2017 Volume 57 Issue 8 Pages 1410-1419
2017 Volume 57 Issue 8 Pages 1410-1419
Kinetics of slag/metal reaction were investigated experimentally using 50 kg electroslag remelting (ESR) furnace in order to clarify the effect of slag containing different CaO and TiO2 content on titanium, aluminum and silicon content during the ESR process with 1Cr21Ni5Ti stainless steel. The results obtained show that the more CaO content in slag is, the more aluminum content in ingots increases. The lg([Al]4/[Ti]3) increases with the increase of slag temperature in the first 13 minutes during the ESR process, and after the slag temperature tends to be stable, the lg([Al]4/[Ti]3) decreases with the increase of TiO2 content in the rest process. The penetration and film theories were employed to analyze the rate determining step of slag/metal reaction, and it was found that the rate determining step of the reaction was the mass transfer of aluminum through the molten steel, silica through the slag and titanium on both of the metal and slag sides. The slag containing low CaO content combined with extra titania constantly added into molten slag in the first temperature-rising period is suitable for electroslag remelting of 1Cr21Ni5Ti stainless steel with high titanium and low aluminum content.
During the electroslag remelting (ESR) with steel containing active alloying elements, the macrosegregation of aluminum, titanium and silicon content along the axial direction of ingot has not been resolved well till today.1,2) Not only the reactions among Al+Al2O3, Si+SiO2 and Ti+TiO2 systems but also the oxidation reactions caused by ferrous oxide, influence the alloying element content, which makes the active alloying element control difficultly. Therefore, it is particularly urgent to provide reliable active alloying element in ingot control technology based on thermodynamic and kinetic analysis during the ESR process.
Until today, research on the macrosegregation of aluminum, titanium and silicon in stainless steel with high titanium and low aluminum content during the ESR process is still continuing, and thermodynamic study of the subject3,4,5,6,7,8,9) and reports on the modeling of the chemical reactions occurring during ESR process10,11) have been published. Hou3,4) studied the effect of slag on titanium, silicon and aluminum content in superalloy based on slag-metal interaction experiment in a resistant furnace, and illustrated that the CaO has large effect on the activities of Al2O3, TiO2 and SiO2 based on thermodynamics. Cheng6) investigated the relationship between titanium and aluminum based on the experiments and thermodynamic equilibrium, by which the TiO2 content was measured in order to make the titanium uniform from top to bottom in ingot during the electroslag remelting process. Nonetheless, these study focused on the slag designing by thermodynamic equilibrium at a fixed temperature, and do not reveal the effect of slag on the change of alloying element along the height of ingot. Schwerdtfeger10) investigated the mechanism of mass transfer occurring in electroslag remelting process, and illustrated the details of processes controlling solute redistribution during the remelting of stainless steel quantitatively. However, the model neglected the change of temperature at the beginning of ESR process, and did not provide an effective way to improve the homogeneity of composition.
As the ESR is a mass transfer process among multiphase reaction system under dynamic conditions of temperature, slag composition and oxygen increment in slag, study of the effect of the each component in the slag consisting of CaO–CaF2–Al2O3–SiO2–TiO2–MgO–FeO on the concentrations of the alloying elements involved in ingot has been insufficient till now. Because of this, kinetics of slag/metal reaction were investigated experimentally using 50 kg electroslag remelting furnace to clarify the effect of CaO and TiO2 content on the concentrations of the aluminum, titanium and silicon in 1Cr21Ni5Ti stainless steel ingot. The present research is expected to provide active alloying elements control technology during the ESR of steel/alloy with high titanium and low aluminum content. Based on the mass transfer model established in Part 1,11) the experiments were carried out by controlling the slag composition and remelting process to improve the macrosegregation of alloying elements from the bottom to top of ingot in this Part 2.
Three experimental heats were carried out using a 50-kg scale ESR furnace under argon atmosphere. The consumable electrode material used in each heat was produced by a vacuum induction furnace and its chemical composition is listed in Table 1. The oxide layer of electrode with a shape of 60 mm in diameter and 2000 mm in length was eliminated mechanically before electroslag remelting. The electroslag remelting equipment is shown in Fig. 1. The inner diameter of water cooled copper mold is 134 mm.
| C | Si | Mn | Cr | Ni | Al | Ti |
|---|---|---|---|---|---|---|
| 0.11 | 0.66 | 0.58 | 20.72 | 5.22 | 0.03 | 0.66 |

