2018 年 58 巻 5 号 p. 823-832
2018 年 58 巻 5 号 p. 823-832
The volumetric shrinking characteristics and kinetics of vanadium titanomagnetite carbon composite hot briquette (VTM-CCB) during isothermal reduction were investigated in this paper. It was found that the volumetric shrinking occurs as a combination of a loss of carbon and oxygen from VTM-CCB as well as sintering of iron oxide, suppression of growth of iron whiskers, formation of molten phases, and residual solid carbon content. In the temperature range from 900°C to 1100°C, the carbon gasification is viewed as the primary factor for the shrinking behavior. In the temperature range higher than 1100°C, the shrinking of VTM-CCB is mainly caused by the formation of molten slag, liquid iron, and residual solid carbon. It also should be pointed out that, in the higher temperature range, the volumetric shrinkage of VTM-CCB decreased with increasing FC/O ratio, which is mainly due to the fact that a higher FC/O ratio leads to a decreasing of molten slag generation proportion and a suppression of aggregation growth of liquid iron by the increasing of residual solid carbon. Based on the kinetics analysis, The formula for the shrinkage of VTM-CCB during reduction can be obtained, and the value of shrinking activation energy of VTM-CCB decreased obviously from 337.86 kJ/mol to 84.85 kJ/mol with increasing FC/O ratio from 0.8 to 1.4.
Vanadium titanomagnetite, as a kind of complex iron ore containing iron, vanadium, titanium, etc., is widely distributed in China, America, Russia, South Africa, and New Zealand. In China, there is about 18 billion tons of vanadium titanomagnetite deposit in Panxi district and Chengde district.1,2,3) Presently, vanadium titanomagnetite concentrate is conventionally used as the raw materials of BF smelting process. It is known that the volumetric swelling of iron-bearing burdens such as sinter and pellet leads to degradation and lowering gas permeability of packed beds and makes blast furnace (BF) smelting difficult.4) Therefore, as the two main iron-bearing burden of blast furnace smelting vanadium titanomagnetite, the volumetric swelling of vanadium titanomagnetite sinter and pellet plays a significant role for BF smooth operation.
Large amounts of previous literatures focused on the volumetric swelling and degradation mechanism of vanadium titanomagnetite sinter and pellet. Pimenta et al.5) believed that the solid solution formed between TiO2 and iron oxides and secondary hematite may be the responsible factors for the intensity of disintegration of sinter during reduction at low temperature. Bristow et al.6) investigated the influence of mineral compositions and microstructure on the reduction degradation behavior and demonstrated that the transition of sinter from hematite to magnetite during reduction is the primary reason of the degradation behavior. Ji et al.7) found the catastrophic swelling of V-Ti bearing pellet during reduction is mainly caused by the growth of iron whiskers and TiO2 content and proposed an index as α=Fe3+/TFe-0.70TiO2 to predict the swelling characteristics of V-Ti bearing pellet. Besides, vanadium titanomagnetite sinter and pellet also show a poor reducibility due to the existence of V and Ti.8) Therefore, the development of a new-type vanadium titanomagnetite BF burden with excellent metallurgical performance has attracted significant interest.
Carbon composite iron ore hot briquette (CCB), as a new type of BF burden for low-carbon and low-temperature ironmaking, is put forward by the researchers in Japan.9,10,11,12,13,14,15) CCB exhibits great reducibility due to the fact that the coal and iron ore in CCB adjoined closely accelerates the carbon gasification and iron oxides reduction.16) Previous literature illustrated that carbon composite iron ore could lower the temperature of the thermal reserve and carbon consumption in a commercial BF.17) Therefore, we applied CCB technologies to the vanadium titanomagnetite utilization and proposed vanadium titanomagnetite carbon composite hot briquette (VTM-CCB) as a new type of BF ironmaking raw material. VTM-CCB is produced from the mixture of fine vanadium titanomagnetite concentrate and fine coal using the thermal plasticity of coal by hot-briquetting and heat treatment processes. Our previous researches illustrated that VTM-CCB exhibited high cold crushing strength and excellent reducibility during reduction.18,19) Besides, the softening-melting-dripping behavior and gas permeability of mixed burden got improved obviously with charging 20% VTM-CCB.20) It is well known that the volumetric change of iron-bearing material during its reduction has a significant effect on BF ironmaking, especially abnormal volumetric swelling. Large amounts of studies illustrated that the abnormal volumetric swelling behavior of iron-bearing material would deteriorate the permeability of BF pecked bed and lower the BF smelting index inevitably. Compared with traditional iron-bearing material, carbon composite pellet performed an obvious volume shrinking during its reduction. K. Ohno et al. explored the shrinking behavior of iron-charcoal composite made out of different kinds of charcoal during its reduction.17) Therefore, as a new-type burden for BF smelting VTM, the volumetric shrinking behavior of VTM-CCB during reduction should be intensively investigated, aiming to evaluate the reduction performance of VTM-CCB comprehensively.
