2017 Volume 57 Issue 8 Pages 1461-1467
2017 Volume 57 Issue 8 Pages 1461-1467
Films of crystalline aluminum, titanium, and amorphous alloy prevent hydrogen entry into steel. Thermal spraying is a convenient method for producing coatings on metal substrates. However, as conventional thermal spray coatings have pores and cracks, they cannot prevent hydrogen entry sufficiently. In this study, with the use of new thermal spray guns that enable nitrogen gas cooling, aluminum-magnesium, titanium, amorphous coatings were fabricated and their anti-hydrogen-embrittlement effects were evaluated by a conventional strain rate tensile test. Passivated alumina film on the aluminum-magnesium coating, passivated chromium oxide film on the iron-chromium-based amorphous coating, and generated nitrogen titanium film on the titanium coating were expected to prevent hydrogen entry and hydrogen embrittlement.
Recently, to save energy and prevent global warming, hydrogen fuel cells for home appliances and automobiles have been developed. Hydrogen fuel cells have two benefits: no carbon dioxide gas emission and energy source with high transformation efficiency. To build hydrogen stations, metal parts are indispensable for sealing hydrogen gas for long durations. Hydrogen embrittlement is the phenomenon that the introduction of diffusible hydrogen lowers the strength, elongations and reduction area of metals.1,2,3,4,5,6,7) Japan Automobile Research Institute has standardized only type 316L stainless steel and type 6061-T6 aluminum alloy as anti-hydrogen embrittlement metallic materials. Because aluminum alloys and stainless steels have stable passivated films to prevent hydrogen entry, they are stronger than carbon steels with respect to hydrogen embrittlement. However, type 316L stainless steel and type 6061-T6 aluminum alloy are not high strength materials.
High strength carbon steels have been used as structural materials. When steels are exposed to atmospheric environments during service, they suffer some extent of aqueous corrosion. During the corrosion of steels in atmospheric environments, hydrogen atoms can be generated on the steel surface by one of the cathodic reactions. Some of the hydrogen atoms formed on the steel surface can be absorbed into the steel and interact with defects and traps, such as vacancies and dislocations, leading to hydrogen embrittlement. Delayed fracture of the high strength bolt is thought to be caused by diffusible hydrogen, which enters via the surface cathodic reactions.8,9,10,11,12,13,14) Thus, hydrogen entry from the environment should be considered. An anti-corrosion-material coatings on a high strength steel is expected to make it possible to prevent delayed fracture due to the prevention of hydrogen entry.
Thermal spraying is a convenient method for producing coatings on metal substrates. For example, the thermal spraying of aluminum-magnesium alloys has recently been utilized to coat marine structures because both aluminum hydroxide and magnesium hydroxide have strong anti-corrosion resistance.15,16) However, as convenient aluminum-magnesium thermal spray coating and titanium coating have many pores, cracks and oxide, it is not enough to prevent hydrogen entry. Furthermore, as conventional iron-chromium based amorphous alloys are fabricated using twin rolling method, it is not stable to coat materials.
In this study, new thermal spray guns that enable nitrogen gas cooling17,18) were used to fabricate aluminum-magnesium, titanium, iron-chromium based amorphous coatings. Their anti-hydrogen-embrittlement effects were evaluated by a conventional strain rate tensile test (Hereafter, the CSRT).19,20,21) Their anti-hydrogen-embrittlement mechanism was discussed from the viewpoints of reduction in pores, barrier effects by passivation films and hydrogen absorption effects.
In the case of a conventional thermal spraying gun, crystal solids are fabricated because of its low cooling rate. However, our original thermal spraying gun has a cooling rate of more than 106 K/s. Figure 1 shows a schematic illustration of the compact ultra-rapid cooling gas flame thermal spraying gun. To control the temperature of particles and the substrate using the outer cooling nitrogen gas, double cylindrical nozzles were developed and attached at the front of the thermal spraying gun. Thermal spray methods using the new gas flame thermal spray gun were explained below. First, alloy powder is melted in gas at a temperature of 2900 K. Then, the melted powder is cooled ultra-rapidly at a rate of more than 106 K/s and an amorphous alloy film is produced on the substrate. The oxidization of melting powders is prevented by introducing external cooled nitrogen gas and reducing the amounts of fuel gas and oxygen gas.

