2017 年 57 巻 5 号 p. 905-912
2017 年 57 巻 5 号 p. 905-912
The effect of Cu addition on the corrosion behavior of 3Cr steel in 3.5 wt.% NaCl solution was investigated by immersion tests and electrochemical measurements. Cu was selected to partially replace Cr in Cu-modified 3Cr steel. Cu addition can improve corrosion resistance of 3Cr steel at the initial stage, and then the phenomenon gradually weakens with increasing immersion time. The reduced corrosion resistance of 3Cr steel caused by Cr decrease suitably offset by the presence of Cu, resulting a reduced-cost Cu-modified 3Cr steel without sacrificing ether its mechanical properties or corrosion resistance.
With the application and promotion of the CO2-enhanced oil recovery (EOR) technique and the exploration of deep oil and gas reserves containing higher CO2, CO2 corrosion of steel has become more severe and is a big concern to scientists and engineers.1,2) Low-alloy steel containing 3% chromium (3Cr) improve CO2 corrosion resistance by a factor of 3–10 while maintain the cost less than 1.5 times that of conventional grades of carbon steel, and then represent the most commonly used construction materials in the oil and gas industry.3,4,5)
At present, ocean oil exploitation is becoming more and more important as oil requirement is rapidly increasing and reserves in land are reducing.6,7) 3Cr steel will be widely used as construction materials in ocean oil exploitation because of good performance in terms of both cost and CO2 corrosion resistance. It is well known that approximately 20% of the total corrosion cost in marine industry is due to Microbially Influence Corrosion (MIC).8,9) However, 3Cr steel does not possess an antibacterial function, and is susceptible to the MIC. Clearly then, there is a need to introduce other alloying elements that can offer a stronger resistance to MIC. The strong antibacterial effects of copper (Cu) ions have been well known for a long time.10) Cu as an alloying element added to 3Cr steel can be expected to produce a unique function of antibacterial, which can solve the MIC problem.10) However, the effect of Cu on the corrosion behavior of low-alloy steel is not sufficiently recognized in seawater, especially for 3Cr steel.
In the present study, a detailed investigation has been undertaken to characterise the changes on the microstructure and corrosion resistance of Cu-modified 3Cr steel, and this investigation can provide some valuable information to utilize the 3Cr steel in ocean oil exploitation.
A Cu-modified 3Cr steel (Cu-3Cr) and 3Cr steel were provided by Baoshan Iron & Steel Co. Ltd., with the composition as listed in Table 1. The steels were melted using a vacuum furnace, casted into ingots and hot-rolled into a 10 mm thick plate. A ferritic microstructure of the plate was produced by 900°C water quenched followed by tempered at 650°C for 1 hour and cooling in air. In considering the economic realities of the practical use of Cu-3Cr steel, Cu was selected to partially replace the element Cr. Meanwhile, the synergistic effects of Cu and Cr on corrosion behaviour of 3Cr steel were investigated. The concentration of Cu addition and Cr subtraction were determined based on our previous researches.
| Alloy | C | Si | Mn | S | P | Cr | Cu | Mo+ Ti+V | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 3Cr | 0.05 | 0.15 | 0.35 | ≤0.005 | ≤0.012 | 3.2 | – | 0.25 | Bal. |
| Cu-3Cr | 0.05 | 0.16 | 0.35 | ≤0.005 | ≤0.012 | 2.6 | 0.6 | 0.25 | Bal. |
The test coupons were machined to a size of 50 mm × 25 mm × 3 mm. Prior to the experiments, the surfaces of the specimens were ground with silicon carbide (SiC) papers progressively up to 1000 grit, rinsed with distilled water and then degreased in acetone. After drying in hot air, the specimens were weighed (precision 0.1 mg) and then stored in a desicator for use. The immersion tests were carried out by suspending the samples in a still solution of 3.5 wt.% NaCl in deionized water exposed to atmospheric air at room temperature, simulating seawater. The specimens were exposed to the test solution for 1, 15, 30 and 60 days. After the test, the specimens were ultrasonically cleaned in 10% hydrochloric acid (HCl) inhibited with 10 g/L hexamethylenetetramine (urotropine), dried and weighed. The corrosion rate of the sample was then normalized in the unit of mm/y, by considering the total surface area of the sample, the density of the sample and immersion time.
