2018 年 58 巻 5 号 p. 929-935
2018 年 58 巻 5 号 p. 929-935
In this paper, a MgO–SiO2–Al2O3–ZnO ceramic-glass coating sprayed on carbon steel was studied at high temperature. The property of the MgO–SiO2–Al2O3–ZnO ceramic-glass coating was analyzed in a range of 900°C and 1150°C a share. The experimental results indicated that the MgO–SiO2–Al2O3–ZnO coating exhibited powerful anti-oxidation property for carbon steel. The MgO–SiO2–Al2O3–ZnO coating could improve the anti-oxidation performance of carbon steel by 84% at 1050°C for 60 min. The Ea of blank and coated samples were 108.65 and 202.55 kJ/mol, respectively. The kp of the blank sample (0.61 mg2·cm−4·s−1) was 7.6 times as much as that of coated sample (0.08 mg2·cm−4· s−1). It demonstrated the coating slowed down oxidation reaction rate and then improved the anti-oxidation performance of carbon steel. The possible protection mechanisms of the MgO–SiO2–Al2O3–ZnO ceramic-glass coating were also investigated using the SEM-EDS, XRD and TG-DTA characterization methods. The mixture formed between the coating and steel substrate (such as MgFe2O4, ZnFe2O4, MgSiO3, FeSiO4, ZnSiO4, SiO2, Al2O3, and (FexMg1−x)2SiO4 (x=0.4, 0.85, 0.94)) played a role in blocking the spread of ions and improved the oxidation resistance of carbon steel during heating treatment. The synergy of the formed Al, Mg and Zn compound layers in the coating could also block spread of oxygen and iron ions and exert an influence on enhancing anti-oxidation property.
Steel is widely applied in engineering, which is based on its excellent performance characteristics (good mechanical properties and low cost). In order to obtain good performance, the steel must undergo corresponding heating treatment. Nevertheless, it is hard to resist oxidation at high temperature during heating treatment, especially carbon steel.1) Carbon steel is the most commonly used materials of the construction of petroleum industry. Recently, the demands of the oil pipe were increasing significantly and the consumption of carbon steel accounted for ~60% of the total consumption of steel pipe.2,3) During the heating process, steel surface reacted with the oxidizing atmosphere and scale was formed on the surface of steel. It would cause many serious problems such as the waste of heating fuel and manpower for cleaning up the scale, low yield and poor surface quality of the carbon steel, and low utilization rate of furnace lining because of the mechanical collision.4,5) Therefore, it was very important to inhibit the oxidation of carbon steel. Coating was a convenient method to prevent carbon steel from oxidation at high temperature.6,7) Till now, people have paid more attention to and done a vast amount of researches to improve the anti-oxidation ability of carbon steel.5)
Glass material (SiO2) has relative low softening point, which can play an important role in preventing the oxidation by forming a dense film at low temperature.5,6) However, ceramic material (Al2O3, MgO) has high melting temperature.5) The ceramic-glass material is formed by high temperature treatment with crystalline and amorphous phase.8,9) Combined with lower temperature protection of glass and high temperature protection of ceramic, the ceramic-glass material having comprehensive properties of glass and ceramic could have a better anti-oxidation performance than a single glass or ceramic material.10,11,12) Silicon would take shape a continuous glassy SiO2 layer at the interface between steel and scale during heating treatment.13) Due to low concentration of defects, the silicon layer had excellent oxidation resistance.14) One of the most important properties of silicon was the good anti-oxidation performance for carbon steel at relative low temperature. To improve the protection temperature, ceramic materials with high melting point was added into the coating. It has been approved that alumina-forming coating has the most effective anti-oxidation performance for carbon steel at high temperatures (>900°C).15) The formation of Al2O3 in coating had a strong resistance performance to oxidation and hot-corrosion at high temperature.16,17) The coating composed of MgO showed good oxidation resistance performance for the steel at high temperature.18,19) Zinc and alloys were also used to prevent the atmospheric corrosion of steel.20)
In this paper, a new MgO–SiO2–Al2O3–ZnO ceramic-glass coating was exploited to improve the oxidation resistance of carbon steel and the anti-oxidation property of the coating was appraised. Additionally, the potential protective mechanism of the MgO–SiO2–Al2O3–ZnO ceramic-glass coating was discussed.
