace. The temperature was monitored by a thermocouple positioned beneath the crucible. Both the crucible and thermocouple were in the uniform temperature zone of the furnace to ensure the slag temperature matched the temperature measured by thermocouple. The crucible and spindle are made of Mo, and their dimensions are shown in Fig. 2(b).
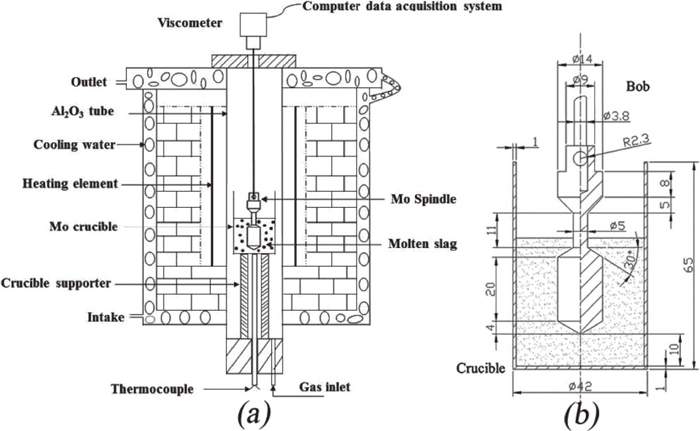
Schematic diagram of experimental apparatus. (a) Viscometer apparatus inside the furnace. (b) Dimensions of the Mo crucible and spindle.
The viscometer was connected to the spindle by a corundum shaft, and calibrated with castor oil with a known viscosity. The crucible with pre-melted sample was placed into the hot zone of the furnace. During the viscosity measurements, a flow of cooling water and a constant stream of pure Ar gas (3 L/min) were maintained throughout the process. The furnace was heated to 1793 K and held for 1 h, to ensure that the samples were fully melted and homogenized. The spindle was then slowly lowered into the slag along the central axis of the melt, until its tip was about 10 mm above the crucible base. The crucible and spindle were aligned with the axis of the viscometer to minimize experimental errors. Viscosity measurements were performed during the cooling cycles, at intervals of 20 K from 1793 K to 1653 K. The temperature was stabilized for 20 min after each temperature drop prior to measurement. The Newtonian behavior of slag can be guaranteed, as there was negligible deviation in the viscosity data obtained with three different rotation speeds. The average value measured across three different rotation speeds was adopted and considered as the final viscosity value at the specific temperature.
After the viscosity measurements, all the slag samples were reheated to 1793 K for 1 h and then quenched in water. The samples were then dried and crushed. Samples were confirmed to be amorphous by X-ray diffraction analysis. Composition of samples were analyzed using X-ray fluorescence spectroscopy, and the composition changes after viscosity measurements were very small, initial composition of samples is seen as the final composition for the sake of convenience. The glassy samples were tested using a Raman spectrometer (JY-HR800, JobinYvon SAS, France), with an excitation wavelength of 532 nm and using a 1 mW semiconductor laser as light source. Raman spectra were recorded in the range 200–1600 cm−1 and the wavenumber precision was 1 cm−1.
