Abstract
The preparation of coating material of welding electrode from marine placer via carbothermic reduction was systematically investigated. Four stages were observed in the carbothermic reduction of marine placer–coal mixture. The release of the volatiles in coal occurred at the first stage (≤600°C), and the solid–solid reduction and gas–solid reduction occurred at the second (600–960°C) and third stages (960–1350°C), respectively. The reduction rate of iron oxide at the third stage was higher than that at the second stage because of the high carbon gasification rate. A slowdown in the total mass loss at approximately 1350°C occurred at the fourth stage. X-ray diffraction patterns show that the reduction process of the marine placer exceeded 1100°C and can be written as: FeTiO3→FeTi2O5→Fe+TiO2. The quality index of the reduced ilmenite was evaluated in terms of the TiO2 and FeO content. The TiO2 content gradually increased as the reduction temperature increased from 1000°C to 1300°C, and the reduction time exerted lesser influence than temperature. The FeO content initially decreased rapidly and then exhibited a downward trend that became slower after 120 min with increasing reduction time. The TiO2 index could be achieved easily because of the high TiO2 content of the raw material. The FeO content could reach 8.45% after reduction at 1300°C for 180 min, which exceeded the technical requirement of 9%.
1. Introduction
Titanium dioxide is the main component of electrode covering, which plays an important role in slagging fluxes, diluents and stabilizers on welding electrodes.1,2,3) Coating material of welding electrode made by titanium dioxide exhibits excellent performance in its mechanical property, arc stability, and viscosity.4,5) Titanium dioxide can prevent the molten metal from being invaded by oxygen in the welding process and decreases its strength and plasticity.6,7) Therefore, titanium dioxide is a key material in preparing coating material of welding electrode.
The standard composition of coating material (YB/T 5141-93) is TiO2 (≥52%), Fe (≤9%), C (≤0.2%), S (≤0.035%), and P (≤0.04%), as determined by the ministry of metallurgical industry of the People’s Republic of China. The reduced ilmenite product contains TiO2 and Fe, which possess oxidizability by TiO2 and restrike of arc property by Fe.8,9) Hence, reduced ilmenite has gradually become an ideal material for electrode coating because of its low price and it can largely substitute natural or artificial rutile in the welding industry. Marine placer belongs to the secondary mineral of ilmenite, which is transformed from rock ore after weathering and river deposits for several years. The structure of marine placer is loose, and the maintained TiO2 content exceeds 50% after beneficiation.10,11) Thus, marine placers are suitable for producing reduced ilmenite for coating material of welding electrode.
In the present study, the reduced ilmenite for welding electrodes was prepared from marine placers via carbothermic reduction, which was performed using a horizontal tube electric furnace. The reduction behavior of the marine placer–coal mixture was checked by thermogravimetry/differential thermal gravimetry/differential scanning calorimetry (TG/DTG/DSC) technology. The phase identification and transformation in each step were characterized by X-ray diffraction (XRD), while the corresponding morphology of the products was analyzed by scanning electron microscopy and energy-dispersive spectrometry (SEM/EDS). The quality of the reduced ilmenite was evaluated by the TiO2 and FeO content.
2. Experimental
2.1. Materials
The marine placer and coal in this study were supplied by Atlantic China Welding Consumables, Inc. (Sichuan Province, China). The chemical compositions and particle size distribution of the raw materials are listed in Tables 1 and 2, respectively. Table 1 shows that the marine placer contains more than 50% titanium dioxide and only 32.18% iron. Coal contains 78.74% C, 8.85% volatile, and 11.64% ash. Table 2 shows that the particle distribution of the marine placer and coal were uneven and mainly in the range of 53–150 μm.