In the present work, the volumetric shrinking behavior of VTM-CCB during isothermal reduction was predominantly investigated to provide more useful information for BF smelting VTM-CCB technology. Firstly, the effects of reduction temperature, FC/O ratios, and reduction time duration on the volumetric shrinkage of VTM-CCB were explored systematically. Next, the shrinking mechanism of VTM-CCB was analyzed and discussed by chemical analysis, SEM-EDS, and Factsage 7.0 package. Finally, the shrinking formula and activation energies of VTM-CCB were obtained by kinetics analysis in the temperature range from 900°C to 1300°C.
The VTM-CCB samples used in the present study was prepared by hot briquetting and heat treatment processes, as shown in Fig. 1. Firstly, the vanadium titanomagnetite and coal were ground to a particle size of less than 75 um. Next, the obtained fine vanadium titanomagnetite and fine coal were mixed uniformly. After that, the mixtures were charged into an ellipsoid die and heated to 200°C and briquetted under a pressure of 40 MPa to form briquettes. Then, the briquettes were charged into heat furnace to take heat treatment at 500°C for 3 hours. Finally, VTM-CCB samples were taken out from heat furnace and placed into coal powder to cool down to room temperature. The weight of each obtained VTM-CCB sample was about 9 g.

Preparation process of VTM-CCB.
In this article, the types of VTM-CCB samples were made out of different FC/O ratios. The FC/O ratio of VTM-CCB, calculated by the ratio of the fixed carbon mol (C) to the reducible oxygen mol (O) in iron oxides, were set in the range from 0.8 to 1.4 (FC/O=0.8, 1.0, 1.2, 14). The chemical compositions of vanadium titanomagnetite concentrate and proximate and ash analysis of the coal used in present study are listed in Tables 1 and 2, respectively. The chemical composition of VTM-CCB with different FC/O ratios are listed in Table 3. It is clearly that the carbon content of VTM-CCB increased from 12.35% to 19.57% with increasing FC/O ratio from 0.8 to 1.4.
| TFe | Fe2O3 | FeO | CaO | SiO2 | MgO | TiO2 | V2O5 | Cr2O3 |
|---|---|---|---|---|---|---|---|---|
| 62.12 | 56.4 | 29.11 | 0.22 | 2.12 | 0.92 | 5.05 | 0.95 | 0.61 |
| Proximate analysis | Ash analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| FC | Vdaf | Aad | Mad | CaO | SiO2 | MgO | Al2O3 | TFe |
| 61.55 | 28.05 | 8.79 | 1.61 | 4.95 | 55.15 | 2.18 | 21.93 | 4.01 |
Note: FC-fixed carbon content; Vdaf-volatile matter content on dry ash free basis; Aad-ash content on air dry basis; Mad-moisture on air dry basis.