Schematic illustration of new gas flame thermal spray gun with ultra-rapid cooling.
Additionally, a new plasma thermal gun attaching double cylindrical nozzles was developed. Figure 2 shows a schematic illustration of the new plasma thermal gun with ultra-rapid cooling. This technology of the plasma thermal gun was the same as that of the gas flame gun. However, because powders are melted by the plasma energy and oxidization of melting powders is prevented by introducing external cooled nitrogen, the new plasma thermal sprayed films have almost no oxidization.
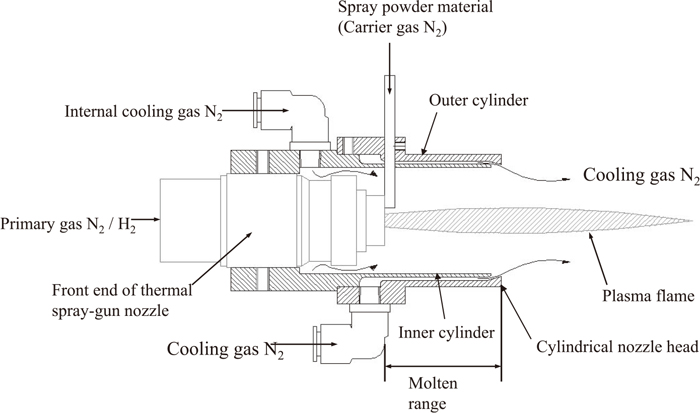
Schematic illustration of new plasma thermal spray gun with ultra rapid cooling.
In this study, aluminum – magnesium films and iron-chromium based amorphous alloy films were fabricated by the new gas flame thermal spray gun and titanium thermal spray films were fabricated by the new plasma thermal spray gun. Base substrate materials were SK85 carbon steels.
2.2. Films Production Methods Using New Thermal Spray Technologies 2.2.1. New Aluminum –Magnesium Thermal Spray FilmsAluminum magnesium alloy powders with a chemical composition of aluminum – 5 mass% magnesium (hereafter Al5Mg) was thermally sprayed on a steel substrate using the thermal spray gas flame gun with ultra-rapid cooling. Prior to the spraying, the steel substrate was blasted by alumina grids and a surface roughness Ra of 3.0 μm was achieved. A reducing flame was used during thermal spraying, where the ratio of acetylene gas to oxygen was 6 to 5. Formed films were investigated using a scanning electron microscopy (SEM) and a transmission electron microscopy (TEM).
Actual high strength bolts with new and conventional Al5Mg thermal spray coatings were investigated for their anti-hydrogen-embrittlement effects. Figure 3(a) shows anew aluminum magnesium coated high strength steel bolt of the 1.29 GPa class which we purchased on the market. Figure 3(b) shows a tool for stretching the bolt in the immersion test. Figure 4 shows a schematic illustration of the tightening bolt and tools, whose torque was 200 Nm. Cathodic hydrogen charge on the high strength bolt was carried out in 0.1 mol/L H2SO4 solution with 1.0 g/L NH4SCN for 120 hours. The current density was 10 A/m2. Using hydrogen charged high strength bolts, the CSRT was carried in the atmosphere. Stretching rate was 0.25 mm/s. The advantage of the CSRT is that it requires no special testing equipment and the testing time is much shorter than those of the previous slow strain rate test methods. The loading rate in the CSRT is similar to that in the conventional tensile test, so that the hydrogen diffusion during the test can be neglected, and it takes only a few minutes to finish the delayed fracture test. Fractured surfaces were observed by SEM.

