2018 年 58 巻 4 号 p. 627-632
2018 年 58 巻 4 号 p. 627-632
The carbothermic reduction experiments were carried out for vanadium-titanium magnetite in alkaline molten in argon atmosphere at high temperatures. The effects of reduction temperature, carbon content and addition agent on the formation of pig iron containing vanadium were studied by X-ray diffraction (XRD) and scanning electron microscope (SEM). The XRD patterns of reduced slags results showed that Fe phase disappeared and the main phase of the reduced sample were Na16Ti10O28, CaTiO3, and Na1.66AlSiO4.33 when the reduction temperature was more than 1473 K when cooled in the air. The SEM pictures show that most of V exists in the crystalline phase, such as Na16Ti10O28, NaAlSiO4 and CaTiO3, when quenched in air slowly; and the vanadium is dispersed in the slag phase when cooled by water quickly. Furthermore, the effects of additive NaOH on the reduction were also studied, results show that NaOH could enhance the separation of iron and slag, promote the transformation of vanadium and titanium and inhibit the vanadium enrichment in metal phase.
Vanadium-titanium-bearing magnetite of Panzhihua, China, is a complex iron ore with the coexistence elements of vanadium and titanium. It accounts for more than 90% of the titanium reserves in China. By the beneficiation process of the ore, titanomagetite concentrates and ilmenite concentrates are produced.1,2) The titanomagnetite concentrates are used as the main materials for the blast furnace process in Panzhihua area now. In this process, most of the iron and partly of the vanadium can be reduced into the hot metal; however, almost all of the titanium remains in the slag, forming the high titanium bearing slag, which contains about 21%–25% of TiO2, and emission in every year is more than 3 million tons.3) This slag is the considerable strategic resources and the precious wealth. There is no appropriate and economic method to deal with such slag so far. Titanium and vanadium are widely used in the aerospace and chemical industrial because of their great use and importance. Many countries regard the deposit of titanium and vanadium as a strategic resource. China is abundant in titanium, account for about 48% of the world. However, the recovery ratio of the titanium is too low in the current smelting process of the vanadium tianomagnetite resource.
In recent years, non-blast furnace process is considered to be one of the great potential of the method. It cannot only be a great utilization of iron, vanadium, titanium, but also is a short process. Over the past several decades, many works have been conducted on the mechanism and kinetics of the carbothermic reduction of titanomagnetite4,5,6,7,8,9,10,11) and titanium-bearing blast furnace slag.12,13,14,15) Although many works have been done, the phase evolution regularity during the carbothermic reduction process of vanadium-titanium-bearing magnetite is still unclear, which is the aim of the present study.
Studies on adding alkali metal additives in reduction process of vanadium-titanium magnetite have been carried out. Much work so far has focused on the effects of a small amount of additives (﹤10%). Results show that it can not only enhance reduction rate, but also reduce the reduction temperature.16,17,18) However, the effects of adding a large number of additives have not been reported extensively. The effect of adding a large number of additive NaOH on reduction process of vanadium-titanium magnetite was presented in this paper.
At the same time, all of these works were focused on the crystalline slags which were cooled in the air slowly after samples are reduced at different temperatures for different time. Then the other problem is that it is very hard to remove the crystalline phase even using acid leaching if extracting pure TiO2 and V2O5 from the treated slag, especially CaTiO3, which is insoluble in both acid and alkali. Therefore, the aim of the present study is to explore the change of phases after reduction cooled by different cooled methods, and then find the best experimental conditions for carbothermic reduction.
Chemical compositions of the vanadium-titanium magnetite were examined by the X-ray fluorescence (ShimazuXRF-1800, current 140 mA, voltage 60 kV), which are presented in Table 1. The practical size distributions of the vanadium-titanium magnetite and graphite powder were presented in Figs. 1 and 2. Figure 3 shows the X-ray diffraction (XRD, conducted using a Cu-Kα source) patterns of the vanadium-titanium magnetite. The main mineral phase of the vanadium-titanium magnetite is Fe3O4, Fe2TiO4 and FeTiO3.
| Components | TFe | TiO2 | V2O5 | SiO2 | Al2O3 | MgO | CaO | MnO |
|---|---|---|---|---|---|---|---|---|
| content | 43.81 | 22.74 | 1.26 | 7.28 | 1.75 | 0.62 | 3.69 | 0.44 |

The practical size distributions of the vanadium-titanium magnetite.

The practical size distributions of the graphite powder.