2.3. Electrochemical MeasurementsThe working cell was a standard three-electrode cell having a Pt net as a counter electrode and a saturated calomel electrode (SCE) as a reference electrode. All the measured potentials presented in the paper were referred to this electrode. For potentiodynamic polarization experiments, the potential was scanned from −0.3 to +0.6 VSCE at a scan rate of 2 mV s−1. Corrosion potentials (Ecorr) and the corrosion current densities (icor) were calculated by using instantaneous Tafel-type fit Gamry-DC105 corrosion analysis software. Electrochemical impedance spectroscopy (EIS) measurements were performed at open circuit potentials from 10−2 to 105 Hz with amplitude of 10 mV.
2.4. Microstructural CharacterizationMicrostructural evaluations of tested samples were carried out using optical microscopy (OM), EVO MA25 scanning electron microscopy (SEM) and JEM 2100F transmission electron microscopy (TEM) equipped with energy-dispersive X-ray spectroscopy (EDX). The external appearances of corroded samples were photographed. Further morphology analysis and reaction product characterization were conducted using OM, SEM and D8 DISCOVER X-ray diffraction (XRD) with Cu Ka radiation.
The microstructures of 3Cr and Cu-3Cr steels are shown in Fig. 1. The microstructure of 3Cr steel consisted of polygonal ferrite, lath ferrite and particulate precipitates distributed within the grain and at the grain boundary (see Figs. 1(a), 1(b). EDX microanalysis exhibited that the particulate precipitates were Cr-rich carbides (white particles in Fig. 1(b)), corresponding to the red arrow in Fig. 1(b). Details of microstructure were further examined by TEM, as shown in Fig. 1(c). EDX microanalysis had been performed on these particles and the results revealed that the first type was equiaxial TiC particle (see Fig. 1(e)) and the second was Cr-rich carbide in a dominate position, corresponding to the red arrow in Fig. 1(c). In addition, no apparent cementite was detected in the microstructure, which could be due to the simultaneous present of low C and relatively high Cr content in the 3Cr steel.
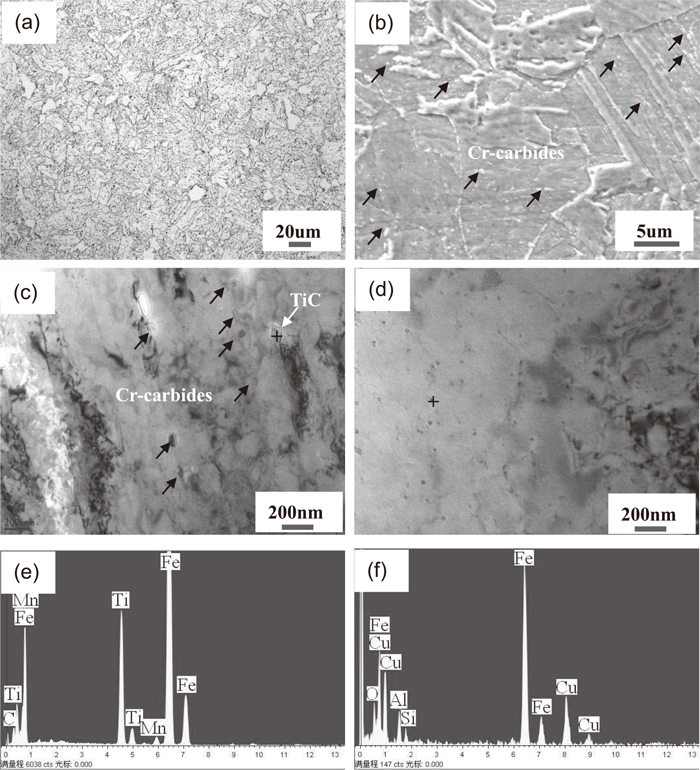
OM and SEM images did not exhibit any visible changes between 3Cr and Cu-3Cr steels. However, significant changes in microstructure were revealed in TEM images. Further TEM observations (Fig. 1(d)) revealed the presence of fine, nearly elliptical nanoparticles with diameters of about 30 nm. EDX analysis showed that these particles were Cu precipitates, as shown in Fig. 1(f). Obviously, Cu precipitates in the microstructure resulted from the addition of 0.6% Cu. Because the solubility of Cu in ferrite is low (~0.2%),11) 0.6% Cu added in 3Cr steel was sufficient for the precipitation of the Cu precipitates during the heat treatment process.