Electroslag remelting equipment: (a) Equipment image, and (b) Schematic diagram.
According to the previous study,3) laboratory experiments were carried out with three kinds of mixtures. The slag in each heat was roasted at 873 K (600°C) in a dry box for at least 4 hours to remove the moisture in the slag before ESR experiment. About 3.2 kg of slag mixtures was used in each experiment by solid slag starting technique. In each heat, the input current, voltage, and outlet temperature of the mold cooling water were kept at about 3000 A, 38 V, and 298 K (25°C) during ESR process, respectively. The remelting rate is approximately 66 kg·h−1 during the process. In experimental A (with slag S1), B (with slag S2) and C (with slag S3), high purity argon gas was introduced into ESR equipment at a gas flow rate of 80 Nl min−1. In experimental C, the extra 200 g TiO2 was continuously added into molten slag in the first 13 minutes, and 20 g deoxidizer aluminum was added into molten slag during the ESR process.
In order to investigate the effect of slag on the chemical composition of ingots during the ESR, the silicon and titanium contents from bottom to the top of the ingots in each experiment were analyzed by direct reading spectrometry, the content of soluble aluminum from bottom to the top of the ingots was determined by the inductively coupled plasma-mass spectroscopy (ICP-MS). Slag samples were taken from the molten slag in the water cooled copper mold using the quartz tube at corresponding height in remelted ingot. The chemical compositions of slag samples at 5 cm height of remelted ingot in each experiment are listed in Table 2.
| Slag | CaF2 | CaO | Al2O3 | TiO2 | SiO2 | MgO | |
|---|---|---|---|---|---|---|---|
| S1 | 63.05 | 4.80 | 17.81 | 4.41 | 1.07 | 8.86 | −2.33 |
| S2 | 54.87 | 16.83 | 17.56 | 4.36 | 1.26 | 5.12 | −2.38 |
| S3 | 55.25 | 4.92 | 19.91 | 8.93 | 1.32 | 9.67 | −1.57 |
It is important to have exact knowledge about the γ values of TiO2 and Al2O3 in ESR slag to control Ti and Al contents in ESR ingots. As stated in the Part 1,11) the γ values of TiO2 and Al2O3 have to be reassessed appropriately.
3.1.1. Thermodynamic Analysis of the Experimental ResultsEquations (1) and (2)8) are employed to investigate the experimental results in order to better understand the effect of slag and remelting process on the change of aluminum, titanium and silicon element concentration in ingot.
| (1) |
| (2) |
| (3) |
| (4) |
Where
Thus, the
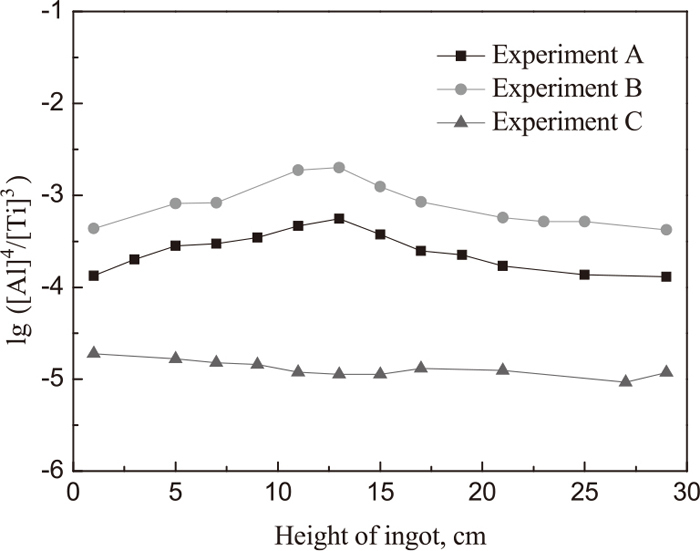
Change of lg([Al]4/[Ti]3) with height of ingot in each heat.
As shown in Fig. 2, both the slag S1 and S2 having the rising trend of lg([Al]4/[Ti]3) in the first 13 cm height of remelted ingot means that the temperature of slag is continuously elevated (assuming from 1750 K to 1950 K according to Part 111)) at the beginning of the ESR process, which makes the lg K(Ti, Al) tend to increase according to the Eq. (2). After the temperature reaches 1950 K (over 13 cm height of remelted ingot), both the slag S1 and S2 have the decreasing trend of lg([Al]4/[Ti]3) in the rest ingot because of the increase of TiO2 that makes the
Based on the analysis above, more TiO2 should be added into slag S1 to prevent titanium loss caused by the reaction of titanium with alumina, such as slag S3. In addition, as the lg K(Ti, Al) in Eq. (2) increases with the increase of slag temperature in the first 13 minutes during the ESR process, the extra TiO2 should be continuously added into the molten slag (the value is the difference between the TiO2 content in slag calculated at 1950 K and 1750 K) in the first 13 minutes during the ESR process, to promote the thermodynamics equilibrium between the Al+Al2O3 and Ti+TiO2 system, which was carried out in experimental C.
3.1.2. Effect of Titania on Activity Coefficients of Titania and Alumina in SlagBased on the Eqs. (40)–(47) in Part 1,11) the