Figure 3(a) shows the effect of Cr2O3 addition on the viscosity of CaO-SiO2-Al2O3-3%MgO-Cr2O3 slags with a C/S ratio of 0.9 at 1793 K. It was found that the slag viscosity increases with increasing Cr2O3 content. When the Cr2O3 content was increased from 0% to 5%, the viscosity increased from 0.51 Pas to 0.95 Pas. A similar trend was observed in previous research.12,13,14)
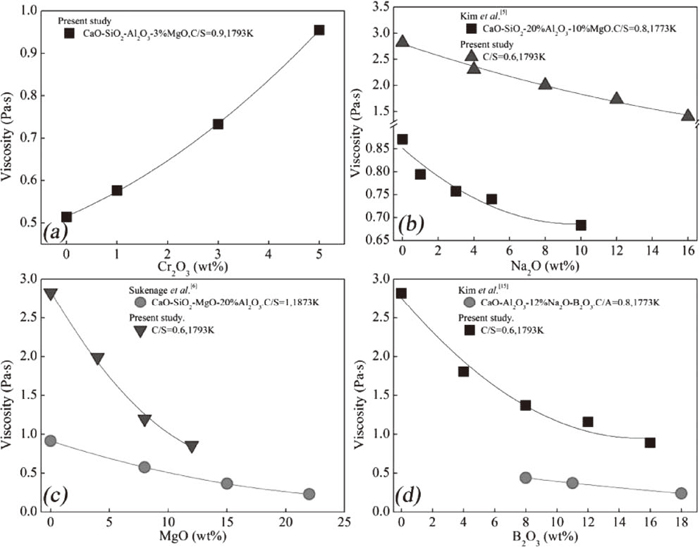
Effect of (a) Cr2O3, (b) Na2O, (c) MgO, and (d) B2O3 on slag viscosity.
The effect of Na2O, MgO and B2O3 on the viscosity of CaO-SiO2-Al2O3-1%Cr2O3 slag with a fixed C/S ratio of 0.6 are illustrated in Figs. 3(b), 3(c) and 3(d), respectively. It can be seen that viscosity decreases with increasing MO content (denoting Na2O, MgO, and B2O3). Similar trends have been observed in Cr2O3 free slags.5,6,15) On increasing Na2O content from 0% to 16%, MgO content from 0% to 12%, and B2O3 content from 0% to 16%, the viscosity of this slag decreased from 2.82 Pa·s to 1.40 Pa·s, from 2.83 Pa·s to 0.86 Pa·s, and from 2.82 Pa·s to 0.89 Pa·s, respectively. All of these decrement were much larger than those observed in Cr2O3 free slags.5,6,15) This suggests that Na2O, MgO, and B2O3 are more useful for reducing the viscosity of Cr2O3-bearing slags than Cr2O3-free slags, probably because the viscosity of Cr2O3-free slag is too low compared to that of Cr2O3-bearing slag. A comparison of the effects of Na2O, MgO, and B2O3 on the slag viscosity values above is shown in Fig 4. This indicates that, in this study, MgO and B2O3 are more effective than Na2O in decreasing the viscosity of the slag. Increasing the MgO content to 12% and the B2O3 content to 16% reduced the viscosity of the slag to below 1.0 Pa·s. In the case of Na2O = 16%, the viscosity of CaO-SiO2-Al2O3-1%Cr2O3-16%Na2O slag was nearly 1.40 Pa·s. This could be due to the MgO, B2O3, and Na2O performing different functions in modifying the structure of CaO-SiO2-Al2O3-1%Cr2O3.

Comparison of the effect of Na2O, MgO, and B2O3 on the viscosity.
The temperature-viscosity relationship can be described by the Arrhenius equation, Eq. (1).
| (1) |
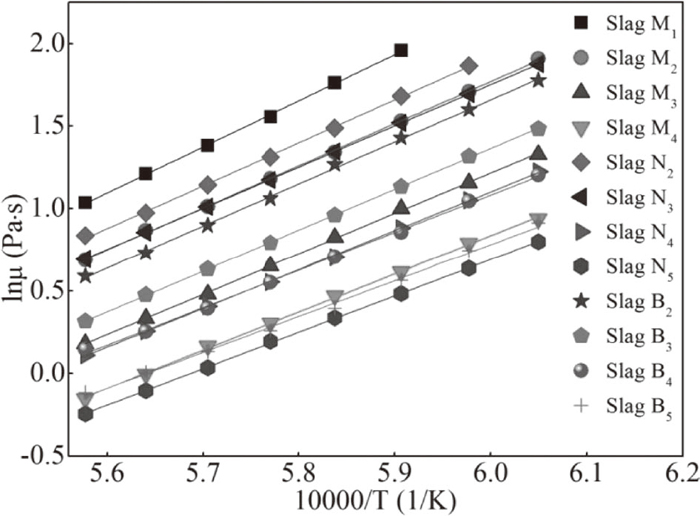
Plots of ln(μ) as a function of (10000/T).