The mechanical properties of 3Cr and Cu-3Cr steels are shown in Table 2. As seen in Table 2, the mechanical properties of 3Cr steel, such as the strength, the elongation and impact toughness, did not experience obvious change due to Cu addition and Cr subtraction.
3.2. Corrosion Rates from the Immersion TestsCorrosion rate was calculated by the following equation:

Corrosion rates of tested steels as a function of immersion time in 3.5 wt% NaCl solution.
Surface product identification was carried out by XRD for 3Cr and Cu-3Cr after 1 day and 60 days immersion tests, as shown in Figs. 3(a), 3(b). In addition to matrix structure of ferrite, FeCr2O4, Fe3O4, and FeCl2 were detected on the surface for both steels. It can be seen from Fig. 3 that as the immersion time increases for both steels, the peak intensities also increase. This simple indicates that the amount of FeCr2O4, Fe3O4, and FeCl2 was increased with increasing immersion time. For Cu-3Cr steel, Cu2O was also found in the corrosion scale and exhibited antimicrobial characteristics,12) as exhibited in Fig. 3.

XRD spectra for corrosion scale of 3Cr and Cu-3Cr steels: (a) 1 day; (b) 60 days.
Figures 4(a), 4(b) shows the potentiodynamic polarization behavior of 3Cr and Cu-3Cr steels at different immersion times in 3.5 wt.% NaCl solution. Their Ecorr, βa, βc and icor values are summarized in Table 3. From Fig. 4 and Table 3, it can be seen that corrosion behavior of the both steels at the first day of immersion was obviously different from the last immersion time. A protective corrosion products scale has not been completely formed at the initial stage. This was the main reason that the obviously difference between the initial stage and the last stage was observed. The anodic and cathodic Tafel constans (βa and βc, respectively) of 15, 30 and 60 days do not obviously change their values, which indicates a similar mechanism for the corrosion reaction of the two steels in the last immersion time. At the first day of immersion, the anodic Tafel constant (βa) of Cu-3Cr is significantly larger than that of 3Cr, indicating that a protective corrosion products scale seem to form rapidly for 3Cr steel due to Cu modification. At the first day of immersion, 3Cr steel has a larger icor than Cu-3Cr steel. This result verifies that the alloy element of Cu exhibited a suppressive effect on the anodic reaction, thus improving the corrosion resistance of 3Cr steel at the initial stage, and then the phenomenon gradually weakens with the increasing of corrosion time.

Polarization curves (a–b) and Nyquist plots (c–d) at different immersion time: (a and c) 3Cr steel; (b and d) Cu-3Cr steel.
Figures 4(c), 4(d) shows the typical Nyquist diagrams as a function of immersion time for 3Cr and Cu-3Cr steels. The impedance spectra measured from the two steels at different immersion times indicated a single semicircle, which means only one reaction existed between specimen and electrolyte. The diameter of the arc can be regarded as a polarization resistance (Rp). The increase in diameter of the arc indicated an increase in the Rp value, and the diameter of arc increased with increasing immersion time. At the first day of immersion, Cu-3Cr steel has a larger Rp than 3Cr steel. This result also verifies that the alloy element of Cu can improve the corrosion resistance of 3Cr steel at the initial stage; the beneficial effect gradually weakens with the increasing of corrosion time. This is consistent with the results of the corrosion rate and potentiodynamic polarization.