The carbon steel sample was cut into a cube (1 cm × 1 cm × 1 cm) by a high speed wire electrical discharge machine. Then the sample was polished by polishing and burnishing machine. Lastly, the sample was rinsed by an ultrasonic washing machine and dried by a drying oven. Table 1 demonstrated the chemical component of carbon steel sample.
| Component | C | Si | Mn | S | P | Fe |
|---|---|---|---|---|---|---|
| Content, wt% | 0.28 | 0.27 | 1.35 | ≤0.030 | ≤0.030 | balance |
The chemical component of MgO–SiO2–Al2O3–ZnO coating was displayed in Table 2. First, water glass with a solid content of 25 wt% was prepared. Then quart powder (20 g), aluminum oxide (5 g), magnesium compound (5 g), zinc oxide (6 g) and water glass (18 g) were placed in a 100 ml beaker. Last, a given volume of water was added to the beaker to ensure the solid content was 66.67%. The coating mixture was dispersed by a high speed homogenizer to make the coating material uniform and thin (below 38 microns). The coating mixture was sprayed on steel about 0.2 mm in thickness by low pressure spray gun (w-77c).
| Component | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | ZnO | K2O |
|---|---|---|---|---|---|---|---|
| Content, wt% | 35.26 | 11.12 | 0.45 | 0.22 | 45.33 | 7.43 | 0.04 |
Weight changes of samples were used as an index of anti-oxidation performance of the MgO–SiO2–Al2O3–ZnO ceramic-glass coating after high-heat treatment from 20°C to different temperatures, such as 900°C, 1000°C, 1050°C, 1100°C and 1150°C, and maintained for 60 min. Then, the coating and scale of the sample were removed and the sample was weighed. The anti-oxidation effect (E) of MgO–SiO2–Al2O3–ZnO ceramic-glass coating depended on the weight changes of the sample, which was given in Eq. (2).5) The anti-oxidation effect of sample for each reported condition was completed for 3 samples and the average value was adopted.
| (1) |
| (2) |
Where, Mbef was the weight of sample before heating treatment, and Maft was the weight of the according sample after heating treatment without scale. αcoated was the yield of the sample with coating and αblank was that of the blank sample.
The non-isothermal and isothermal kinetics were also given in this study. It was taken using a thermogravimetry (TG). It was possible to get a continuous record of weight changes during the heating treatment. In the non-isothermal kinetic, the reaction rate (v) was shown in Eq. (4).5)
| (3) |
| (4) |
Where, Δm was the mass loss per unit area (cm2), m1 was the mass per unit area of sample during heating treatment at certain temperature and m2 was that of sample after heating treatment constant for sustain time (t). For non-isothermal kinetic, t was 5 min.
For the isothermal kinetic, the weight change of sample with time was given by Eq. (5)
| (5) |
Here, Δw was weight change per unit area, w0 was the weight per unit area of sample at certain temperature and wi was that of sample constant for sustain time at that temperature.
The microscopic characteristics of blank and coated samples were identified by SEM-EDS (SEM-EDS; JSM-6700, JEOL, Japan). Energy and weight changes of the sample during the heating treatment were investigated by thermal gravity-differential thermal analysis (TG-DTA; STA449, Netzsch, Germany), which was implemented from 20°C to 1200°C at a speed of 10°C/min. The XRD patterns (XRD, X`Pert Pro, Philips, Netherlands) was also used to verify phase changes of coated sample after heating treatments.
The anti-oxidation performance of MgO–SiO2–Al2O3–ZnO ceramic-glass coating was shown in Fig. 1. It demonstrated that the anti-oxidation property of the coating was improved from 900°C to 1050°C, and which could up to 84.2% at 1050°C. But the anti-oxidation property of the MgO–SiO2–Al2O3–ZnO ceramic-glass coating decreased rapidly to 65% over 1150°C. This result approved that the MgO–SiO2–Al2O3–ZnO ceramic-glass coating could efficiently protect the carbon steel from oxidation below 1150°C.