| Sample | N1 | N2 | N3 | N4 | N5 |
| Eμ (kJ/mol) | 232 | 216 | 208 | 196 | 183 |
| Sample | B1 | B2 | B3 | B4 | B5 |
| Eμ (kJ/mol) | 232 | 213 | 206 | 191 | 181 |
| Sample | M1 | M2 | M3 | M4 | |
| Eμ (kJ/mol) | 232 | 212 | 204 | 192 |
It can be seen that the addition of MgO, Na2O, and B2O3 results in a decrease of Eμ. Similar Eμ values were observed in the case of B2O3 and MgO with the same addition levels, while that of Na2O is noticeably higher. This also suggests that B2O3 and MgO are more effective than Na2O at decreasing the energy barrier for viscous flow, which decreases the slag viscosity. This could be due to their different structural modification behaviors on the CaO-SiO2-Al2O3-1%Cr2O3 slag.
3.2. Structural Characteristics of Cr2O3-bearing CaO–SiO2–Al2O3 SlagThe Raman spectra for Cr2O3 content in the range of 0 to 5%, are shown in Fig. 6(a). The relative intensity of bands at around 450 cm−1 and 550 cm−1 increases as the Cr2O3 content increases. This indicates that the number of bridging oxygen atoms (BO) increases with the addition of Cr2O3, and that the network structure polymerizes. The peak at around 950 cm−1 shifts to lower frequency with the addition of Cr2O3. This peak shift indicates that the added Cr2O3 is introduced into the silicate network as a network former, which increases the degree of polymerization (DOP), but decreases the polymerization strength. The typical silicate Raman band is in the region of 800–1200 cm−1, which corresponds to tetrahedral [SiO4] structural units. The relative intensity of the stretching vibration QnSi (n is the number of bridging oxygen per tetrahedral unit, n = 0 to 4),17,18,19) increases with the addition of Cr2O3. According to Jiang et al.,20) the variation of QnSi structural units is considered as the main reason affecting the DOP of the network structure, and viscosity.

Effect of varying Cr2O3 content on the (a) Raman spectra and (b) structural units of the CaO-SiO2-13 Al2O3-3%MgO-Cr2O3 slag with C/S = 0.9.
In order to carry out a detailed investigation into the structure of slags, quantitative peak deconvolution of the Raman band from 800 to 1200 cm−1 was conducted using the Gaussian-deconvolution method (Mysen et al.21,22)). The recommended frequencies of Raman bands are summarized in Table 3. The deconvolution results for CaO-SiO2-13%Al2O3-3%MgO-Cr2O3 slags are shown in Fig. 7. The relative fractions of QnSi units as a function of bulk composition are shown in Fig. 6(b).
| Units | Frequency (cm−1) | Structure assignment | Ref. |
|---|---|---|---|
| Q0Si | 850–880 | Zero bridging oxygen in [SiO4] | [23,24,25,26,27,28,29] |
| Q1Si | 900–920 | One bridging oxygen in [SiO4] | [23,24,25,26,27,28,29] |
| Q2Si | 950–980 | Two bridging oxygen in [SiO4] | [23,24,25,26,27,28,29] |
| Q3Si | 1040–1060 | Three bridging oxygen in [SiO4] | [23,24,25,26,27,28,29] |
| Q4Si | 1060, 1190 | Four bridging oxygen in [SiO4] | [23,24,25,26,27,28,29] |
| Si–O–Si | 500–650 | Symmetric Si–O0 bending | [30] |
| Al–O–Al | 550 | Vibration of Al–O0 | [31] |
| Cr–O–Cr | 420–440 | Asymmetric Cr–O0 stretching | [32] |

Deconvoluted Raman spectra of the slag samples with C/S=0.9 and different Cr2O3 contents. Cr2O3 content=(a) 0 wt.%, (b) 1 wt.%, (c) 3 wt.%, and (d) 5 wt.%.