3.5. Scale Microstructure InvestigationMacroscopic views of the corrosion scale formed on 3Cr and Cu-3Cr steels are shown in Figs. 5(a), 5(b), and the corresponding appearances of the substrate after scale removal are shown in Figs. 5(c), 5(d). The uniform attack is the most common form of corrosion of both steels. Compared to the rough surface of 3Cr steel, Cu-3Cr steel surface is considerably smoother. This indicates that the corrosion of Cu-3Cr was likely a more homogenous dissolution compared to the 3Cr steel.

Surface morphologies before (a–b) and after corrosion scale removal (c–d) in 3.5. wt% NaCl solution for 60 days: (a and c) 3Cr steel; (b and d) Cu-3Cr steel.
Details of corrosion scale morphologies are further examined by SEM, as shown in Figs. 6, 7. In considering the obvious difference in corrosion rate and electrochemical behavior between 3Cr and Cu-3Cr steels at the initial stage (see Fig. 2), the morphologies of the corroded surface of the both steels exposed for 1 day were observed in Fig. 6. A flat darkened surface (corrosion products scale) lightly covered by many clusters and exhibited good protection of the steel substrate. According to EDX and XRD results, the flat darkened surface was FeCr2O4 for 3Cr steel and a mixture of FeCr2O4 and Cu2O for Cu-3Cr steel and many clusters were a mixture of Fe3O4 and FeCl2 for both steels. Thus, the difference of the corrosion resistance of the both steels was related to the darkened rust layer. It is worth noting that some cracks in the scale are induced by the dehydration effect13) when the specimen is taken out for SEM observation. A loose outer layer, consisting of Fe3O4 and FeCl2, was seen on the surface with increasing immersion time to 60 days. Meantime, a continuous, dense and adherent inner layer was observed on the surface. The inner layer was identified to be FeCr2O4 for 3Cr steel and a mixture of FeCr2O4 and Cu2O for Cu-3Cr steel by EDX and XRD analysis. The surface morphologies of 3Cr are basically the same as that of Cu-3Cr, as shown in Figs. 7(a), 7(b). In order to obtain further insight into corrosion mechanism, the surface morphologies of studied steels after removing the corrosion product scale are characterized by SEM (Figs. 7(c), 7(d)). Many small holes are observed clearly in 3Cr steel (Fig. 7(c)), whereas in Cu-3Cr steel, there is still no hole with increasing immersion time to 60 days (Fig. 7(d)).

SEM micrographs of the tested steel in 3.5 wt% NaCl solution for 1 day: (a) 3Cr steel; (b) Cu-3Cr steel; (c) EDX from (a); (d) EDX from (b).

SEM morphologies before (a–b) and after corrosion scale removal (c–d) in 3.5 wt% NaCl solution for 60 days: (a and c) 3Cr steel; (b and d) Cu-3Cr steel.
Figure 8 presents cross-sections of the corroded specimens exposed for 60 days. 3Cr and 3Cr-Cu steels all show uniform corrosion with a typical duplex layer formation. The duplex layer is comprised of the outer corrosion layer contact with the solution, mainly consisting of Fe3O4 and FeCl2, and the inner layer in contact with the steel substrate, mainly consisting of FeCr2O4 (3Cr steel) or FeCr2O4 and Cu2O (Cu-3Cr steel) by EDX and XRD analysis. This is consistence with the surface morphologies of the both steels. SEM cross-section image of 3Cr steel (Fig. 8(a)) revealed a highly serrated interface between the inner layer and substrate, corresponding to many small holes in Fig. 7(c). It should be mentioned that the gaps of the inner layer are induced by the dehydration effect due to SEM observation.