Table 1. Chemical composition of marine placer and coal (wt.%).
| Marine placer | Coal |
|---|
| TiO2 | TFe | FeO | MgO | SiO2 | Al2O3 | CaO | C | Volatile | Ash |
|---|
| 50.1 | 32.18 | 25.77 | 1.05 | 0.79 | 0.69 | 0.28 | 78.74 | 8.85 | 11.64 |
Table 2. Particle size distribution of marine placer and coal (wt.%).
| Size/μm | >150 | 105–150 | 75–105 | 61–75 | 53–61 | <53 |
| Marine placer | 1.58 | 27.28 | 15.74 | 24.70 | 25.36 | 5.34 |
| Coal | 10.36 | 17.93 | 35.36 | 21.21 | 10.57 | 4.57 |
The mineralogical phase of marine placer was investigated with an XRD analyzer (D/Max 2200 X, Rigaku, Japan). The working line was CuKα radiation (λ = 1.5418 Å, 35 kv, 20 mA) over the 2θ range of 20°–75° and calculated by applying the Scherrer equation. TG/DSC were recorded by a TG analyzer (STA 409, NETZSCH, Germany) with a 10°C/min heating rate in the argon protection environment. The morphology of marine placer was examined with an SEM (Nova NanoSEM 450, FEI, America). A magnetic separator (XCGQ50, YongSheng, China) was employed to remove carbon residue at 1200 Gs. The reduction experiment was investigated using a horizontal tube electric furnace (13Q-YC, YiFeng, China), and its schematic is shown in Fig. 2.

The marine placer was first mixed homogenously with the coal under the molar ratio of Cfixed/O(bonded with Fe) of 1.2 to guarantee the complete reduction of iron-bearing oxides to metallic iron. Appropriate distilled water and 1% organic binder (methylcellulose) were added into the mixture and then made into a spheroidic briquette under the pressure of 15 MPa with a briquette maker. Each briquette was approximately 30 mm in size and approximately 22 g in weight. All the briquettes were dried for 12 h at 105°C before use in the reduction experiments.
The furnace has an isothermal zone at approximately 100 mm in length in the corundum tube. The temperature was measured with an S-type thermocouple. The temperature increase was controlled by the programs where the heating rate was 10°C/min. Argon was pumped into the tube once the desired temperature was reached to release air. A single briquette was then placed in a cymbiform corundum crucible, which was placed in a horizontal tube electric furnace and introduced to the isothermal zone of the furnace after ventilation with argon for 10 min. Argon was purged to the tube with the rotameter at 0.2 L/min to provide inert ambience until the reduction experiment was completed. Finally, the briquette was removed rapidly from the tube and cooled in the absence of air, and then prepared by magnetic separation after grinding.
3. Results and Discussion
3.1. Reduction Behavior of Marine Placer-Coal Mixture
The TG/DTG/DSC techniques were applied to evaluate the reduction behavior of the marine placer–coal mixture in argon; the results are shown in Fig. 3. The TG curve shows that the heating behavior of the mixture involved the weightlessness process and slowdown in the total mass loss at approximately 1350°C. Four stages of mass loss were exhibited in the TG curve, which can be described as four different reaction processes that consolidated the DTG and DSC.12,13)

The mass decreased at a slow rate at the first stage when the temperature was less than 600°C, which can be attributed to the release of the volatiles in coal. The DSC curve exhibited an endothermic peak at approximately 800°C at the second stage (600–960°C). However, the mass loss from the TG curve was still small, which illustrates that the main reductive reaction at this stage was a solid–solid reduction. The carbon gasification reaction started concurrently, and the reduction rate of iron oxide–coal composites was reportedly controlled by the gasification reaction rate at a temperature lower than 1000°C.
The DTG curve at the third stage (960–1350°C) exhibited a clear peak at approximately 1210°C, and the DSC curve exhibited a corresponding endothermic peak. The total mass loss was more than 16.66% at the temperature range of 960–1350°C, which indicates that the reduction rate was high because of the high carbon gasification rate. The carbothermic reduction mechanism of iron oxides has been suggested to be a two-stage mechanism with the participation of CO and CO2.14,15,16) The two-stage mechanism can be expressed as follows:
|
F
e
x
O
y
+C=F
e
x
O
y-1
+CO;
| (1) |
|
F
e
x
O
y
+CO=F
e
x
O
y-1
+C
O
2
;
| (2) |
The total mass loss gradually slowed according to the TG curve during the fourth stage (≥1350°C), which indicates that the reduction of iron oxides was nearly completed.