(a) New aluminum magnesium coated high strength bolt and (b) stretching tool.
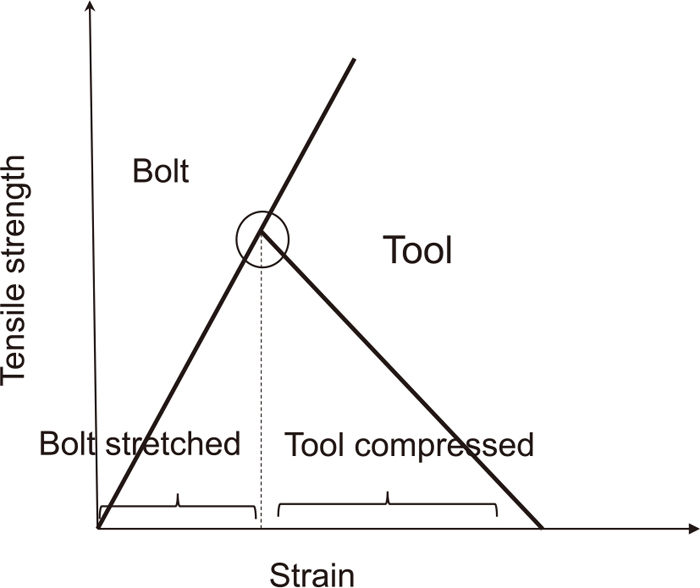
Schematic illustration of tightening bolt and tool.
Iron-chromium based alloy powders with a chemical composition of 70Fe-10Cr-13P-7C atom% was thermally sprayed on a steel plate using the gas flame thermal spray gun with ultra-rapid cooling. The steel substrate was blasted by alumina grids and a surface roughness Ra of 3.0 μm was achieved. A reducing flame was used during thermal spraying, where the ratio of acetylene gas to oxygen was 5 to 5. The cooling-gas flow rate can be controlled up to 60 m/s by adjusting the gas pressure in the cylindrical nozzle. Formed films were investigated by SEM and X-ray.
Anti-corrosionability and anti-hydrogen-embrittlement ability of iron-chromium based thermal sprayed coating was estimated. Measurement conditions are explained below. First, films were cleaned with acetone ultra-sonic waves in 5 min and deposited by 1 cm2. Then, after 30 min of nitrogen bubbling in 5 mass% NaCl solution at room temperature, natural polarization voltage was measured in 30 min. Next, the CSRT was carried out for iron-chromium amorphous alloy coated SK85 steel using a JIS-5 grade-size tensile specimen in the atmosphere. The thicknesses of SK85 steel and iron-chromium amorphous alloy were 3.2 mm and 200 μm, respectively. Hydrogen was introduced into the specimens by cathodic hydrogen charging in 0.1 mol/L H2SO4 with 1.0 g/L NH4SCN aqueous solution at the current density of 10 A/m2 for 48 hours prior to the CSRT. Fractured surfaces were observed by SEM.
2.2.3. New Plasma Sprayed Titanium FilmsTitanium powders were plasma sprayed on a steel plate using the plasma thermal spray gun with ultra-rapid cooling. The steel substrate was blasted by alumina grids and a surface roughness Ra of 3.0 μm was achieved. Titanium plasma thermal spraying conditions are explained in Table 1. Formed films were investigated by SEM and X-ray.
| Plasma | Voltage (V) | 40 |
| Current (A) | 600 | |
| Primary gas | Argon or Nitrogen | |
| Cooling | Flow of internal cooling nitrogen gas (L/min) | 600 |
| Flow of outer cooling nitrogen gas (L/min) | 600 | |
| Material | Powder | Titanium <45 μm |
Figure 5(a) shows a scanning electron microscope (SEM) of new Al5Mg alloy with a fine microstructure. Figure 5(b) shows SEM of conventional Al5Mg alloy using the conventional thermal spray gun. It can be seen that a new Al5Mg thermally sprayed coating has few pores than conventional Al5Mg. Figure 6 shows an image of passivation film, obtained with a transmission electron microscope (TEM) of the new Al5Mg film. It can also be seen that the new Al5Mg thermally sprayed coating has fine, less cracked passivation films. We assume that a partially amorphous phase in the Al5Mg film was produced as a result of thermal spraying with rapid cooling.22,23) Then, ultra-fine grains of Al5Mg alloy were generated from the amorphous phase owing to the recuperative heat provided by spraying.

(a) Scanning electron microscope images of new and (b) conventional aluminum 5 mass% magnesium (Al5Mg) thermal spray films.

Transmission electron microscope image of new aluminum- 5 mass% magnesium thermal spraying passivation film.
Figure 7 shows the results of elongation measurements of the new Al5Mg coated and bared 1.29 GPa class high strength bolts. Because the elongation of the coated bolt was greater than that of the naked bolt, less hydrogen embrittlement occurred in the coated bolt than in the naked bolt. Figure 8 shows SEM images of fractured surfaces of high strength bolts with and without thermal spray coating after hydrogen charging and stretching. It can be seen that both had intergranular fractures.

Load-distance curves of coated and naked high strength bolts.

SEM images of fractured high strength bolts after hydrogen charging and stretching.
Figure 9 shows an iron-chromium amorphous thermal spray film prepared using the ultra-rapid cooling thermal spraying gun on the steel substrate and a halo peak of X-ray analysis proved to be amorphous. Figure 10 shows the result of natural polarization measurement. A quickly repaired passivate film of an iron-chromium amorphous alloy was seen. Figure 11 shows the results of elongation measurements of as as-rolledSK85, and the hydrogen charged bared and amorphous coated SK85. The elongation of the amorphous alloy coated SK85 was higher than that of the bared SK85 and almost as same as that of the as-rolled one. Figure 12 shows SEM images of fracture surfaces of bared and coated SK85 after hydrogen charging and stretching.