| FC/O ratio | TFe | FeO | CaO | SiO2 | MgO | Al2O3 | TiO2 | C |
|---|---|---|---|---|---|---|---|---|
| 0.8 | 54.64 | 25.60 | 0.28 | 2.84 | 0.85 | 3.20 | 4.44 | 12.35 |
| 1.0 | 52.82 | 24.75 | 0.29 | 2.98 | 0.83 | 3.19 | 4.29 | 14.92 |
| 1.2 | 51.11 | 23.95 | 0.30 | 3.11 | 0.81 | 3.17 | 4.16 | 17.32 |
| 1.4 | 49.51 | 23.20 | 0.31 | 3.23 | 0.79 | 3.16 | 4.02 | 19.57 |
As shown in Fig. 2, the experimental apparatus comprise electrical furnace, gas control system, computer control system, and digital camera. A working Al2O3-tube with inner diameter of 30 mm was mounted inside the electrical furnace and was heated on both sides. One end of the working Al2O3-tube was supplied by a nitrogen flow. The other end of the tube was left open for placement of a Al2O3-pad with VTM-CCB sample and capturing the sample size changes by digital camera. Before every experiment, the working Al2O3-tube was sealed and purged with high purity nitrogen at 4 NL/min for 1 hour. After the purging period, the tube was heated to the specified isothermal reduction temperature. The isothermal reduction temperatures were set as 900°C, 1000°C, 1100°C, 1200°C, and 1300°C. The Al2O3-pad with VTM-CCB sample was inserted in the constant temperature zone of the tube when the temperature of the tube reached the desired value. At the five selected reduction temperatures, the VTM-CCB sample was reduced for different time ranging from 0 min to 30 min. After the predetermined reduction time, the VTM-CCB sample was took out from the tube and was cooled to room temperature under the protection of high purity nitrogen.

Schematic diagram of experimental apparatus.
The reduction index of metallization degree of the reduced VTM-CCB samples was calculated by the following formula:
| (1) |
The volumetric shrinkage of reduced VTM-CCB Sh was calculated by Eq. (2),21) given as follows:
| (2) |
Figure 3 shows the effect of reduction temperature and FC/O ratio on metallization degree and FeO content of VTM-CCB samples reduced for 30 min. It can be seen that the metallization degree of VTM-CCB highly depends on reduction temperature. The metallization degree of VTM-CCB reduced at 1200°C for 30 min is about 90% and increases slowly with further increase in the reduction temperature. Besides, the metallization degree also increases with increasing FC/O ratio duo to the fact that a higher FC/O ratio provides more reductant and promotes the reduction of VTM-CCB. In contrast, the FeO content of VTM-CCB decreases with increasing FC/O ratio and reduction temperature. At the four selected FC/O ratios, the FeO contents of VTM-CCB reduced at 900°C for 30 min are all higher than 60%. However, it decreases sharply from higher than 60% to lower than 10% with increasing reduction temperature to 1300°C. Especially, the FeO content of VTM-CCB reduced at 1300°C for 30 min is only about 2% when the FC/O ratio is 1.4.
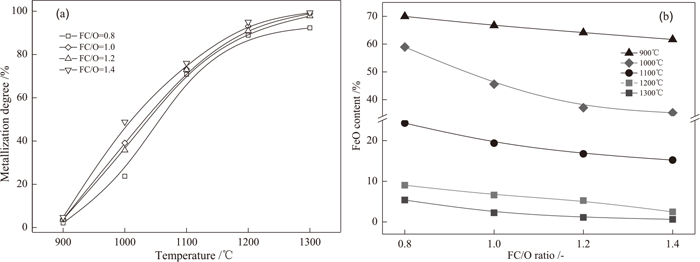
Effect of temperature and FC/O ratio on metallization degree and FeO content of VTM-CCB reduced for 30 min.
Figure 4 shows the volumetric shrinkage of VTM-CCB as a function of time at different reduction temperature and FC/O ratios. It can be seen clearly that the volumetric shrinkage highly depends on reduction temperature. When FC/O ratio is constant, the shrinkage of VTM-CCB increases with increasing reduction temperature and prolonging reduction time. The VTM-CCB sample with a FC/O ratio of 0.8 reaches a highest volumetric shrinkage when it is reduced at 1300°C for 30 min. It should be pointed out that the volumetric shrinkage of reduced VTM-CCB is relatively small when the reduction temperature is lower than 1100°C, and an obvious increasing of the shrinkage can be observed when the reduction temperature is higher than 1100°C.