Change of the
Based on the Eqs. (40)–(47) in Part 1,11) the influence of TiO2 content on activity coefficients of Al2O3, TiO2 and FeO were calculated by using the slag (the ratio of each component is, CaF2: CaO: Al2O3: MgO: SiO2: FeO: TiO2 = 62: 5: 20: 10: 1: 0.1: X) at the temperature of 1850 K and 1950 K (1577°C and 1677°C), as shown in Fig. 4(a). Then the change of

Change of the activity coefficients with TiO2 content in slag.
In order to determine the addition of TiO2 into slag during the ESR process, three experimental heats of interaction between slag and metal in a MoSi2 resistance furnace were carried out with 500 g 1Cr21Ni5Ti stainless steel and 120 g slag mixtures at the temperature of 1850 K (1577°C), and the slag composition is listed in Table 3. In each heat, the aluminum particles were added into slag mixtures before the experiment, and the addition of aluminum particles is listed in Table 3. The details of the experimental process can be seen in the previous study.3)
| Slag | CaF2 | CaO | SiO2 | Al2O3 | TiO2 | MgO | Al added |
|---|---|---|---|---|---|---|---|
| T1 | 62.5 | 5 | 0.5 | 20 | 2 | 10 | 0.5 g |
| T2 | 59.5 | 5 | 0.5 | 20 | 5 | 10 | 0.2 g |
| T3 | 54.5 | 5 | 0.5 | 20 | 10 | 10 | 1.3 g |
Steel samples No.1 through No.4 were taken from the molten steel after a prescribed amount of slag listed in Table 3 was put onto the surface of the molten metal for 15, 25, 35 and 45 minutes, respectively. Slag sample was taken from the molten slag after the slag had melted for 45 minute. Then the contents of titanium and soluble aluminum in each steel sample were determined, and the results of steel samples were shown in Fig. 5. It is clear that the reaction between slag and metal nearly reaches the thermodynamic equilibrium at 45 minute. Thus, the contents of TiO2 and Al2O3 in slag, and the Al, Ti in steel samples at 45 minute in each experiment were used to determine the TiO2 content in Fig. 6.