Anti-oxidation property of coating at 900°C−1150°C for 60 min.
The non-isothermal kinetic for the coated and blank sample was shown in Fig. 2. The non-isothermal kinetic was calculated according to Arrhenius Eqs. (6) and (7).21)
| (6) |
| (7) |

Non-isothermal kinetic of coated and blank sample: (a) weight loss and (b) the reaction rate at different temperatures maintained for 5 min.
The Ea value of blank sample was 108.65 kJ/mol and that of coated sample was 202.55 kJ/mol. The higher value of Ea indicated that it was difficult to carry out the oxidation of the coated sample. It meant the MgO–SiO2–Al2O3–ZnO ceramic-glass coating played a protective role for the anti-oxidation of carbon steel.
As the MgO–SiO2–Al2O3–ZnO ceramic-glass coating indicated oxidation resistance for carbon steel at 1050°C, the isothermal kinetic was studied at 1050°C. For isothermal kinetic, the temperature was constant and t was the variable. The oxidation kinetics of metals or alloys followed the parabolic law at high temperature, which indicated the oxidation reaction was controlled by the diffusion.22) The reaction rate constant (kp) was given in Eq. (10).23)
| (8) |
Where, H was a constant, ti was oxidation time and Δw was the weight gain per area (mg/cm2).
Pieraggi had analyzed the relationship of (Δw)2 and t. But they did not get the true parabolic reaction rate constant (kp).24) In this study, the relationship of Δw and t1/2 was used to evaluate the kp. Figure 3(a) showed the coated sample had good anti-oxidation performance for the carbon steel at 1050°C. A relationship of Δw and t1/2 for blank and coated samples was shown in Fig. 3(b) for comparison. The value of kp for blank sample (0.61 mg2·cm−4·s−1) was 7.6 times as much as for that of coated sample (kp=0.08 mg2·cm−4·s−1), which indicated coated sample had better anti-oxidation property than that of blank sample.

Isothermal kinetic oxidation at 1050°C: (a) weight gain per area for 600 min, (b) a relationship between weight gain per area and t1/2.
Figure 4 was the morphology of the coating after heating treatment at certain temperatures (900°C, 1000°C, 1050°C, 1100°C) for 60 min. As shown in Fig. 4(a), the surface of the coating was porous structure at 900°C. The MgO–SiO2–Al2O3–ZnO coating formed a compact structure at 1000°C, which might be attributed to solid state sintering reaction in the coating and lead to good anti-oxidation performance for the coating. Figure 4(b) was the inner surface of the scale stripped from the sample. It demonstrated that the structure contained certain porosity at 900°C. The material components had the sintering reaction and formed a dense structure with the increasing temperature. It clearly indicated the inner surface of the scale had the compact structure at 1050°C. Based on the anti-oxidation results, the suitable temperature for the MgO–SiO2–Al2O3–ZnO ceramic-glass coating was 1000–1100°C. Compacted structure was formed for the coating to prevent the diffusion of ions.

SEM images of coated sample at different temperatures: (a) the outer surface (b) the inner surface.
Figure 5 was the EDS results of outer surface for the coated sample. The outer surface of coated sample was composed of Si, Al, Mg and Zn elements. The molten material (point C) was mainly composed of Si element, while the non-molten material (point A and B) in the coating were mainly composed of Mg, Al and Zn elements. Combined the anti-oxidation effect with EDS results of the outer surface for the coating, it exhibited that the compounds maintaining Si, Mg, Al and Zn elements had major contribution to improve the anti-oxidation effect for the carbon steel.