3.2. Quality Index of Reduced Ilmenite
The quality index of reduced ilmenite was evaluated in terms of the TiO2, iron (II) oxide (FeO), carbon (C), sulfur (S), and phosphorus (P) contents. The C, S, and P contents in this study were far below the technical requirements after magnetic separation. Therefore, the TiO2 and FeO contents were investigated at different reduction temperatures and times, and the results are shown in Figs. 4 and 5.
Figure 4 shows that the TiO2 content of reduced ilmenite gradually increased from 54.27% to 58.84% as the reduction temperature increased from 1000°C to 1300°C. The reduction time exerted lesser influence than temperature. After reduction at 1000°C for 30 min, the TiO2 content could reach 52.8%, which exceeded the technical requirement of 52%. The TiO2 index could be achieved easily because of the high TiO2 content of the raw materials.
The FeO content is an important quality index of reduced ilmenite, and the results are shown in Fig. 5. The reduction temperature clearly influenced the FeO content of the reduced ilmenite. The FeO content initially decreased rapidly and then exhibited a downward trend that became slower after 120 min with increasing reduction time. The FeO content was larger than that of the raw material of 25.77% at 1000°C. The reason is that the reduction reaction had only begun and most of the high-valent iron particles transformed into low-valent iron particles at the initial reaction. The reduction process of iron oxides can be described as follows: Fe2O3→Fe3O4→FeO→Fe, which is consistent with the results of the XRD analysis at 1000°C. The FeO reduced into metallic iron and the FeO content decreased with strengthening reaction intensity. The FeO content could reach 8.45% after reduction at 1300°C for 180 min, which exceeded the technical requirement of 9%. The FeO content subsequently followed a slow downward trend.
3.3. Phase Transitions of Reduced Ilmenite
The phase transformation of the reduced ilmenite after reduction at different temperatures and different times was characterized using the XRD technique; the results are shown in Figs. 6 and 7. Figure 6 shows that the main phases of the reduced ilmenite at 1000°C were FeTiO3, Fe2O3, TiO2, and Fe. FeTi2O5, MgTi2O5 and (Mg,Fe)Ti2O5 (ferrous–pseudobrookite, where some Fe2+ is replaced by Mg2+) became clear with increasing reduction temperature from 1100°C to 1300°C. The rutile TiO2 of 27.42° shifted to the anatase TiO2 of 25.37° and 37.88° with increasing reduction temperature from 1100°C to 1200°C. Traces of magnesium aluminum spinel (MgAl2O4) were detected in the XRD patterns when the temperature increased to 1200°C. Comparing with the XRD phase transformation and combining with existing research,12,17,18) the reduction process of the marine placer that exceeded 1100°C can be written as: FeTiO3→FeTi2O5→Fe+TiO2.

The phase transformation of the reduced ilmenite at 1300°C at different times is shown in Fig. 7. The Fe and TiO2 diffraction intensity of the peaks significantly enhanced with increasing reduction time at 1300°C. After reduction at 1300°C for 180 min, the main phases of the reduced ilmenite were Fe, TiO2, FeTi2O5 and a minor amount of MgTi2O5 and (Mg,Fe)Ti2O5.
3.4. Micro Morphology of Reduced Briquettes
SEM and EDS were employed to investigate the morphology changes of the reduced briquettes after polishing; the SEM results are shown in Fig. 8. Figure 8(a) shows that the marine placer was composed mainly of ilmenite and had a denser and smoother surface morphology after polishing. Figures 8(b) to 8(e) show that the polishing of the reduced briquettes revealed two distinct regions that appeared as bright and dark gray phases. The bright phase became large with increasing time from 30 min to 180 min at 1300°C.