It can be seen in Fig. 6(b) that the relative fraction of simpler Q0Si units decreases with increasing Cr2O3 content, and the relative fractions of Q1Si, Q2Si and Q3Si units then increase. Increasing Cr2O3 content makes the network structure more complex, which results in increased slag viscosity. The viscosity decrease of Cr2O3 containing slags in section 3.1, could be attributed to the variation of multiple structural units, and was caused by the addition of MgO, Na2O, and B2O3 to the silicate network structure.
3.3. Effect of Na2O Addition on Slag StructureThe Raman spectra for glass samples with different Na2O content are shown as a function of wavenumbers in Fig. 8(a).

Effect of varying Na2O content on the (a) Raman spectra and (b) structural units of the CaO-SiO2-15 wt.% Al2O3-1 wt.%Cr2O3-Na2O slags with C/S=0.6.
It can be seen in Fig. 8(a) that the Raman peak near 1050 cm−1 shifts towards lower frequency with increasing Na2O. This implies that the degree of polymerization (DOP) of the silicate network structure decreases at higher Na2O content. The Raman band near 440 cm−1, which relates to the stretching vibration of Cr–O–Cr, is not discernible. The peak at 510 cm−1 vanishes, and the peak at 630 cm−1 turns into a shoulder with the addition of 4% Na2O, both bands are assigned to the symmetric bending of Si–O–Si. The addition of 4% Na2O leads to a decrease in relative intensity, which indicates that the number of bridging oxygen bonding with Si radically decreases, and the silicate network structure is partly broken. The peak at around 550 cm−1, which is related to the transverse motion of bridging oxygen in Al–O–Al, becomes more pronounced with the addition of Na2O, and an increase in intensity means that the number of bridging oxygen increases, this phenomenon could be ascribed to the incorporation of [AlO4] into the silicate network structure as a result of charge balancing mechanisms arising from the addition of Na2O. The band near 694 cm−1,33) corresponding to [CrO6], is also not discernible. The bands corresponding to [CrO4] and QAl4 stretch vibrations at 873 cm−1 33) and 850 cm−1 31) respectively, are overlapped with the Si–O stretching vibration in Q0Si units. Because the combined capacity of Al3+, Cr3+ and O2− is less than that of Si4+.32,33) In consequence, the effect of [AlO4] and [CrO4] units on the network is much less than the predominant [SiO4] units.
All the spectra in high-frequency region from 800 to 1200 cm−1 were fitted properly in Fig. 9. The deconvolution results as a function of Na2O content are shown in Fig. 8(b). The fraction of fully polymerized Q4Si units decreases as Na2O content increases from 0 to 16%. The predominant Q3Si units are also found to significantly decrease with increasing Na2O content. The relative fraction of Q2Si units varies slightly with the increasing Na2O content. The relative fractions of Q1Si and Q0Si units both increase. When the Na2O content reaches 16%, Q1Si becomes the predominant unit present in the slag systems, and the relative fractions of Q0Si and Q3Si are equal.

Deconvoluted Raman spectra of the slag samples with C/S = 0.6 and different Na2O contents. Na2O content = (a) 0 wt.%, (b) 4 wt.%, (c) 8 wt.%, (d) 12 wt.% and (e) 16 wt.%.
The basic oxide Na2O (a so-called network modifying oxide) dissociates into ‘free oxygen’ O2− anions in silicate melts. With the addition of Na2O, the O2− can react with ‘bridging oxygen’ O0 (connected with two Si atoms)and break the existing bonds.The silicate network structure polymerized by O0 can be modified into discrete structural polymeric units by the formation of ‘non-bridging oxygen’ O− (connected with one Si atom), as shown in the reaction below:
| (2) |
| (3) |
This reaction describes the breaking event of complex structural units Q4Si and Q3Si into simple Q1Si and Q0Si units, which can be attributed to the supply of Q2− anions to the silicate network from the addition of Na2O.