Change of aluminum and titanium content in steel with time after each slag addition.
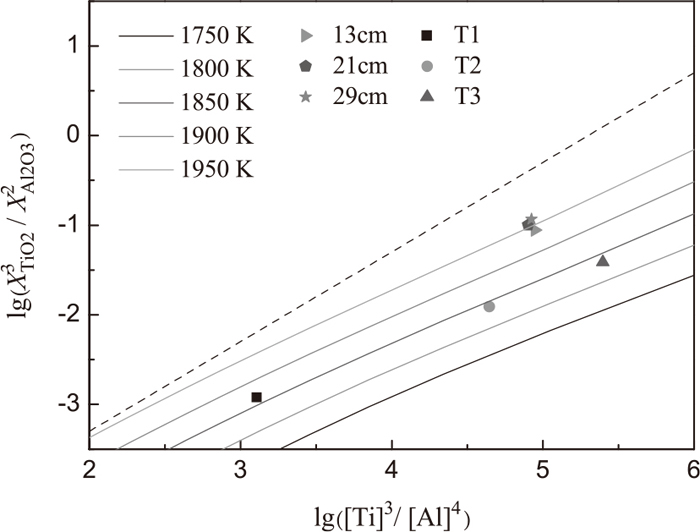
Change of the
Based on the Eqs. (40)–(47) in Part 111) and Eq. (2), the TiO2 content in slag (the ratio of each component is, CaF2: CaO: Al2O3: SiO2: MgO: TiO2 = 62: 5: 20: 1: 10: X) used for remelting of stainless steel with different titanium and aluminum contents can be calculated, and the change of
Above all, the TiO2 content in initial slag during the ESR experimental C can be determined, and the extra addition of TiO2 in the first 13 minutes can also be acquired. Thus, in experimental C, the concentration of aluminum and titanium along the axial direction of ingot was uniform, and the change of lg([Al]4/[Ti]3) with height in ingot is close to the straight line, as shown in Fig. 2. It also can be concluded that the reaction 3[Ti] + 2(Al2O3) = 4[Al] + 3(TiO2) at the interface of metal pool/slag in Exp.C is nearly close to the thermodynamic equilibrium. As shown in Fig. 6, the experimental points at 13, 21, 29 cm height of Exp.C ingot located at the line of 1950 K (1677°C) provide the best proof that the temperature of the reactions at the interface of metal pool/slag is 1950 K (1677°C), which was estimated in Part 1.11)
3.2. Concentration Change of Al, Ti and Si 3.2.1. Experimental A and BFrom the results described above, it was understood that the concentrations of the elements vary from the bottom to the top of the ingot are influenced by the slag composition and remelting process, and this process is also an exchange reaction among Al+Al2O3, Si+SiO2, Ti+TiO2 and Fe+FeO systems. Thus, the mass transfer model in Part 111) is employed to investigate the details of the processes controlling solute redistribution in the molten metal, and estimate the rate-determining step in these respective element processes.
Experience has shown that during the ESR process iron oxide is continuously added into the slag due to the consumable electrode oxidation with air and oxygen transfer in the air/slag/metal.12) If assuming the remelting rate during the whole ESR process is 66 kg/h, the area of the electrode tip and volume/reaction time Vm/te can be taken as 36.91 cm2 and 2.55 cm3/s, respectively, according to Eqs. (52) and (53) in the Part 1.11) The increment of iron oxide (IFeO) during the ESR process can be expressed by the following Eq. (5), and the change of ferrous oxide increment with time can be acquired, as shown in Fig. 7.
| (5) |

Change of ferrous oxide mass increment with time during the ESR process.
Where: Wm is the mass remelting rate; Mi is the molar mass of i element.
As can be seen in Figs. 7(a) (Experimental A) and 7(b) (Experimental B), the increment of iron oxide (IFeO) in the first 780 seconds (13 cm height of the ingot) during the ESR process can be expressed by ellipse Eq. (6) in order to obtain the smooth curve. After the 780 second, the tangent line of ellipse is employed to illustrate the stable increment of iron oxide.
| (6) |
Where: Ttime is the remelting time in the ESR process, s.
Same as the iron oxide, the increment of slag temperature (TSlag) at the interface of droplet metal/slag and pool metal/slag in the first 780 seconds (13 cm height of the ingot) can be expressed by ellipse Eq. (7) to obtain the smooth curve. If assuming that the approximate average depth of the cylinder hr in metal pool is 15 mm, the depth of the arc he is 40 mm, and the radius of crystallizer RC is 67 mm, the area and volume of the molten metal pool can be calculated as 141.03 cm2 and 399.57 cm3, respectively. Thus, the change of the molten metal pool volume (VPool) with time can be determined by ellipse Eq. (8), as shown in Fig. 8.
| (7) |
| (8) |

Change of metal pool volume and temperature with time during the ESR process.
Based on the analysis above, the mass-transfer model of oxidation of alloying elements during ESR of stainless steel has been established. Figures 9, 10, 11 show the observed results of the change of the Ti, Al and Si content with height of the ingots in experimental A and B, in comparison with the results calculated based on the model described above. In Figs. 9, 10, 11 the experimentally determined concentration curves are shown together with the calculated results.

Concentration of titanium in ingot, and of titania oxide in slag for experimental A and B.