XRD patterns for vanadium-titanium magnetite.
The vanadium-titanium magnetite and the graphite powder were mixed homogenously with the addition of the alcohol. By considering Eqs. (1), (2), (3), the mass ratios of C to vanadium-titanium magnetite in mixtures were set to be 20:100 (corresponding to the carbon molar ratio of 2.0), 24:100 (corresponding to the carbon molar ratio of 2.4), 28:100 (corresponding to the carbon molar ratio of 2.8), 35:100 (corresponding to the carbon molar ratio of 3.5), respectively.
| (1) |
| (2) |
| (3) |
Then the mixtures were dried to get rid of the alcohol at 393 K for 2 hours. The powder was placed in an alumina crucible. Vertical tube furnace with silicon molybdenum as the heating element was used. The temperature was accurately controlled within ±1 K by PID controller under a flowing argon atmosphere. The alumina crucible was put into the hot zone of the vertical tube furnace quickly when the furnace temperature reached the desired value. The isothermal reduction reactions at three different temperatures were studied, as 1373 K, 1423 K, 1473 K, 1523 K and 1573 K respectively. The reaction time at each reaction temperature was 2 h respectively. The addition agents was NaOH, and the mass ratios of addition agent to vanadium-titanium magnetite in mixtures were set to be 30%, 35%, 45%. The samples reduced at different temperatures for different time were cooled in the air slowly and in the water quickly after the alumina crucibles were took out from the furnace. The reduced slags were examined by XRD and SEM (Mineral Liberation Analyzer, voltage 200 V–30 KV) analyses to study its phase composition and morphology.
The separations of Fe and slag after carbothermic reduction were shown in Fig. 4. It can be seen that metal and slag can be well separated when the reduction temperature more than 1473 K. The reduced samples’ fractional degree with different NaOH and different carbon ratio at 1473 K was shown in Figs. 5–6. In the shaded area, metal and slag can be well separated. Figure 5 shows that when the ratio of carbon is 2.0 to 2.8 and the content of NaOH is 30% to 45% at 1473 K, metal and slag can be well separated. But when the ratio of carbon is more than 2.8, it needs more additive NaOH to make metal and slag be well separated. Figure 6 shows that when adding the same content of NaOH at 1473 K, the fractional degree decreases as increasing the carbon ratio (more than 2.4). When adding the same ratio of carbon, the fractional degree increases as increasing the content of NaOH. The reason might be that carbon is infusible solid, and addition of excess carbon results in a increase in melt viscosity.19,20,21)

The reduced samples with 45% NaOH at different temperatures, a): 1373 K; b): 1423 K; c): 1473 K; d): 1523 K.

The Separated Area of samples with different NaOH and different carbon ratio at 1473 K.

The reduced samples’ fractional degree (3D) of samples with different NaOH and different carbon ratio at 1473 K.
In the case of adding NaOH, the XRD patterns of the samples reduced with carbon ratio of 2.4 and 45% NaOH at different temperatures are presented in Fig. 7. Figure 8 gives the XRD results of reduced samples with carbon ratio of 2.4 at 1473 K for different percentage of NaOH. The XRD patterns results show that the main phases of the reduced samples are CaTiO3, NaAlSiO4, Na1.45Al1.45SiO4, Na16Ti10O28 and Fe. The XRD patterns of reduced slags results showed that Fe phase disappeared and the main phase of the reduced sample were Na16Ti10O28, CaTiO3, and Na1.66AlSiO4.33 when the reduction temperature was more than 1473 K and the mass ratios of NaOH was more than 35%, which also means that metal and slag can be well separated when the reduction temperature more than 1473 K (shown in Fig. 4). Figure 9 gives the XRD results of reduced samples adding 45% NaOH at 1473 K for different carbon ratios. It is shown that Fe phase disappeared when the carbon ratio was 2.4. From Figs. 7, 8, 9, it can be seen that there was no obvious change in the main phases as increasing the carbon ration and reduction time at 1473 K.

XRD results of reduced sampvles with carbon ratio of 2.4 and 45% NaOH at different temperatures.

XRD result of reduced sample with carbon ratio of 2.4 at 1473 K for different percentage of NaOH.

XRD results of reduced samples with 45% NaOH at 1473 K for different carbon ratio.
In addition, in order to keep the phase composition at high temperature, the quenching is necessary. Figure 10 gives the XRD results of reduced samples quenched by water. It is shown that the reduction product almost was glass phase, that is, there little crystalline phase was formed when quenched by water.