The DOP was used to characterize the structural change of the network. The parameter NBO/Si9,10,11) (average number of non-bridging oxygen per Si atom) is adopted to explain DOP in the silicate slags. The effect of multiple QnSi species on the modification of silicate network structure, and consequently on the variation of DOP, can be calculated from the following equation:
| (4) |

Calculation results of NBO/Si for studied samples.
It can be seen that the value of NBO/Si increases as Na2O content increases from 0 to 16%. This can be explained by the Na2O playing the role of network modifier and causing depolymerization of the network structure, which decreases the DOP of silicate network and results in a decrease in slag viscosity.
3.4. Effect of MgO Addition on Slag StructureThe Raman spectra of the CaO-SiO2-15%Al2O3-1%Cr2O3-MgO slags (C/S = 0.6) with MgO content in the range of 0 to 12% are shown in Fig. 11(a).

Effect of varying MgO content on the (a) Raman spectra and (b) structural units of the CaO-SiO2-15 wt.% Al2O3-1 wt.%Cr2O3-MgO slags with C/S = 0.6.
As seen in Fig. 11(a), the intensity of the Raman band at around 550 cm−1 (which is related to the Al–O–Al bonds as discussed in section 3.3) slightly increases with the increased MgO content, this increase of bridging oxygen contributes to the formation of [AlO4] units charge balancing by MgO. The peak at around 640 cm−1 is related to Si−O−Si bending, and the increase of its relative intensity indicates an increase of bridging oxygen. The typical silicate Raman peak between 800 and 1200 cm−1 shifts significantly to lower frequency, which implies that higher MgO content leads to severe depolymerization of the silicate network structure.
The results of Gaussian deconvolution were shown in Fig. 12, the variation of QnSi species (derived by Gaussian deconvolution) is shown in Fig. 11(b). It can be seen that the relative fraction of fully polymerized Q4Si units varies slightly as MgO content increase. The fraction of Q3Si units greatly decreases. The fraction of Q2Si units slightly decreases with the addition of 8% MgO, but then increases. The relative fraction of Q1Si increases with increasing MgO. Finally, for Q0Si units, the relative fraction increases to a maximum at MgO content of 8% and then remains almost constant. The NBO/Si values are found to increase with the addition of MgO, as shown in Fig. 10.
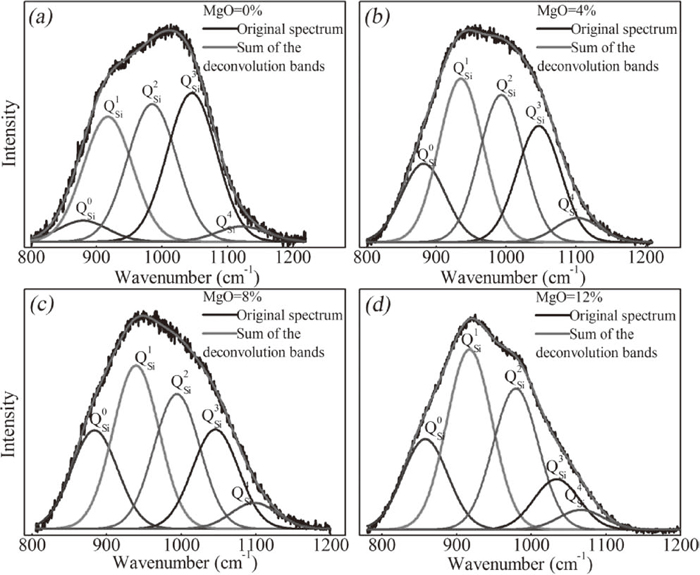
Deconvoluted Raman spectra of the slag samples with C/S = 0.6 and different MgO contents. MgO content = (a) 0 wt.%, (b) 4 wt.%, (c) 8 wt.%, and (d) 12 wt.%.