Concentration of aluminum in ingot, and of alumina in slag for experimental A and B.
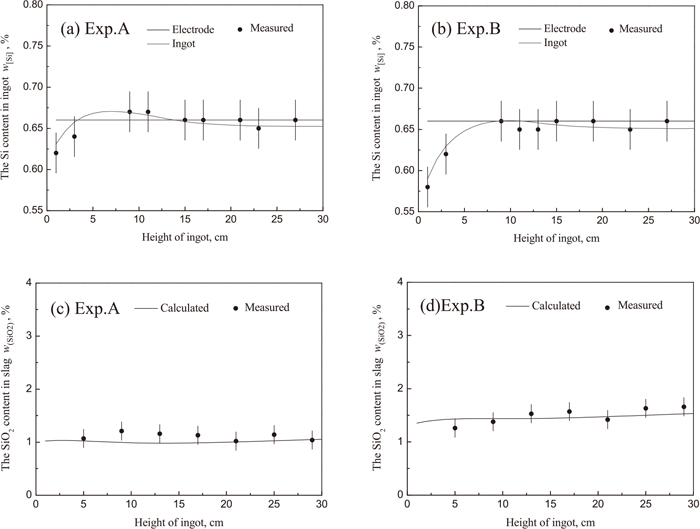
Concentration of silicon in ingot, and of silica oxide in slag for experimental A and B.
As shown in Fig. 9(a), in experimental A the oxidation of titanium has occurred to a less extent compared to that in the experimental B (Fig. 9(b)). While in experimental A the increase of aluminum content (Fig. 10(a)) is less than that in experiment B (Fig. 10(b)). Compared slag S1 with S2, even the
As can be seen in Figs. 10(a) and 10(b), both in experimental A and B the aluminum content in ingot have the rising trend in the first 13 cm height of ingot, while after reaching the peak value at the 13 cm height of ingot, the aluminum content have the downward trend in the rest ingot. As the temperature increases in the first 13 cm height of ingot, the lg K(Ti, Al) in Eq. (2) increases, which makes the aluminum content has the rising trend. While the temperature and lg K(Ti, Al) in Eq. (2) are stable after the 13 cm height, the aluminum content decreases with the increase of TiO2 content in slag.
As shown in Fig. 11(d), even the slag S2 has higher silica content than slag S1 (Fig. 11(c)), the silicon content of ingot in experimental B (Fig. 11(b)) is less than that in the experimental A (Fig. 11(a)). According to the previous study,4) the CaO has a great effect on the activities of TiO2, SiO2 and Al2O3. With the increase of CaO mass fraction in slag, the activity coefficient of SiO2 decreases significantly, whereas, slightly change happens for Al2O3 and TiO2. Thus, in order to prevent the losses of aluminum and titanium caused by reaction with the silica of the ESR slag, the slag with low CaO (slag S1) should decrease the SiO2 content in slag. Especially for remelting steel and/or alloy with high titanium, low aluminum and low silicon content, not only should the slag have the low CaO content, but also decrease the silica content to a very low level.
It also can be concluded that at the beginning of the ESR process, the silicon content increases with the decrease of ferrous oxide shown in Fig. 7(a), and during the 4–12 cm height of ingot the silicon content in ingot being larger than that in electrode according to the Eq. (9) combined with the large increase of silica content in slag in the first 4 cm height of ingot. After 12 cm height of ingot, the silicon content decreases as the ferrous oxide at metal-slag interface increases, as shown in Fig. 12(a). At last, the Eq. (9) reaches the thermodynamic equilibrium in the rest process, both silicon and titanium content have the rising trend close to chemical composition in electrode according to the Eqs. (10), (11), (12), (13).
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |

Concentration of ferrous oxide at metal-slag interface for experimental A and B.
Based on the analysis above, the molar volume of FeO at the slag-metal interface during the molten metal pool
Both in experimental A and B, the titanium in electrode plays a role of deoxidizer to decrease the ferrous oxide in slag. As shown in Fig. 4, the
Then, the mass transfer resistance of the respective components on the metal side and slag side at the molten metal pool-slag in experimental A and B can be obtained using Eqs. (23)–(26) in Part 1,11) as shown in Table 4, Figs. 13(a) (Experimental A) and 13(b) (Experimental B). From this table,13) it can be inferred that the chemical reaction Eq. (1) in Part 1, which is due to slag-metal reactions, is the rate-determining steps of mass transfer on the metal side, the reaction in Eq. (2) in Part 1 is rate-determining for mass transfer on the slag side, and the reaction in Eq. (3) in Part 1 is rate-determining for mass transfer on both of the metal and slag sides.
| Mass transfer Resistance | Each element in molten steel | SiO2 In molten slag | AlO1.5 in molten slag | TiO2 in molten slag |
|---|---|---|---|---|
| Expression |