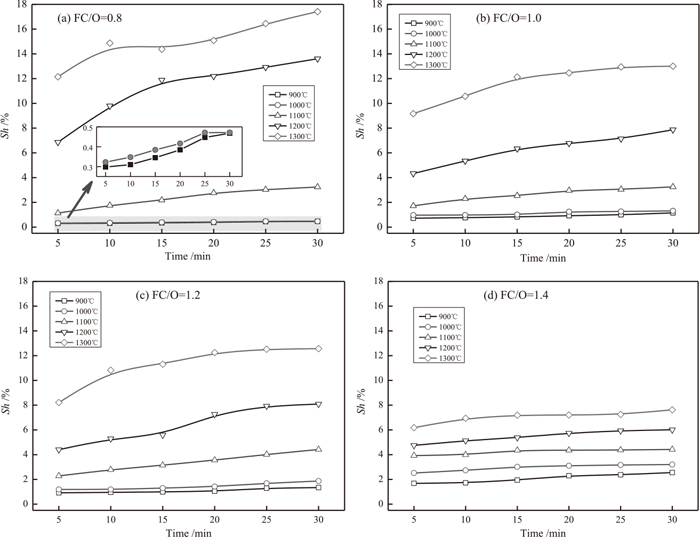
Volumetric shrinkage of VTM-CCB during reduction.
The macro-morphologies of reduced VTM-CCB samples are presented in Fig. 5. It can be observed that VTM-CCB samples after reduction remains intact and there is no serious disintegration occurred. Besides, it is found that there are some metallic iron particles generated on the surface of reduction products when the FC/O ratio of VTM-CCB sample is higher than 1.2 and the reduction temperature is up to 1300°C. The amount of the iron particles on the surface of VTM-CCB samples increases with increasing FC/O ratio from 1.2 to 1.4.

Effect of reduction temperature and FC/O ratio on the macro-morphology of VTM-CCB.
It is generally agreed the abnormal swelling occurs during the reduction of iron ore pellet due to the transformation of the pellets from the hematite to magnetite and the growth or iron whiskers in the reduction of wustite. However, the volumetric shrinkage of the VTM-CCB samples during reduction in the temperature range from 900°C to 1100°C is relatively small, which is mainly due to a combination of vanadium titanomagnetite particles sintering, iron oxides reduction swelling, iron whiskers growth suppression, and carbon gasification. Figure 6 shows the evolution of microstructure of VTM-CCB (FC/O=1.4) before and after reduction at 900°C, 1000°C and 1100°C for 30 min. Compared with the VTM-CCB before reduction, it was observed that vanadium titanomagnetite particles (bright phase) lost the identities and spherodised gradually and the sintering of iron ore occurred with increasing reduction temperature. With the sintering of vanadium titanomagnetite particles occurring, the volume of vanadium titanomagnetite particles shrunk gradually and then some fine cracks generated at the edge of vanadium titanomagnetite particles, as shown in Fig. 6. Therefore, the sintering of vanadium titanomagnetite particles in VTM-CCB promotes it shrinking during its reduction.

Evolution of microstructure of VTM-CCB after reduction at different temperature.
Numerous investigators illustrated that the iron whiskers growth has been extensively regarded as the major cause of the reduction swelling of iron ore pellet.22,23,24) Figure 7 presents the SEM images of VTM-CCB (FC/O=1.4) reduced at 1100°C for 30 min. As shown in Fig. 7(a), the iron whiskers in VTM-CCB were near-spheroidal and quite fine and exists only over a small region on the wustite surface, which indicates the growth of iron whiskers is suppressed effectively. The suppression of the growth of iron whiskers can be attributed to the following reasons. (1) VTM-CCB containing coal as reductant are less susceptible to present swelling due to the presence of residual volatile matter. Our previous research25) illustrated that, the volatile matter, mainly containing hydrogen, has an obvious suppression on the growth of iron whiskers. (2) Generally, the growth of iron whiskers is greatly depended on the iron oxide sample surface characteristics. It is well known that the annealing of iron oxide sample would smooth its surface, which results in the iron whiskers prefer to form as sponge iron or iron layer rather than conical and cylindrical. The VTM-CCB samples used in this research were prepared by heat treatment at 500°C for 3 hours. After heat treatment, the surface characteristics of iron oxide in VTM-CCB would be altered and then the growth of iron whiskers would be suppressed.26,27)

SEM images of VTM-CCB (FC/O=1.4) reduced at 1100°C for 30 min.
