2017 Volume 57 Issue 4 Pages 630-633
2017 Volume 57 Issue 4 Pages 630-633
High manganese-aluminum steel have obtained great attention because of their excellent strength, ductility and formability. However, a high manganese content can increase the solubility of nitrogen significantly and result in the formation of the AlN inclusions during the process of steelmaking. Therefore, a better understanding and control of nitrogen content and AlN inclusions in Fe–Mn–Al melts is essential for the process of steelmaking. In this work, the nitrogen solubility in Fe–Mn–Al melts ([%Mn]<20, [%Al]<2) was measured at 1873 K under the nitrogen partial pressure range of 0.1 atm to 0.4 atm. It was concluded from the experimental results that nitrogen solubility decreased with the increase of aluminum content, but increased with the increase of manganese content. Moreover, AlN inclusions were found in the samples with the aluminum content of 1.3 mass% and 1.4 mass% under the nitrogen partial pressure of 0.4 atm. It was also confirmed that the interaction parameters of thermodynamic model for Fe–Mn–Al–N system determined by Paek et al. were effective over a wide range of aluminum content and nitrogen partial pressure.
In the past decades, advanced high strength steels (AHSS), including Twinning Induced Plasticity (TWIP), have obtained great attention because of their excellent strength, ductility and formability.1) The TWIP effect means twin crystal formation by plastic deformation. Such formation mechanism is the reason for the extraordinary behavior of steels containing 15–25 mass% manganese and 1–2 mass% aluminum. In these steels, high manganese and aluminum have great influences on their properties. However, manganese can increase the solubility of nitrogen significantly2,3) to form the AlN inclusions in TWIP steel.4) These will bring a large amount of problems during the steelmaking and casting process. Therefore, a better understanding and control of nitrogen content and AlN inclusions in Fe–Mn–Al melts is essential for modern steel grades.
In the literatures, many investigations have been done on the thermodynamics of Fe–Mn–N system and Fe–Al–N systems.3,5,6,7,8,9,10,11,12,13,14) However, few researchers have studied the effects of high manganese and aluminum contents on nitrogen solubility in Fe–Mn–Al–N system. Using Wagner’s formalism,15) many researchers8,9,13,14) have systematically determined the first and second order interaction parameters of manganese or aluminum on nitrogen, and manganese on aluminum, based on the data obtained under high nitrogen partial pressures in liquid Fe–Mn–Al melts with a high manganese content and a low aluminum content. In this study, the effect of manganese (5–20 mass%) and aluminum (0–2 mass%) on nitrogen solubility were measured at 1873 K under the low nitrogen partial pressures. The obtained results were used to check the validity of the interaction parameter values determined by Paeks’ over a wide range of aluminum content in Fe–Mn–Al melts.
The gas-liquid metal equilibration experiments were carried out to measure nitrogen solubility in Fe–Mn–Al melts. Electrolytic iron (99.99 mass% purity), manganese (99.99 mass% purity) and aluminum (99.9999 mass% purity) were used as raw materials.
In the experiments, the mixture of electrolytic iron, manganese and aluminum was about 20 g. They were accommodated in an alumina crucible (outer diameter (OD): 17 mm, inner diameter (ID): 14 mm, height (H): 30 mm), which was placed in a graphite crucible (outer diameter (OD): 32 mm, inner diameter (ID): 28 mm, height (H): 25 mm). The graphite crucible was used as the protected crucible, meanwhile, it was beneficial to pull out the graphite crucible with sample in a short time. The temperature was measured by a Pt/Pt-13 mass% Rh thermocouple. At first, the furnace was raised to 1873 K with the heating rate of 5 K/min. During the heating period, a mixed gas (500 ml/min, Ar+3%H2, controlled by a mass flow controller) was introduced into the furnace to decrease the oxygen content in the furnace. When the temperature was raised to the desired temperature, the crucible filled with sample was placed in the furnace and the gas was switched to a mixture of Ar-3%H2-N2 with the nitrogen partial pressures ranged from 0.1 atm to 0.4 atm. Before the mixed gas entered into the furnace, it was dehydrated by silica gel in a dehydrator. After the melted metal was in an equilibrium state with the gas phase, the crucible with the sample were picked out quickly and quenched in a high purity Ar stream in a short time. The schematic diagram of the experimental appratus was shown in Fig. 1.

The schematic diagram of the experimental appratus.
After each experiment, the sample was polished and cleaned in alcohol by ultrasonic cleaning before detection. The contents of nitrogen, manganese and aluminum were measured for each sample. Among them, the content of manganese and aluminum was analyzed by the inductively coupled plasma atomic emission spectroscopy (ICP-AES, OPTIMA 7000DV, USA). The content of solubility nitrogen was measured by inert gas fusion-infrared absorptiometry technique (OHN-3000, Beijing), and all measured samples were taken from the middle part. The micro-morphology of samples were examined by scanning electron microscope (SEM, Mineral Liberation Analyzer 250, voltage 200 V-30 KV), which was equipped with an Energy Disperse Spectroscopy (EDS).