Cross-sections of corrosion scale in 3.5 wt% NaCl solution for 60 days: (a) 3Cr steel; (b) Cu-3Cr steel.
Precipitation of copper in ferritic steels has been studied extensively,14,15,16,17,18) particularly in reference to pressure vessel steels19,20,21) which are used for nuclear reactors. It is now generally accepted that a complicated sequence involving the formation of two intermediate structures is characteristic of precipitation in this process: bcc→9R→3R→fcc.22,23) Initially, the copper precipitates nucleate and grow with the bcc crystal structure and are coherent with the bcc ferrite matrix. When the precipitates reach a critical size, they lose their full coherency due to the large coherency strain energy, transforming first to a twinned 9R structure (fcc with stacking faults) and then to 3R structure (a distorted fcc) and finally to the fcc structure (ε-Cu). The present results, together with previous research,14,15,16,17,18,19,20,21,22,23) suggests that Cu-rich precipitates were ε-Cu. Cu alloying in combination with appropriate heat treatment is used to produce a special class of antibacterial steels that have antimicrobial activity due to ε-Cu precipitation of on the steel surface.24)
The uniform dispersion of the ε-Cu nanoparticles provides stronger interactions and obstacles to the dislocation motion, thus resulting in an increase in the strength of the steel. The elongation and impact toughness of the steel did not experience an apparent decrease due to the particle size remaining extremely small (~30 nm). Therefore, the mechanical properties of 3Cr steel did not experience apparent change due to the decrease of Cr content.
4.2. Corrosion Behaviour 4.2.1. Corrosion Mechanism of 3Cr SteelIn this case, the corrosion process is controlled by the three following electrochemical anodic reactions, Eqs. (1), (2) that are supported by an cathodic reaction. Equation (3).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
In the solution system, the standard electrode potential of Reaction (2) is lower than that in Reaction (1). Consequently, continuously selective dissolution of Fe from the surfaces of 3Cr steel will result in an enrichment of Cr at the surface, indicating that a slightly protective Cr-rich surface scale (FeCr2O4) is formed, inducing a “transient passivation”. When the product of the concentrations of Fe2+, OH− and Cl− exceeds the critical value in the solution due to the dissolution and diffusion of Fe outward through the defects of FeCr2O4 scale in 3Cr steel, the Fe3O4 and FeCl2 precipitate on the top of the inner FeCr2O4 layer, forming the outer Fe3O4 and FeCl2 layer (see Figs. 7(a), 7(b)).
Chromium carbides precipitated in the microstructure of 3Cr steel can be clearly seen in Figs. 1(a)–1(c), once useful alloying element Cr is tied up as carbides, the ferrite matrix near carbides will deplete of Cr element. These regions, due to the absence or reduced presence of Cr, act as dissolution sites during the formation of the passive film, suggesting that preferential corrosion has possibly occurred in the vicinity of Cr-rich carbides, in particular in the vicinity of large Cr-rich carbides. Therefore, many small holes are observed clearly in 3Cr steel (see Fig. 7(c)) and a highly serrated interface between the inner layer and substrate are shown in Fig. 8(a).
4.2.2. The Role of Alloy Element CuThe anodic dissolution process of copper in NaCl solution is a complex process, which has been reported elsewhere.25,26,27) If the 3Cr steel contains Cu, other anodic reactions could occur:
| (7) |
| (8) |
| (9) |
In the first step, CuCl is formed as an insoluble product, and can be easily adsorbed on the steel surface. In the next step, the soluble CuCl2 is formed from the dissolution of the adsorbed CuCl products or of copper itself.