Figure 8 shows the effect of reduction temperature on residual carbon content of VTM-CCB samples with different FC/O ratio and the calculated equilibrium pressure of carbon gasification reaction. Compared with the initial carbon content in VTM-CCB, the residual carbon content decreases slightly at the reduction temperature of 900°C due to the fact that the carbon gasification reaction rate is relatively slow. However, the gasification reaction rate almost reaches the maximum value in the reduction temperature range higher than 900°C, which implies a higher rate of carbon and oxygen loss from VTM-CCB. As a result, the large cracks and voids generated in VTM-CCB, as shown in Fig. 7(b), and these cracks and voids free up space for the growth of iron whiskers and result in an efficient suppression of the volumetric swelling of VTM-CCB. Thus, under the comprehensive function of vanadium titanomagnetite particles sintering, iron oxides reduction swelling, iron whiskers growth suppression and carbon gasification, the value of volumetric shrinkage of VTM-CCB is relative small when reduced in the temperature range from 900°C to 1100°C.

Effect of reduction temperature on residual carbon content of VTM-CCB samples with different FC/O ratio.
Figure 9 shows the effect of FC/O ratio on the terminal shrinkage of VTM-CCB at different reduction temperature. In the reduction temperature range lower than 1100°C, the volumetric shrinkage of VTM-CCB increases gradually with increasing FC/O ratio. However, in the reduction temperature range higher than 1100°C, the volumetric shrinkage of VTM-CCB decreases with increasing FC/O ratio. The difference of the shrinkage tendency is caused by different shrinking mechanism in the two reduction temperature range. In the reduction temperature range lower than 1100°C, a higher FC/O ratio indicates more carbon and oxygen would be removed during reduction, which benefits the shrinking of VTM-CCB. However, the shrinking behavior of VTM-CCB in the temperature range higher than 1100°C is mainly concerned with the formation of molten phases including liquid slag and liquid iron.

Effect of FC/O ratio on the terminal shrinking of VTM-CCB at different temperature.
To investigate the effect of FC/O ratio and temperature on the phase composition of VTM-CCB thermodynamically, thermodynamics equilibrium analysis was carried out on the VTM-CCB of 100 g by FactSage 7.0. During the thermodynamics equilibrium calculation, the selected database is FToxide. The calculated chemical compositions of VTM-CCB made out of different FC/O ratios are listed in Table 3. The possible products were identified in inert atmosphere under total pressure of 1.01325×105 Pa in the temperature range from 800°C to 1400°C. The products selected from the calculated results were determined by the principle of minimal gibbs free energy. Figure 10 presents the thermodynamics calculation results of the reduction of VTM-CCB with different FC/O ratio. The generated molten phases during the reduction of VTM-CCB include liquid slag and liquid iron. When FC/O ratios are 0.8, 1.0, 1.2, and 1.4, the molten slag start generating temperature are about 1100°C, 1250°C, 1300°C and 1350°C, respectively. The liquid iron only generates with a FC/O ratio higher than 1.0 because a higher FC/O ratio provides sufficient carbon to promote carburization of reduced iron and realize the decreasing of the melting point temperature of reduced iron. With a FC/O ratio of 0.8, the reduction products comprises solid iron, molten slag and Ti-spinel at 1300°C. In contrast, with a FC/O ratio of 1.4, the main phases of VTM-CCB reduced at 1300°C include liquid iron, solid slag and residual solid carbon.