In order to measure the nitrogen solubility in Fe–Mn–Al melts, the obtained nitrogen content must be in an equilibrium condition. Thus, the equilibrium time should be determined first. The nitrogen content, in Fe-15mass%Mn-0.5mass%Al melt under nitrogen partial pressure of 0.1 atm at 1823 K, was measured for different reaction time. As shown in Fig. 2, 18 hours was enough to reach equilibrium condition

Variation of nitrogen content with reaction time in Fe-15mass%Mn-0.5mass%Al melt under nitrogen partial pressure of 0.1 atm at 1823 K.
The contents of nitrogen, aluminum as well as manganese for different samples were measured and shown in Table 1. When AlN was not formed during the reaction process, the solubility equilibrium was charged by the dissolution reaction of nitrogen in liquid Fe–Mn–Al melts. The solution can be described by the following reaction:
| (1) |
| (2) 3) |
| (3) |
| (4) |
| Temp. (K) | PN2 (atm) | [%Mn] | [%Al] | [%N] |
|---|---|---|---|---|
| 1863 | 0.1 | 9.99 | 0.42 | 0.0288 |
| 9.79 | 0.84 | 0.0223 | ||
| 9.73 | 1.40 | 0.0223 | ||
| 9.52 | 1.90 | 0.0261 | ||
| 0.1 | 14.84 | 0.44 | 0.0332 | |
| 14.75 | 0.93 | 0.0328 | ||
| 14.80 | 1.43 | 0.032 | ||
| 14.84 | 1.93 | 0.0312 | ||
| 0.1 | 19.86 | 0.44 | 0.0401 | |
| 19.54 | 0.88 | 0.0398 | ||
| 18.97 | 1.61 | 0.0355 | ||
| 0.4 | 19.44 | 0.42 | 0.072 | |
| 18.89 | 0.78 | 0.0693 | ||
| 19.24 | 1.30 | 0.0684 | ||
| 18.17 | 1.40 | 0.0631 |
The standard Gibbs free energy change for reaction were shown in Eq. (2), in which KN is the equilibrium constant for Eq. (1); PN2 is nitrogen partial pressure during the reaction process, atm; [%N] is the equilibrium nitrogen content dissolved in the melt; T is absolute temperature, K; aN is the activity of nitrogen; and fN is the activity coefficient of nitrogen, for which the referenced state is the infinitely dilute solution.
According to Wagner model,15) the activity coefficient for the dissolved nitrogen can be derived as follow:
| (5) |
| (6) |
When AlN was saturated and formed on the surface, the solubility equilibrium was charged by the reaction of the dissolution of AlN in liquid Fe–Mn–Al melt. The reaction and the corresponding standard Gibbs free energy change were shown in Eqs. (7) and (8):
| (7) |
| (8) 14) |
The equilibrium constant of reaction (7) can be represented as follows:
| (9) |
By using Wagner model, the activity coefficient of Al can be described in terms of first and second order interaction parameters as follows:
| (10) |
As substituting Eqs. (5) and (10) into Eq. (9) , the equilibrium constant KAlN can be obtained as follow:
| (11) |
Although several researchers have measured the thermodynamic parameters of Fe–Mn, Fe–Al or Fe–Mn–Al melts by different methods, there were large discrepancies among the values of interaction parameters. Considering the similar experimental method and composition range, the interaction parameters from Paek et al.9,14,17) was adopted. The first and second order parameters, including
Thus, the Eqs. (6) and (11) can be simplified as shown in follows:
| (12) |
| (13) |
Figure 3 shows the effects of aluminum, manganese and nitrogen partial pressure on the nitrogen solubility in Fe–Mn–Al melts. In Fig. 3, the dotted lines were estimated by using the interaction parameters from Paek et al.9,14,17) It was easy to find that the nitrogen solubility was determined by reaction (1) when nitrogen partial pressure was 0.1 atm. However, nitrogen solubility decreased sharply and was determined by the dissolution reaction of AlN under 0.4 atm nitrogen partial pressure when aluminum content was higher than 1.2 mass%. It indicated that the nitrogen partial pressure had an important effect on the formation of AlN. The data with probable formation of AlN, described later in detail, were surrounded by the squares in Fig. 3. Due to the negative value of the interaction parameter of

Effect of aluminum, manganese and nitrogen partial pressure on the nitrogen solubility in Fe– Mn–Al melts.
In order to confirm the validity of the interaction parameter values determined by Paek et al.9,14,17) over a wide range of aluminum content and nitrogen partial pressure in liquid Fe–Mn–Al melts, the estimated nitrogen contents were compared with the experimental results. As shown in Fig. 4, the experimental data agreed well with the estimated nitrogen content. Among these estimated nitrogen contents, the data surrounded by the squares were calculated by Eq. (13) due to the formation of AlN particles while the others were calculated by Eq. (12). It can be concluded that the model is applicable to the composition with a high aluminum content at a low nitrogen partial pressure.