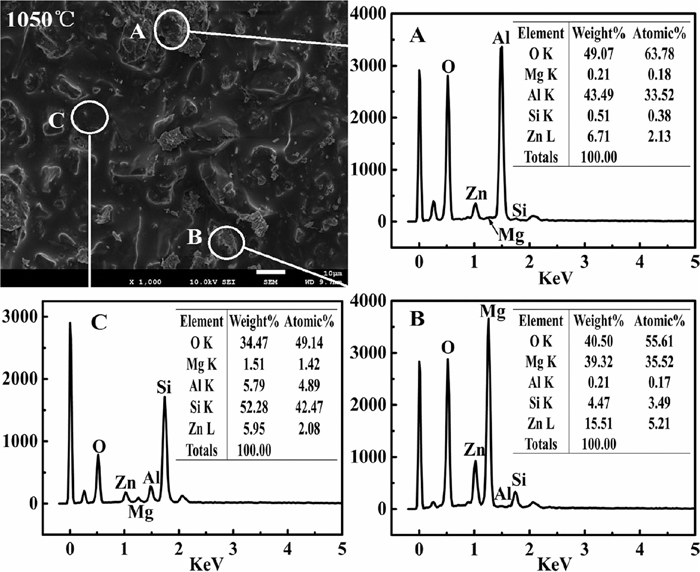
EDS results of outer surface for the coated sample at 1050°C.
The TG-DTA result was shown in Fig. 6. The Endo and Exo were abbreviated for endothermic and exothermic, respectively, which represented for the endothermic and exothermic direction in Fig. 6. In Fig. 6(a), there was a sequence of exothermic peaks from 500°C to 1000°C, which indicated the oxidation reactions were occurred for blank sample. In Fig. 6(b), the exothermic peaks were appeared below 600°C, which indicated that other reactions had occurred in coating (such as phase transformation). Compared comprehensively, the formations by some reactions (such as reactions of coating materials, reactions between coating materials and carbon steel) potentially prevented the oxidizing reaction of carbon steel, which could indicate the MgO–SiO2–Al2O3–ZnO coating played an important role in improving the anti-oxidation performance for carbon steel.

TG-DTA curves of (a) blank and (b) coated sample.
Figure 7 was the XRD curves of the coated sample heated at different temperatures. As shown in Fig. 7(a), the coating was composed of SiO2, Al2O3, MgO, ZnO and some trace amounts of FeO and Fe3O4. The amorphous silica had significant impact on the improvement of anti-oxidation property.25) MgO also played an important role in improving anti-oxidation performance, which was due to the diffusion coefficient of oxygen ion in MgO was smaller than that in iron oxides.26) With increasing temperature, the magnesium and silicon began to react with iron ions, and MgSiO3, MgFe2O4 and (Fe0.4Mg0.6)2SiO4 were formed at 1000°C. According to reactions (a), (b) and (c), MgFe2O4 was formed due to counter-diffusion of Mg2+ and Fe2+ through the oxide lattice. In this case, reaction (a) showed the total reaction between the iron element and magnesium element; reaction (b) showed the reaction between iron-rich phase and MgFe2O4; reaction (c) showed reaction between MgO-rich phase and MgFe2O4.27,28) MgFe2O4 could slow down the spread rate of oxygen and iron ions, which was due to its smallest diffusion coefficient of iron ion than that in Fe2O3, FeO and Fe3O4. Therefore, it could directly decrease the oxidation of the steel at high temperature.18,19)

XRD result of the coated sample heated at (a) 900°C, (b) 1000°C, (c) 1050°C and (d) 1100°C for 60 min.
As temperature increased to 1050°C, compounds containing Zn and Al elements were formed, such as ZnSiO4, ZnFe2O4 and Al86Fe14. Meanwhile, (Fe0.4Mg0.6)2SiO4 changed into (Fe0.85Mg0.15)2SiO4 with more iron ions participated in the oxidation reaction. SiO2 was the main composition of the coating when temperature was below 1050°C, which made a contribution to improve the anti-oxidation of steel. When temperature was up to 1100°C, the main compositions of the scale were MgFe2O4, ZnFe2O4, Fe2SiO4, Al86Fe14 and (Fe0.94Mg0.06)2SiO4. The Fe2SiO4 could improve the anti-oxidation performance of carbon steel below 1173°C.18) The formation of Fe2SiO4 was demonstrated in reaction (d). Combined the anti-oxidation performance with the XRD result of the coated sample, it indicated that Mg, Zn, Si and Al elements had major contribution to enhance the anti-oxidation property of carbon steel.