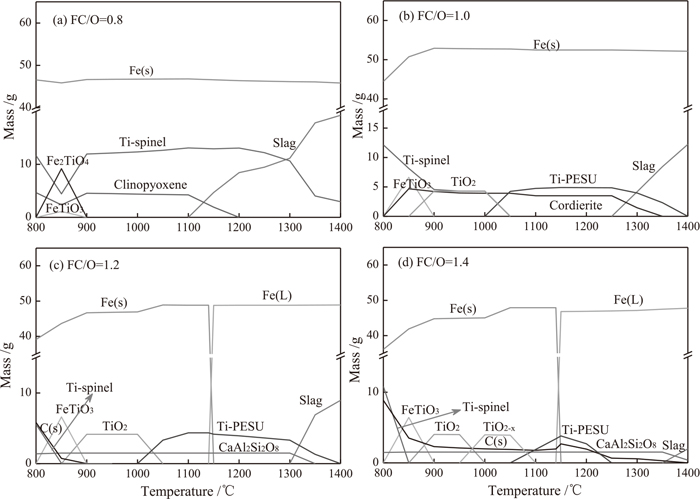
Thermodynamics calculation results of the reduction of VTM-CCB with different FC/O ratio.
The chemical compositions of VTM-CCB slag after reduction were detected and listed in Table 4. Based on the detected chemical compositions of VTM-CCB slag, the MgO–Al2O3–TiO2–SiO2–FeO quinary system of VTM-CCB slag were calculated by FactSage 7.0 with setting an oxygen partial pressure of 10−5. Figure 11 shows the isothermal sections of the MgO–Al2O3–TiO2–SiO2–FeO quinary system of VTM-CCB with different FC/O ratio at 1300°C. In Fig. 11, the red zone, brown zone and white zone are purity molten slag zone, purity solid slag zone and solid-liquid coexistence slag zone, respectively. The solid condensed phase mainly includes spinel, olivine, monoxide, pseudobrookite, rutile, and cordierite, as shown in Fig. 11. It can be seen that the four type VTM-CCB slag systems are all located in the solid-liquid coexistence zone. However, the purity liquid slag zone only exists when FC/O ratio is 0.8, and the purity solid slag zone expands with increasing FC/O ratio. Obviously, the increasing of the solid phase would inhibit the shrinking of VTM-CCB.
| FC/O | SiO2 | MgO | Al2O3 | TiO2 | FeO |
|---|---|---|---|---|---|
| 0.8 | 16.67 | 4.97 | 18.80 | 26.11 | 31.81 |
| 1.0 | 22.76 | 6.34 | 24.35 | 32.79 | 11.51 |
| 1.2 | 25.27 | 6.59 | 25.81 | 33.80 | 6.05 |
| 1.4 | 27.02 | 6.66 | 26.45 | 33.62 | 3.64 |
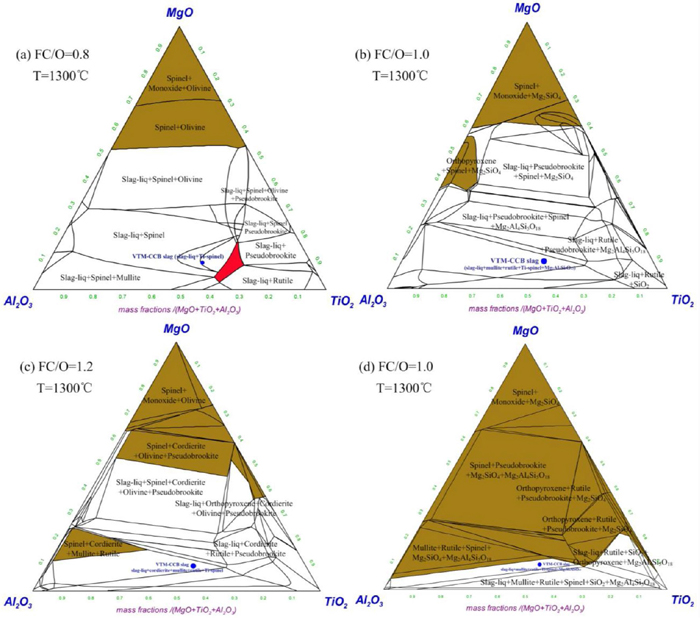
Isothermal sections of the MgO–Al2O3–TiO2–SiO2–FeO quinary system of VTM-CCB with different FC/O ratio. (Online version in color.)
