Volume 57, Issue 4
Displaying 1-26 of 26 articles from this issue
- |<
- <
- 1
- >
- >|
Fundamentals of High Temperature Processes
Regular Article
-
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 593-601
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Advance online publication: March 07, 2017Download PDF (1968K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 602-608
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Advance online publication: February 28, 2017Download PDF (1047K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 609-614
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (1174K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 615-624
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (2315K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 625-629
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (745K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 630-633
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (503K) Full view HTML
Note
-
Article type: Note
2017 Volume 57 Issue 4 Pages 764-766
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (502K) Full view HTML
Ironmaking
Regular Article
-
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 634-642
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Advance online publication: February 21, 2017Download PDF (1484K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 643-648
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Advance online publication: March 10, 2017Download PDF (615K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 649-655
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Advance online publication: February 23, 2017Download PDF (935K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 656-664
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Advance online publication: March 07, 2017Download PDF (938K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 665-672
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (1324K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 673-680
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Advance online publication: March 07, 2017Download PDF (1197K) Full view HTML -
Reducibilities of Wüstite and Calcio-wüstite in Terms of High Temperature X-ray Diffraction AnalysisArticle type: Regular Article
2017 Volume 57 Issue 4 Pages 681-688
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (1772K) Full view HTML
Steelmaking
Regular Article
-
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 689-696
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (1701K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 697-705
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (1339K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 706-712
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Advance online publication: March 17, 2017Download PDF (914K) Full view HTML -
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 713-722
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (1179K) Full view HTML
Note
-
Article type: Note
2017 Volume 57 Issue 4 Pages 767-769
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Advance online publication: March 10, 2017Download PDF (478K) Full view HTML
Forming Processing and Thermomechanical Treatment
Note
-
Article type: Note
2017 Volume 57 Issue 4 Pages 770-773
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Advance online publication: March 07, 2017Download PDF (671K) Full view HTML
Welding and Joining
Regular Article
-
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 723-729
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (2095K) Full view HTML
Surface Treatment and Corrosion
Regular Article
-
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 730-736
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Advance online publication: March 07, 2017Download PDF (997K) Full view HTML
Transformations and Microstructures
Regular Article
-
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 737-745
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Advance online publication: March 07, 2017Download PDF (1719K) Full view HTML
Mechanical Properties
Regular Article
-
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 746-754
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (2390K) Full view HTML
Physical Properties
Regular Article
-
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 755-757
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (487K) Full view HTML
Social and Environmental Engineering
Regular Article
-
Article type: Regular Article
2017 Volume 57 Issue 4 Pages 758-763
Published: April 15, 2017
Released on J-STAGE: April 19, 2017
Download PDF (1211K) Full view HTML
- |<
- <
- 1
- >
- >|

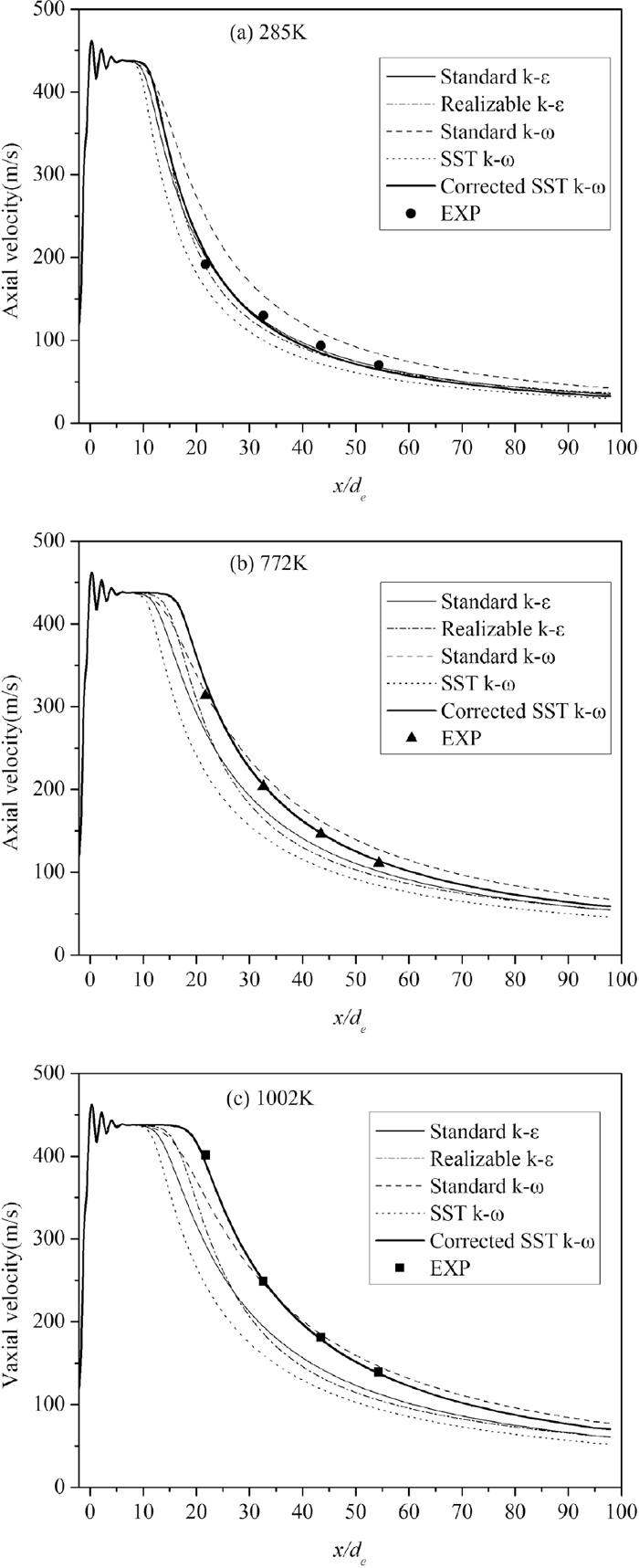
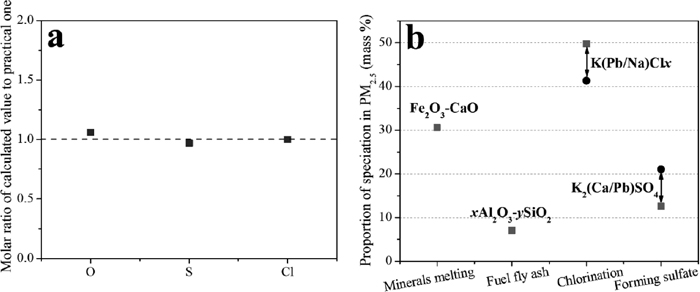
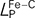 , were measured. The results demonstrated
, were measured. The results demonstrated