It has been suggested that at high concentrations ([Cl−]>0.3 mol dm−3), the soluble CuCl2 is hydrolysed to forma passive Cu2O layer.27,28) The hydrolysis reaction is shown as follows:
| (10) |
It is generally known that the chloride concentration is 0.6 mol dm−3 in 3.5 wt.% NaCl. So it is reasonable to assume that the corrosion of Cu-3Cr steel proceeded as above. Therefore, Cu2O was detected in the corrosion scale of Cu-3Cr steel. However, no CuCl is detected in the corrosion scale which could be due to relatively low CuCl content in the corrosion scale.
On the other hand, the corrosion potential of Cu is more positive than the ferrite matrix, Cu and the ferrite matrix will form a micro battery in the corrosion process, and the ferrite matrix near the Cu will accelerate the dissolution. The accelerated dissolution of the ferrite matrix, in particular Cr dissolved in ferrite matrix, can play a role in acceleration of the anodic polarization. Our polarization data show that Cu facilitated the onset of passivation, which could be considered a beneficial effect. This effect is connected with the presence of cathodic element of Cu dispersed in the ferrite matrix stimulating the anodic polarization of ferrite and enhancing the passivation process at the initial stage.24,29,30,31,32) However, the beneficial effect gradually weakens with increasing immersion time, because of the enough accumulation of protective corrosion products scale. Clearly, Cu demonstrates a positive effect in terms of enhancing the corrosion resistance of 3Cr steel, which is characterized by the low weight loss at the initial stage during immersion testing and a Cu-rich passive film on the steel surface.
Because of selective oxidation, the alloy elements Cr and Cu enriched in the inner layer, and these elements promoted the formation of compacted inner layer,33) which contacted the steel substrate and obstructed the corrosion mediums from penetrating to achieve further corrosion resistance of steel. The synergistic effect of Cr and Cu increased the corrosion resistance of steel due to the formation of a Cr- and Cu-rich layer. This excellent structure of inner layer maybe give a reason why Cu-3Cr steel, then even reducing Cr content, does not cause a reduction in the corrosion resistance compared to 3Cr steel.
Compared with the passive film, the presence of Cu2O in inner layer of Cu-3Cr steel should influence the composition and structure of passive film, indicating that preferential corrosion occurred in the vicinity of Cr-rich carbides has possibly been changed. In addition, the presence of the ε-phase Cu in the ferrite has affected the quality of the passive film. It is probable that more defects are introduced in the growing film due to the presence of dispersed inclusions of this phase, resulting in the increasing number of preferential corrosion sites. The preferential corrosion of 3Cr steel in limited sites, in particular in the vicinity of large Cr-rich carbides. In contrast, the preferential corrosion of Cu-3Cr steel initiates in several sites. As compared to Cu-3Cr steel, 3Cr steel exhibited a highly serrated interface between the inner layer and substrate.
3Cr steel will be widely used as construction materials in ocean oil exploitation because of good performance in terms of both cost and CO2 corrosion resistance. The microstructure, mechanical properties and corrosion resistance of Cu-modified 3Cr steel in 3.5 wt.% NaCl solution was investigated and this investigation can provide some valuable information to utilize the 3Cr steel in ocean oil exploitation. Specific conclusions are as follows:
(1) The mechanical properties of 3Cr steel, such as the strength, the elongation and impact toughness, did not experience obvious change due to Cu addition and Cr subtraction.
(2) Cu facilitated the onset of passivation, which could be considered a beneficial effect. This effect is connected with the presence of cathodic element of Cu dispersed in the ferrite matrix stimulating the anodic polarization of ferrite and enhancing the passivation process at the initial stage. However, the beneficial effect gradually weakens with increasing immersion time, because of the enough accumulation of protective corrosion products scale.
(3) As compared to Cu-3Cr steel, 3Cr steel exhibited a highly serrated interface between the inner layer and substrate.
(4) The reduced corrosion resistance of 3Cr steel caused by Cr decrease suitably offset by the presence of Cu, resulting a reduced-cost Cu-modified 3Cr steel without sacrificing ether its mechanical properties or corrosion resistance.
This research was supported by Shanghai Municipal Natural Science Foundation (no. 16ZR1414900).