Amphoteric aluminum oxide (Al2O3) usually exists as a four-coordinated tetrahedral configuration [AlO4] in a silicate network structure, when the mole ratio of Al2O3/Basic oxides is less than unity.34,35,36) Na2O is more capable of catalyzing the formation of network former [AlO4] units than MgO, due to the higher priority of Na+ over Mg2+ for charge balancing Al3+.37) More [AlO4] units produced by the consumption of Na2O would polymerize with the silicate network, and a small proportion of the Na2O would modify the silicate network structure. In consequence, the NBO/Si values of MgO are higher than that of Na2O, as shown in Fig. 10. The slag viscosity therefore increases when Na2O is substituted by equal amounts of MgO.
3.5. Effect of B2O3 Addition on Slag StructureFigure 13(a) shows the Raman spectra for CaO-SiO2-15%Al2O3-1%Cr2O3-B2O3 slags with different B2O3 content. The intensity of the Raman band at around 500 cm−1 greatly increases with the addition of B2O3. This Raman band corresponds to Si–O–Si bending. Fan and Li et al.38,39) suggested that the Raman band between 430 and 600 cm−1 also represented the Al–O–Al vibrations within [AlO4] tetrahedral units. An increase in the relative intensity of the Raman band between 430 and 600 cm−1 indicates that the number of bridging oxygen is increased by increasing the B2O3 content.

Effect of varying B2O3 content on (a) Raman spectra and (b) structural units of CaO-SiO2-15 wt.% Al2O3-1 wt.%Cr2O3-B2O3 slags with C/S = 0.6.
The typical silicate Raman band between 800 and 1200 cm−1 shifts towards higher frequency, which implies the DOP of the silicate network increases with increased B2O3 content. Taking into consideration the effect of B2O3 on the network structure, some assignments of B2O3 related Raman bands that are of interest to the present work are summarized in Table 4. According to Cochain et al.,40) the stretch band reflecting B−O0 vibrations in [BO4] tetrahedral units also exists in this region, at the frequency region of 900 to 920 cm−1. As shown in Fig. 13(a), the continuous addition of B2O3 reduces the peak intensity at around 920 cm−1, which indicates a decrease of bridging oxygen in the three dimensional [BO4] tetrahedral units. The relative intensity of the Raman band between 1360 and 1520 cm−1 significantly increases with increased B2O3. This band is related to the stretch vibrations of B−O− non-bridging oxygen bonds in two dimensional [BO3] triangular structural units.40,41,42,43,44) An increase in the relative intensity of this band indicates the [BO3] units and non-bridging oxygen content increase with increasing B2O3 content. The [BO3] triangular structure is simpler compared to the [BO4] tetrahedral structure, and consequently, the network structure becomes inherently less complex at higher B2O3 content.
The Gaussian deconvolution of the Raman band between 800 and 1200 cm−1 has been carried out in Fig. 14, the variation of structural units QnSi is shown as a function of B2O3 content in Fig. 13(b). It can be seen that Q0Si units only exist in the B2O3 free slag, and are not observed in any other samples containing B2O3. The relative fraction of Q1Si units significantly decreases with the addition of 4% B2O3 and slightly decreases with a further increase of B2O3. The Q2Si fraction increases with the addition of 4% B2O3 and then slightly decreases with increased B2O3 content. The fraction of Q3Si units significantly increases with the addition of 4% B2O3 and then increases slightly at higher B2O3 content. The fraction of Q4Si units is also found to increase slightly with increased B2O3 content. Consequently, an increase in B2O3 content must cause the polymerization of simple Q0Si and Q1Si units into complex Q3Si and Q4Si ones. B2O3 behaves as a network former and may capture non-bridging oxygen in [SiO4] to form B−O bonds, which causes the decrease of NBO/Si in Fig. 10 due to the formation of Si–O–B bonds. Consequently, the DOP of borosilicate network structure increases with increased B2O3 content.