Comparison of estimated and measured nitrogen solubility at different conditions in Fe–Mn–Al melts.
From Fig. 3, it can be found that nitrogen content decreased sharply under 0.4 atm nitrogen partial pressure when aluminum content was around 1.3–1.4 mass%. It was indicated that the AlN phase was formed under this condition. In order to confirm the existence of AlN, the micro-morphology of the top part of the quenched samples having 1.3 mass% and 1.4 mass% aluminum, are shown in Figs. 5(a) and 5(b). According to the EDS analysis shown in Fig. 5(c), it can be seen that AlN was found. Figure 5(d) showed the SEM result of the middle part of samples, which demonstrated that no AlN inclusion was found, thus the measured nitrogen content should be the nitrogen solubility of the corresponding sample at the high temperature. It was also found that there is no AlN particles on the top parts of the quenched samples which had 0.42 mass% and 0.78 mass% aluminum as shown in Figs. 5(e) and 5(f). Consequently, the AlN particles in Figs. 5(a) and 5(b) corresponding to the samples with 1.3 mass% and 1.4 mass% aluminum should be formed at the experimental temperatures.
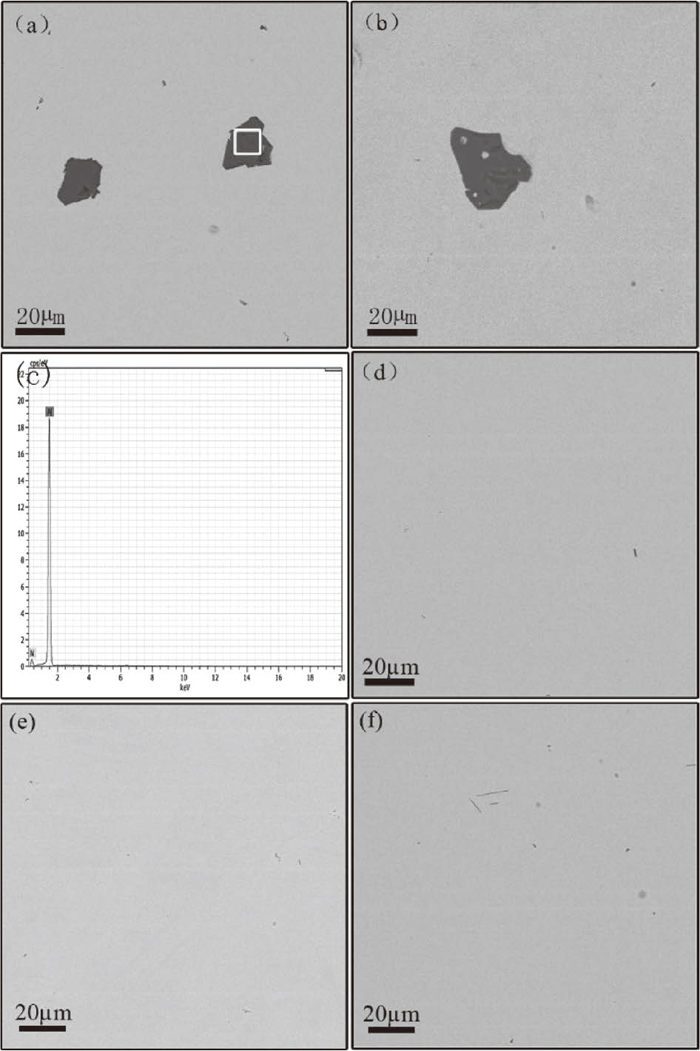
(a) and (b) SEM of the top parts of samples with 1.3 mass% and 1.4 mass% aluminum under 0.4 atm nitrogen partial pressure, (c) EDS pattern of AlN, (d) SEM of the middle of sample with 1.3 mass% aluminum under 0.4 atm nitrogen partial pressure, (e) and (f) SEM of the top parts of samples with 0.42 mass% and 0.78 mass% aluminum under 0.4 atm nitrogen partial pressure.
The nitrogen solubility was measured for Fe–Mn–Al melts with the aluminum content from 0.5 mass% to 2 mass% and manganese content from 5 mass% to 20 mass% at 1873 K under the low nitrogen partial pressures. It was found that manganese and aluminum significantly affected the solubility of nitrogen. The nitrogen solubility decreased with the increase of aluminum content, but increased with an increase of manganese content. The experimental data of nitrogen solubility agreed well with the estimated nitrogen content by model of Paek et al. The AlN phase was formed for composition having 1.3 mass% and 1.4 mass% aluminum when the nitrogen partial pressure is 0.4 atm.
Thanks are given to the financial supports from the Fundamental Research Funds for the Central Universities (FRF-TP-15-009A3).