SEM images of iron-chromium amorphous film and X ray analysis result.

Natural polarization result of iron-chromium amorphous film.
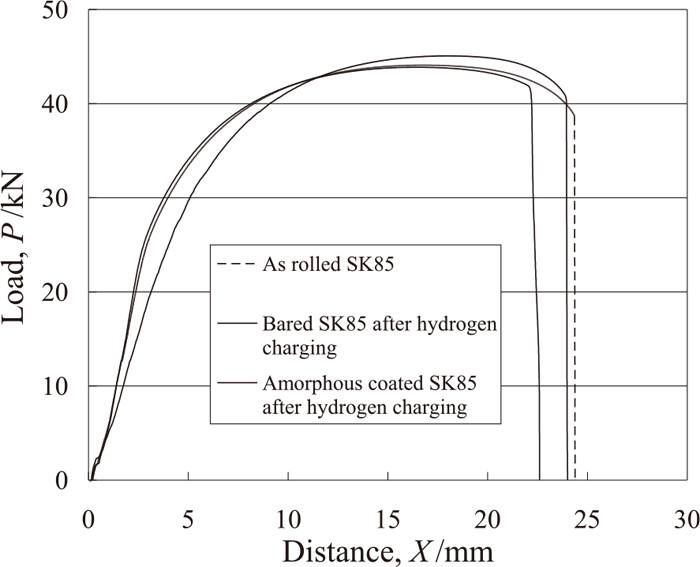
Load-distance curves of amorphous coated SK85 after hydrogen charging.

Fracture surfaces of hydrogen charged and stretched SK85.
Two types of primary plasma gas were used such as argon or nitrogen in Table 1. Figure 13 shows a SEM image of titanium plasma thermal sprayed film using argon gas with fine microstructure. Figure 14 shows an X ray result of the film after hydrogen charging whose conditions were mentioned in chapter 2.2.2. It was observed that titanium hydride was produced in the film.

SEM image of titanium plasma sprayed films using argon gas.
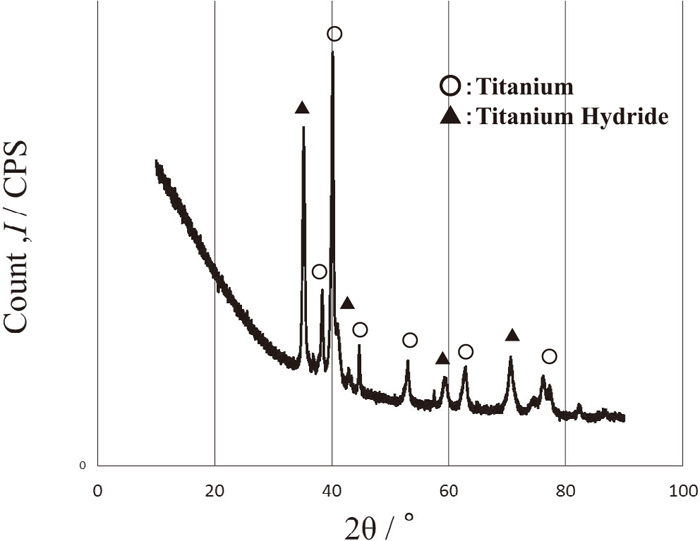
X ray result of hydrogen charged titanium plasma sprayed films using argon gas.
Figure 15 shows a SEM image of titanium plasma thermal sprayed film using nitrogen gas with fine microstructure. X ray analysis results of as-sprayed and hydrogen charged films were shown in Figs. 16 and 17, respectively. It can be seen that no hydride was formed after hydrogen charging.
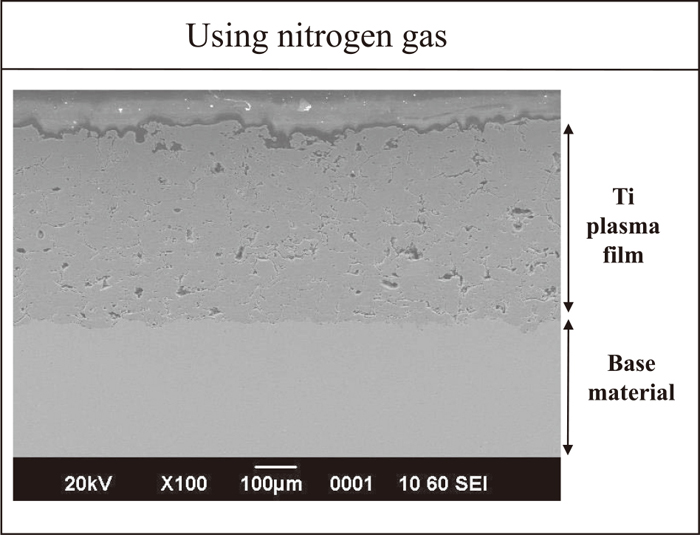
SEM image of titanium plasma sprayed films using nitrogen gas.
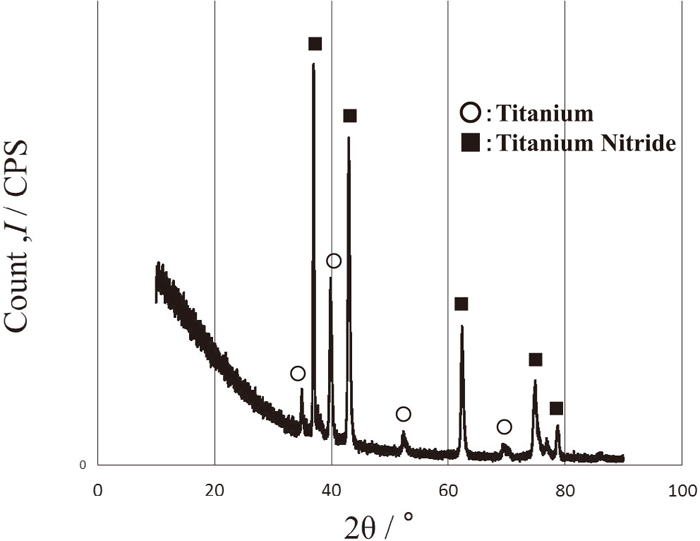
X ray result of titanium plasma sprayed films using nitrogen gas.