Deconvoluted Raman spectra of the slag samples with C/S = 0.6 and different B2O3 contents. B2O3 content = (a) 0 wt.%, (b) 4 wt.%, (c) 8 wt.%, (d) 12 wt.%, and (e) 16 wt.%.
B2O3 is seen as a network former, however, it was observed that viscosity decreased as B2O3 content increased. This means that the decrease of viscosity (but not the lower DOP) might be caused by three phenomena described below.
Firstly, B–O bonds are weaker than Si–O bonds, and the flow units containing B–O bonds are easily broken. The polymerization of B2O3 into the silicate network will give rise to a higher DOP but a weaker polymerization strength. With the continuous addition of B2O3 above 4%, the relative fraction of simpler two dimensional [BO3] triangular structural units increases in borosilicate melts, which is advantageous for decreasing the connectivity of the network. Secondly, by increasing B2O3 content, and decreasing SiO2 content, the silicate network structure is less dominant. Even if the [SiO4] structural units become more complex, the DOP of the silicate network structure is enhanced. Thirdly, due to the lower melting point of B2O3, B2O3 could combine with basic oxides and form some eutectics, which will help lower the melting point of the slags and enhance the fluidity of molten slags.
3.6. Error Sources of Viscosity ValuesThe error sources are inaccuracies of the temperature measurement, compositional analysis and viscosity measurement apparatus. Firstly, there is a difference between the slag temperature and thermocouple reading (variation less than ± 2°C), though the thermocouple and the crucible were both in the uniform temperature zone of the furnace. Secondly, slag samples were prepared using pure oxides with about 1% impurities. Though, the weighing was done carefully, there is still an error in the composition of the slags. Thirdly and most importantly, the spindle may be not completely in the central region of the crucible, the nonuniform gap between the walls of crucible and spindle will magnify the boundary effects. The heavy spindle in the tip of corundum shaft will sway, especially easily at a higher rotational speed and measurement of low viscosity. Therefore, taking the average of measured values in three rotational speeds could minimize the error.
The effects of MgO, Na2O and B2O3 addition on the viscosity and structure of CaO–SiO2–Al2O3–Cr2O3– slags were investigated in this study. The following conclusions were made:
(1) The viscosity of the slags investigated in this study increased with the addition of Cr2O3 and decreased as the MgO, Na2O, and B2O3 content increased. The activation energy decreased with increased MgO, Na2O, and B2O3. MgO and B2O3 are confirmed as more effective fluxing agents than Na2O for decreasing the viscosity of CaO–SiO2–Al2O3–Cr2O3 slag under experimental conditions.
(2) The introduction of basic oxides Na2O and MgO into Cr2O3 bearing CaO–SiO2–Al2O3 slags as network modifiers, helps to destroy the silicate network structure. Thus, the DOP of silicate network decreases, proved by the decrease in the relative fractions of Q4Si and Q3Si as well as the increase of Q1Si and Q0Si species.
(3) As Na+ has a higher priority than Mg2+ for charge compensation of Al3+ and its surrounding O2−, the formation of network former [AlO4] units catalyzed by Na2O results in a higher DOP. This leads to the increase of viscosity and Eμ in Cr2O3 bearing CaO–SiO2–Al2O3 slags, when comparable amounts of Na2O and MgO are added.
(4) The addition of B2O3 into the silicate network, as a network former, gives a higher DOP for silicate network. But, the silicate network is weakened by the introduction of B2O3, and the existence of two dimensional [BO3] units reduces the complexity of the network. Additionally, low melting point eutectics are formed. These factors contribute to the decrease of slag viscosity and Eμ with increasing B2O3 content.
The authors are grateful for the financial support for this work from National Nature Science Foundation of China (No. 51474021, No. 51674022).