XRD results of reduced samples cooled by water.
The BSED (Backscattered Electron Detector) images of the samples reduced with carbon ratios of 2.4 and 45% NaOH at different temperatures cooled by air are shown in Fig. 11. The BSED image reveals three distinct regions which appear as bright, light gray, dark gray, some black. In order to identify the phases, EDS analyses were performed at different regions as shown in Table 2. Example of the samples reduced at 1473 K, the region 1 consists of 99.56% Fe, which indicates that the bright phase is composed of Fe phase. The region 2 is made up of 37.18% Ti, 22.88% Ca and 37.41% O, which indicates that the light gray phase is mainly composed of CaTiO3 phase. The region 3 is made up of 40.81% Ti, 19.73% Na, and 37.25% O, which indicates that the dark gray phase is mainly composed of Na16Ti10O28 phase. The region 4 consists of many elements, such as 30.42% O, 3.44% Al, 1.38% Ca, 19.63% Si, 19.78% Ti, 20.66% Na and so on, which implies that the black phase is mainly composed of Na16Ti10O28 and NaAlSiO4 phase. The region 5 is made up of 36.77% O, 15.42% Al, 17.35% Si, 5.52% Ti, 19.45% Na and so on, which indicates that the region 5 is the same compose of region 4. Furthermore, the element of V consists in all of the five regions, it means that V mainly occurs in the Na16Ti10O28, NaAlSiO4 phase and some exists in the CaTiO3 phase. That may imply that V into the slag phase could be helpful for recovering of V, but the crystalline phase could be harmful for the recycling of V.

BSED images of the samples reduced with carbon ratios of 2.4 and 45% NaOH at different temperatures cooled by air: a) 1423 K; b) 1473 K; c) 1523 K; d) 1573 K.
| T/K | Phase | Fe | V | Ti | Na | Si | Ca | Al | O | C |
|---|---|---|---|---|---|---|---|---|---|---|
| 1423 | 1 | 98.87 | 0.18 | 0.95 | ||||||
| 2 | 0.63 | 34.73 | 3.22 | 25.31 | 36.11 | |||||
| 3 | 0.68 | 36.61 | 18.82 | 5.56 | 1.16 | 2.48 | 34.67 | |||
| 1473 | 1 | 99.56 | 0.26 | 0.18 | ||||||
| 2 | 0.48 | 37.18 | 2.05 | 22.88 | 37.41 | |||||
| 3 | 1.09 | 40.81 | 19.73 | 1.12 | 37.25 | |||||
| 4 | 2.93 | 1.75 | 19.78 | 20.66 | 19.63 | 1.38 | 3.44 | 30.42 | ||
| 5 | 2.07 | 0.52 | 5.52 | 19.45 | 17.35 | 15.42 | 36.77 | |||
| 1523 | 1 | 99.43 | 0.47 | 0.10 | ||||||
| 2 | 0.81 | 36.52 | 4.00 | 26.00 | 32.27 | |||||
| 3 | 1.38 | 0.89 | 34.74 | 16.84 | 5.27 | 2.45 | 38.42 | |||
| 4 | 0.24 | 10.11 | 18.78 | 16.12 | 13.87 | 40.88 | ||||
| 1573 | 1 | 99.32 | 0.68 | |||||||
| 2 | 72.66 | 5.50 | 21.84 | |||||||
| 3 | 0.34 | 36.24 | 4.37 | 1.92 | 24.32 | 32.81 | ||||
| 4 | 0.14 | 5.81 | 16.89 | 19.24 | 0.63 | 15.50 | 40.47 |
BSED images of the samples reduced with carbon ratios of 2.4 and 45% NaOH at 1473 K quenched by water are shown in Fig. 12. There are two distinct regions. In order to identify the phases, EDS analyses were performed at different regions as shown in Table 3. Compared to quench by air, some phases disappeared, which means the main phase of the products was Fe and some CaTiO3 phases may be formed when quenched slowly. EDS analyses also shown that V and CaTiO3 were obviously found to be mixed together tightly.

BSED images of the samples reduced with carbon ratios of 2.4 and 45% NaOH at 1473 K cooled by water.
| Phase | O | Na | Al | Si | Ca | Ti | V |
|---|---|---|---|---|---|---|---|
| 1 | 26.78 | 25.18 | 3.56 | 7.45 | 4.1 | 22.7 | 1.72 |
| 2 | 29.32 | 3.63 | 28.59 | 37.07 | 1.38 |
During the reduction of vanadium-titanium magnetite by graphite, the chemical compositions of the reduction slags were examined by the XRF and ICP-OES (Optimal 5300DV, Perkin-Elmer, USA), which are presented in Figs. 13, 14.