X ray result of hydrogen charged titanium plasma sprayed films using nitrogen gas.
New Al5Mg thermal spray film has a fine microstructure with few pores as shown in Fig. 5(a). Additionally, little oxidization is also expected to be obtained.24,25) Because the elongation of the coated bolt was greater than that of the naked bolt, it was confirmed that less hydrogen embrittlement occurred in the coated bolt than in the naked bolt as shown in Fig. 7.
The mechanism of the prevention of hydrogen-embrittlement by the Al5Mg alloy films was discussed as follows. Al5Mg alloy films prepared with the new thermal spray gun using nitrogen gas cooling had a partially submicron microstructure because residual strains induced by rapid cooling acted as solid nuclei.26) It was considered in another report that the decrease in grain size leads to less hydrogen embrittlement.27) Additionally, it was reported that the anti-corrosion ability of Al5Mg alloy films was high.26) Thus, we consider that the fine Al5Mg structure had a strong passive layer. It is well-known that preventing corrosion and hydrogen permeation, oxides of metals, including steel, reduce the permeability of hydrogen and its isotopes by at least one order of magnitude. It was also reported that in laboratory experiments, aluminum enhanced the reduction of permeation.28) We guess that fine microstructure, passive layer and aluminum included in the new Al5Mg films, prevented hydrogen embrittlement due to the suppression of hydrogen permeation.
4.2. Prevention of Hydrogen Embrittlement by Iron-chromium Based Amorphous Alloy Thermal Spray FilmsIron-chromium coatings using the new gas flame thermal spray gun were confirmed to be amorphous as shown in Fig. 9. The elongation of the amorphous alloy coated SK85 was higher than that of the bared SK85 as shown in Fig. 11. We guess that iron-chromium amorphous coatings prevented hydrogen embrittlement by both hydrogen barrier effect in passiavtion films and solid solubility of hydrogen in amorphous alloy as described below.
At first, the mechanism of anti-hydrogen-embrittlement properties of the iron-chromium based amorphous alloy was discussed from the viewpoint of barrier effect in passivation films. According to the polarization measurement shown in Fig. 10, the iron-chromium amorphous alloy was considerably more stable than a crystallized alloy with the same composition. It is generally reported that amorphous iron-chromium alloys have superior anti-corrosion ability.29) This extremely high corrosion resistance of the amorphous iron alloys containing 8 at% or more chromium has been interpreted in terms of the rapid formation of thick, uniform, highly corrosion resistant passive film because of the characteristics of the amorphous alloys. When amorphous alloy coatings on the steel were used to prevent corrosion, the passive film formed on the amorphous alloy consists mainly of hydrated chromium oxyhydroxide, which is a common major constituent of stable passive films on ordinary stainless steels.30,31,32,33) Thus, an amorphous alloy coating on high strength steel is expected to prevent hydrogen embrittlement due to the similar mechanism to the suppression of corrosion.