Mass transfer resistances of each component in metal and slag.
Thus, the mass transfer of aluminum can be expressed by Eq. (14), and the
| (14) |
| (15) |
| (16) |
It also can be seen from Fig. 4 that the
The agreement of the simulation created in the Part 111) with the measured values proves that the values cited from the previous studies, including the reaction time td, droplet velocity and slag velocity at the droplet, are quite valid.
3.2.2. Experimental CIt is the final goal to produce an ESR ingot with a homogeneous distribution especially in Al and Ti contents. Based on the mass transfer model established in Part 1,11) the experimental results of experimental C were investigated, as shown in Figs. 14(a) and 14(c), 15(a) and 15(c), 16(a) and 16(c), respectively. In order to investigate the effect of TiO2 content in the initial slag on the concentration of each alloying element, the slag SS1 and SS2 (the TiO2 content in each slag are calculated by thermodynamic equilibrium at 1750 K and 1950 K, respectively) listed in Table 5 were investigated by mass transfer model under the condition of no TiO2 added into molten slag during the remelting process, as shown in Figs. 14(b) and 14(c), 15(b) and 15(d), 16(b) and 16(d), respectively.

Concentration of titanium in ingot, and of titania oxide in slag for experimental C, SS1 and SS2.

Concentration of aluminum in ingot, and of alumina oxide in slag for experimental C, SS1 and SS2.

Concentration of silicon in ingot, and of silica oxide in slag for experimental C, SS1 and SS2.
| Slag | CaF2 | CaO | Al2O3 | TiO2 | MgO |
|---|---|---|---|---|---|
| SS1 | 57 | 5 | 20 | 8 | 10 |
| SS2 | 49 | 5 | 20 | 16 | 10 |
As shown in Figs. 14(a) and 15(a), the macrosegregation of aluminum and titanium content along the axial direction of ingot in Experimental C are largely improved compared with the ingots in Experimental A and B. Aluminum content in ingot ranges from 0.036 wt% to 0.048 wt%, and titanium content in ingot ranges from 0.53 wt% to 0.63 wt%, respectively. And the change of TiO2 content in slag with height caused by extra addition of TiO2 during the remelting process is shown in Fig. 14(c).
At the beginning of the ESR process, the titanium and silicon was reduced by Eqs. (11) and (13), as shown in Figs. 14(a) and 16(a). As the temperature and TiO2 content in slag increase, the silicon content in ingot decreases in the first 5 minutes, and after reaching the valley value, silicon content has the rising trend to chemical composition in electrode.
As shown in Figs. 15(a) and 15(b), the aluminum content in experimental C is homogeneous along the height of ingot compared with that in traditional experimental SS1 and SS2, which illustrates the superiority of the remelting process by adding extra TiO2 into molten slag in the first temperature-rising period.
According to the previous study,3) several kinds of slag containing different CaO content combined with steel samples of 1Cr21Ni5Ti were employed to illustrate the effect of CaO on
In order to clarify the effect of slag containing different CaO and TiO2 content on titanium and aluminum content in ESR ingots, kinetics of slag/metal reaction were investigated experimentally using a 50 kg ESR furnace. The results were presented as follows.
(1) The lower CaO in slag is, the smaller numerical value of lg([Al]4/[Ti]3) in ingot is. The lg([Al]4/[Ti]3) would increase with the increase of slag temperature at the beginning of the ESR process, while decrease with the increase of TiO2 content after the temperature reaches stable. Slag with low CaO content is suitable for 1Cr21Ni5Ti stainless steel electroslag remelting.
(2) The value of
(3) The mass transfer model based on penetration and film theories shows good agreement with the experiments. The rate determining step of the reaction was the mass transfer of alumina through the molten steel, silica through the slag and titania on both of the metal and slag sides.
(4) The
(5) The results of experimental C show that the appropriate slag combined with extra TiO2 added into molten slag in the first temperature-rising period is applied to effectively control alloying elements content during the ESR process.
This project is supported by Joint Research Fund of National Nature Science Foundation of China and Baosteel Group Corporation with the grant No. U1560203, and it is also supported by the National Nature Science Foundation of China with the grant No. 51274266.