| (a) |
| (b) |
| (c) |
| (d) |
Figure 8 was the SEM-EDS results of the coated sample heated at 900°C, 1000°C, 1050°C and 1100°C for 60 min. It indicated the silicon element formed a compact structure and the aluminum element formed a relative enrichment layer at the outside of the ceramic-glass coating in Figs. 8(a) and 8(b). Comparing the anti-oxidation performance with XRD results, it indicated that Si and Al element played an important role in improving anti-oxidation performance below 1000°C. As shown in Figs. 8(a) and 8(b), silicon in the coating formed compact structure which could prevent the diffusion of ions. Silicon in the coating reacted with FeO to form Fe2SiO4, which could improve the anti-oxidation performance of carbon steel below 1173°C.18,29) With increasing temperature, the silicon compound changed into a loose and porous structure. Meanwhile, a layer of aluminum was formed on the surface of coating, which enhanced the oxidation resistance of carbon steel.30) Mg and Zn layers were also formed at the surface of coating when the temperature was over 1000°C. The Fig. 8 also clearly indicated MgO–SiO2–Al2O3–ZnO coating prevented the diffusion of iron ions at 1050°C, which was correspond to the previous good anti-oxidation performance under this temperature condition. The formation of layer was due to the selective oxidation and diffusion of the corresponding elements, which could prevent the destructive oxidation reaction for the steel substrate.31) MgO and MgFe2O4 effectively slowed down the inward diffusion of oxygen ions and outward diffusion of iron ions, respectively.19,26) Hence, MgO and MgFe2O4 played an important role in improving the anti-oxidation performance of carbon steel. For Zn element, it could react with the agents in environment and form a chemically inert thin layer, which could prohibit carbon steel from further corrosion. Furthermore, Zn had higher oxidation potential than iron which could slow down the oxidation of iron ions.31)

SEM-ESD results of the coated sample at different temperatures for 60 min: (a) 900°C, (b) 1000°C, (c) 1050°C and (d) 1100°C.
Combined with the protection of silica matrix, the Al, Mg and Zn compound layers that were on the outer side of the coating also played an important role in improving the anti-oxidation performance of carbon steel. Fe2SiO4, MgO, MgFe2O4, ZnFe2O4 formed by the reaction between the coating and steel substrate could form a protective phase to prevent the diffusion of iron and oxygen ions.
In present work, a MgO–SiO2–Al2O3–ZnO ceramic-glass coating sprayed on carbon steel was studied. The conclusions of this study were as follows:
(1) The MgO–SiO2–Al2O3–ZnO ceramic-glass coating was able to enhance anti-oxidation effect of the carbon steel by 84.2% at 1050°C for 60 min. The kp value (0.61 mg2·cm−4·s−1) of blank sample was 7.6 times as much as that of coated sample (0.08 mg2·cm−4·s−1), which indicated the MgO–SiO2–Al2O3–ZnO ceramic-glass coating decreased oxidation rate of carbon steel.
(2) The formations of MgFe2O4, ZnFe2O4, MgSiO3, Fe2SiO4, ZnSiO4, SiO2, Al2O3 and (FexMg1−x)2SiO4 (x=0.4, 0.85, 0.94) by reactions between coating and steel substrate could slow down the spread of ions and enhanced the anti-oxidation performance of the carbon steel.
(3) The formations of (Al, Mg and Zn compound layer) in the MgO–SiO2–Al2O3–ZnO ceramic-glass coating played an important role in improving the anti-oxidation performance of carbon steel.
The authors expressed gratitude for the financial support from the Natural Science Foundation of China (No. 51202249) and 863 Project (No. 2011AA06A104).