Secondly, the beneficial effect of the iron-chromium based amorphous alloy was discussed based on solid solubility of hydrogen. In conventional crystalline materials, hydrogen dissolves principally in the tetrahedral interstitial sites in body centered cubic structures and in the octahedral interstitial sites in face centered cubic structures. The defects in the metallic microstructure act as hydrogen traps and result in a significant reduction in hydrogen mobility since hydrogen tends to diffuse to defects and becomes bonded to them. The elevated cooling rates utilized during the fabrication of amorphous metals generate innumerable defects of different potentials energies in the metallic structure.34) Such defects, generated by rapid cooling, contributes to a large increase in the solid solubility of hydrogen and a reduction in its diffusivity. The hydrogen diffusion coefficient of the iron-based amorphous alloys is several orders of magnitude lower than that of carbon steel. The lower diffusivity of hydrogen in the amorphous alloy is expected to suppress hydrogen permeation. The amorphous alloys dissolve greater amounts of hydrogen in the solid solution and this phenomenon may improve anti-hydrogen-embrittlement properties.
4.3. Prevention of Hydrogen Embrittlement by Plasma Sprayed Titanium FilmsTitanium nitride formation in the new plasma thermal sprayed titanium films was confirmed by X-ray analysis as shown in Fig. 16. Titanium nitride thin films were obtained by new plasma-spraying techniques with reaction between molten titanium and nitrogen gas.35,36,37,38,39,40,41,42,43) Thus, we guess that the new plasma thermal spray coatings prevented hydrogen embrittlement due to hydrogen barrier effect by titanium nitride.
The hydrogen permeation inefficient barrier layers would be controlled by surface rather than bulk diffusion. Nitrogen implantation significantly affected hydrogen absorption in titanium and this effect was caused by the chemical transformation of the near surface layer to titanium nitride. Hydrogen migration in titanium nitride coatings is sufficiently slow that they are promising candidates for hydrogen permeation barriers at such a small thickness.
Estimated mechanism of anti-hydrogen-embrittlement properties using the new thermal spray technologies is summarized in Table 2. The beneficial mechanism is categorized into barrier effect and absorption effect. Bulks and coatings have same effects on anti-hydrogen-embrittlement properties. Barrier layers on the steel, for example, Cr2O3, Al2O3 and TiN, prevent hydrogen entry due to the suppression of hydrogen permeation. Absorption mechanism has two types. One is formation of hydrogenide, such as TiH3. The hydride formation is expected to have storage effect of hydrogen in it. The other is store of hydrogen in tetrahedral-site and octahedral-site of crystalline, random site of amorphous alloy. The storage effect is expected to lead to hydrogen trap or reduction in hydrogen diffusivity.
| Chemical Reaction | Morphology | Material | Principle |
|---|---|---|---|
| Barrier | Bulk/Coating | Al–Mg | Passivation film (Cr2O3, Al2O3, TiN) |
| SUS | |||
| Ti | |||
| Fe–Cr Amorphous | |||
| Absorption | Ti | Hydrogenide alloy (TiH3, MgH2, ZrH3) | |
| Mg | |||
| Zr | |||
| FCC | Tetra-site, Octa-site, Gap of random atoms | ||
| BCC | |||
| Amorphous |
Note: Bold styles in the table are shown in this research.
Using nitrogen cooling method with double cylindrical nozzles, the new thermal spray technologies were developed. Anti-hydrogen-embrittlement properties of three types of films produced by the new process were investigated. Thermal spray coatings with ultra-rapid cooling such as aluminum – 5 mass% magnesium, iron-chromium based amorphous and titanium are all formidable barriers to the permeation of hydrogen. It was confirmed that the thermal spray coatings prevented hydrogen embrittlement. The main mechanism of the beneficial effect of the thermally sprayed films with ultra-rapid cooling is considered to have fewer defect than conventionally sprayed films, barrier effect by passivation films and hydrogen storage effect.