The percentage of V2O5 with different carbon ratios at 1473 K for NaOH added.
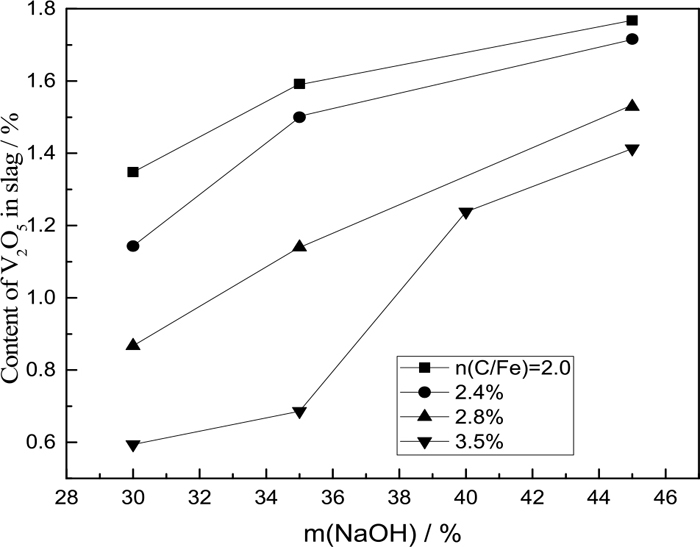
The percentage of V2O5 with different percentage of NaOH at 1473 K.
In the case of adding NaOH, the chemical compositions of the samples reduced are presented in Figs. 13, 14. Figures 13, 14 show that the percentage of V2O5 decreases as increasing the carbon ratios and increases as increasing the content of NaOH. That may imply that NaOH not only benefits the separation of metal and slag, but also benefits vanadium enrichment in slag phase.
(1) From the results of XRD and SEM, it can be seem that most of V entered into the slag phases after reduction. It exists in the crystalline phase, such as Na16Ti10O28, NaAlSiO4 and CaTiO3, when quenched in air slowly; on the other hand, the vanadium is dispersed in the slag phase which it is no crystalline phases when cooled by water quickly. All of works were focused on the crystalline slags which were cooled in the air slowly after samples are reduced at different temperatures for different time. The slag can produce crystalline phases when quenched in air slowly, which may make V and Ti be wrapped in crystalline phases. And that will be harmful for the next extraction process. Therefore, to obtain the glass slags and to avoid the precipitation of crystalline phase may be a new idea.
(2) Many studies22,23) pointed that V ions are occupied in the octahedral position of the magnetite in the form of +3. The main reactions about V probably occurred during the carbothermic treatment of vanadium-titanium magnetite are given as follows:
| (4) |
| (5) |
| (6) |
The results of thermodynamics calculation by FactSage6.0 were given in Fig. 15. From Fig. 15, it could be indicated that the all of the Eqs. (4), (5), (6) would occur, and then V ions entered into the slag phases in the form of +5. Moreover, from Fig. 13, it can be seen that the percentage of V2O5 decreases as increasing the carbon ratios. The reason may be that theV5+ ions were reduced to V3+ ions by the excessive carbon, and then enter into the Fe phase, then the percentage of V2O5 decreases.
(3) From Fig. 14, it can be seen that more V2O5 could be produced by improving the content of NaOH. That is, the reaction rate would be improved by increasing the addition agents. NaOH was added for its strong ability of decreasing viscosity which is beneficial for the diffusion transport of ions.24,25) In addition, they promoted the oxidation of V3+ iron.
The carbothermic reduction process of vanadium-titanium magnetite was investigated in argon atmosphere. Reduction experiments with different carbon ratios at different temperatures for different additives were carried out to investigate their influences on the reduction. The following conclusions were obtained:
(1) The XRD patterns results show that Fe phase disappeared and the main phase of the reduced sample were Na16Ti10O28, CaTiO3, and Na1.66AlSiO4.33 when the reduction temperature was more than 1473 K when cooled in the air. The SEM pictures show that most of V exists in the crystalline phase, such as Na16Ti10O28, NaAlSiO4 and CaTiO3, when quenched in air slowly; and the vanadium is dispersed in the slag phase when cooled by water quickly.
(2) The percentage of V2O5 in the slags decreases as increasing the carbon ratios and increases as increasing the content of NaOH.
(3) NaOH can be helpful for the reduction. NaOH can enhance the separation of iron and slag, promote the transformation of vanadium and titanium and inhibit the vanadium enrichment in metal phase.
Thanks are given to the financial supports from National Natural Science Foundation of China (21506233, 21606241), Key Research Program of Frontier Sciences of Chinese Academy of Sciences (QYZDJSSW-JSC021), Science and Technology Service Network Initiative (KFJ-STS-ZDTP-040) and Nonprofit Industry Research Subject of Environmental Protection (No. 201509053).