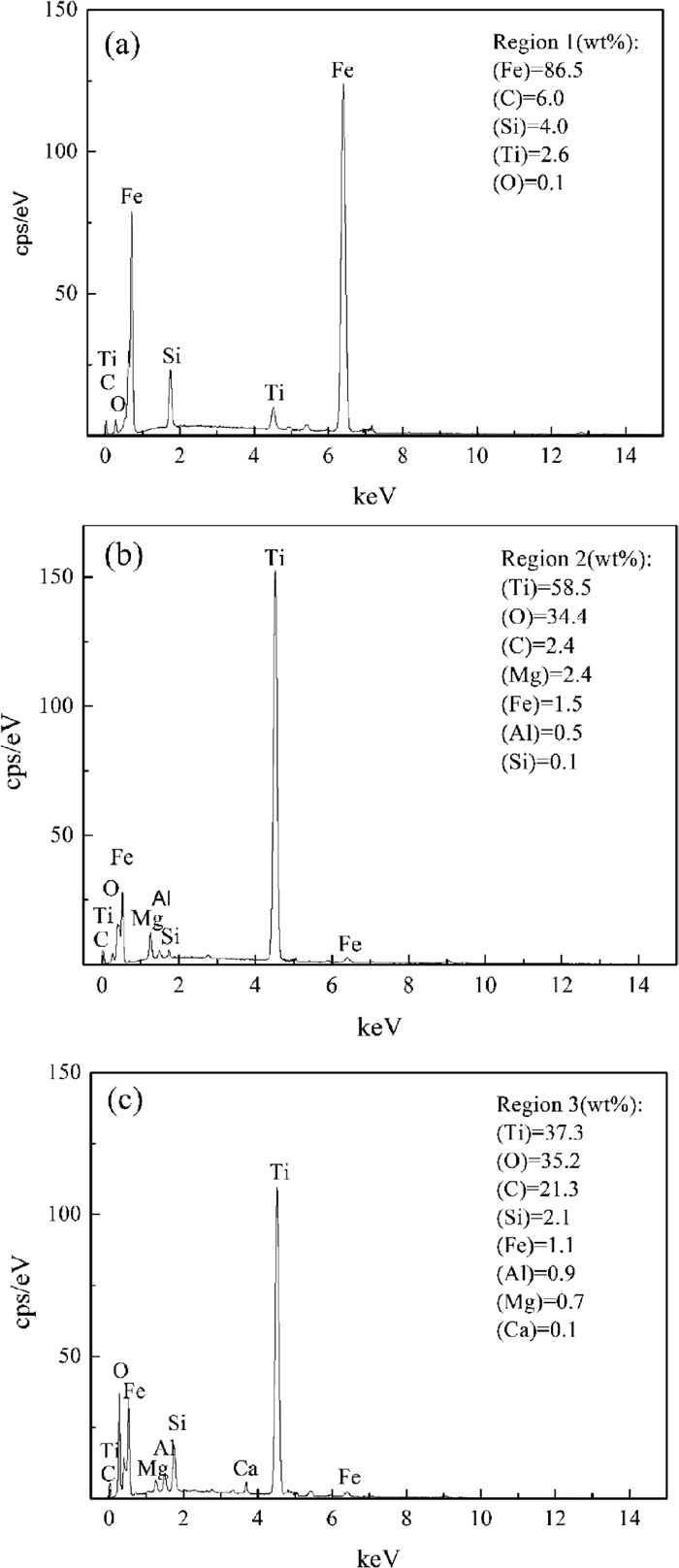
EDS was implemented at the regions marked in Fig. 8(e) to authenticate the bright and dark gray phases; the results are shown in Fig. 9. Region 1 mainly comprised approximately 91.8% Fe, which could be identified as the iron phase. Region 2 consisted of 59.7% Ti, 31.3% O, and minor elements such as Mg, Al, and Si, which indicates that the dark gray phase primarily involved titanium oxides. Region 3 was significantly different from the other two phases, which could be made up of approximately 58.5% Ti, 34.4% O, 21.3% C, and other mineral elements.19,20,21) Therefore, this phase is mainly the carbon phase mixed with a few pseudobrookite.
4. Conclusion
The reduced ilmenite for coating material of welding electrode was prepared from marine placers via carbothermic reduction. The obtained conclusions are as follows:
(1) Four stages were observed in the carbothermic reduction of the marine placer–coal composites. The release of the volatiles in coal occurred at the first stage (≤600°C), and the solid–solid reduction and gas–solid reduction occurred at the second (600°C–960°C) and third stages (960–1350°C), respectively. The reduction rate of iron oxide at the third stage was higher than that at the second stage because of the high carbon gasification rate. A slowdown in the total mass loss occurred at approximately 1350°C at the fourth stage.
(2) The TiO2 index can be achieved easily because of the high TiO2 content of the raw material. The FeO content could reach 8.45% after reduction at 1300°C for 180 min, which exceeded the technical requirement of 9%.
(3) The reduction process of the marine placer that exceeded 1100°C can be written as: FeTiO3→FeTi2O5→Fe+TiO2.
Acknowledgment
The authors acknowledge the financial supports from the National Scientific Foundation of China (No: 51504113).
References
- 1) W. Zhang, Z. Zhu and C. Y. Cheng: Hydrometallurgy, 108 (2011), 177.
- 2) M. Fattahi, N. Nabhani and M. R. Vaezi: Mater. Sci. Eng. A, 528 (2011), 8031.
- 3) A. M. Paniagua, V. M. Lopez and H. J. Dorantes: Mater. Charact., 60 (2009), 36.
- 4) A. G. Vodop’yanov and G. N. Kozhevnikov: Russ. J. Appl. Chem., 86 (2013), 124.
- 5) M. Aghakhani, M. R. Ghaderi, A. Karami and A. A. Derakhshan: Int. J. Adv. Manuf. Technol., 70 (2014), 63.
- 6) S. St-Laurent and G. L’Esperance: Mater. Sci. Eng. A, 149 (1992), 203.
- 7) L. Aucott, S. W. Wen and H. Dong: Mater. Sci. Eng. A, 622 (2015), 194.
- 8) S. Mohan, S. P. Sivapirakasam and M. C. Kumar: J. Mater. Process. Technol., 219 (2015), 237.
- 9) B. G. Tang and S. K. Yin: Welding Material of Low Carbon and High Strength Low Alloy Steel, China Machine Press, Beijing, (1987), 59, (in Chinese).
- 10) U.S. Geologieal Survey: Mineral Commodities Summaries 2012, Reston, VA, (2012), 178.
- 11) G. Z. Deng: Titanium Metallurgy, Metallurgical Industry Press, Beijing, (2010), 11, (in Chinese).
- 12) T. Hu, X. Lv and C. Bai: Metall. Mater. Trans. B, 44 (2013), 252.
- 13) M. Chen, A. T. Tang and X. Xiao: Trans. Nonferr. Met. Soc. China, 25 (2015), 4201.
- 14) H. P. Gou, G. H. Zhang and K. C. Chou: Metall. Mater. Trans. B, 46 (2015), 48.
- 15) R. J. Fruehan: Metall. Trans. B, 8 (1977), 279.
- 16) S. K. Dutta and A. Ghosh: Metall. Mater. Trans. B, 25 (1994), 15.
- 17) E. Park and O. Ostrovski: ISIJ Int., 44 (2004), 74.
- 18) A. F. Buddington and D. H. Lindsley: J. Petrol., 5 (1964), 310.
- 19) Y. M. Wang, Z. F. Yuan and Z. C. Guo: Trans. Nonferr. Met. Soc. China, 18 (2008), 962.
- 20) J. Zhang, G. Zhang and Q. Zhu: Metall. Mater. Trans. B, 45 (2014), 1.
- 21) B. Song, X. Lv and J. Xu: Int. J. Miner. Process., 142 (2015), 101.