The molten slag generation ratios were calculated by the equilibrium module in FactSage 7.0. Figure 12 shows the variation of generation slag ratio for different FC/O ratio of VTM-CCB. When the reduction temperature is 1300°C and FC/O ratio is 0.8, the generation slag ratio is up to 80%, which is beneficial to the shrinking of VTM-CCB during reduction. In contrast, at the same reduction temperature, the liquid slag ratio of VTM-CCB with a FC/O ratio of 1.4 is less than 5%. Therefore, a lower FC/O ratio is favorable to the shrinking of VTM-CCB in the reduction temperature range higher than 1100°C.

Variation of liquid slag ratio for different FC/O ratio of VTM-CCB. (Online version in color.)
To explore the effect of molten phase and solid phase on the volumetric shrinking of VTM-CCB, SEM-EDS analysis of VTM-CCB reduced at 1300°C for 30 min was carried out, as shown in Fig. 13. The microstructure evolution of VTM-CCB with increasing FC/O ratio is assumed to undergo the following step. With a FC/O ratio of 0.8, as shown in Fig. 13(a), the fresh metallic iron grow and aggregated by progressive metallic iron bridging firstly. According to the EDS of point 1–3, it can be deduced that the iron oxides can not be reduced completely due to the insufficient reductant when FC/O is 0.8. Therefore, the unreduced iron oxides, mainly wustite, started to react with gangue components (CaO, SiO2, Al2O3), forming low melting point compounds. At 1300°C, the vanadium titanomagnetite particles softened and partially melted and metallic iron particles were thus liberated from the gangue network and coalesced together. Besides, due to the melted slag phase bonding and aggregation growth, the sintering and shrinking of VTM-CCB became more apparent. With increasing FC/O ratio from 0.8 to 1.0, the unreduced wusitite decreased and the low melting point compounds phase decreased inevitably. As shown in Fig. 13(b), the decreasing molten slag is dispersively distributed in the solid metallic iron and solid slag, which is obviously unfavorable for the shrinking of VTM-CCB.

SEM-EDS analysis of VTM-CCB with different FC/O ratio reduced at 1300°C for 30 min.
With a FC/O ratio of 1.2, VTM-CCB could be completely reduced at 1300°C. Under this condition, molten slag phases nearly disappeared and the liquid iron generated due to sufficient carburization. Theoretically, the aggregation growth of liquid iron would also favor the shrinking of VTM-CCB. However, the solid gangue and residual carbon are obviously inlayed in the liquid iron, as shown in Fig. 13(c), which leads a mass of pores generated and results in a decreasing of the volumetric shrinkage of VTM-CCB. Figure 14 presents the elemental distribution images of VTM-CCB (FC/O 1.2) reduced at 1300°C for 30 min. It can be seen clearly that the liquid iron, solid gangue and residual carbon are wrapped each other and mutual doping, which causes the inhibition of the shrinking of VTM-CCB during reduction. When FC/O ratio of VTM-CCB is 1.4, the aggregation growth of the liquid iron is much more difficult due to the excessive residual carbon. As shown in Fig. 13(d), the excessive residual carbon particles distribute at the boundary of the liquid iron particles, which inhibits the aggregation and growing up of liquid iron. Additionally, the excessive residual carbon, acting as framework role in VTM-CCB, causes large pores and cracks formed, which results in the shrinkage of VTM-CCB decreases perceptibly.
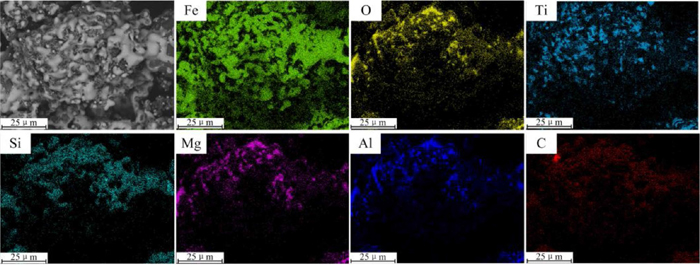
Elemental distribution images of VTM-CCB (FC/O 1.2) reduced at 1300°C for 30 min. (Online version in color.)
In conclusion, the shrinking of VTM-CCB is closely related to the reduction temperature, FC/O ratio and reduction time. In the temperature range from 900°C to 1100°C, the shrinkage value is relatively small due to a combination of iron ore sintering, iron oxides reduction swelling, iron whiskers growth suppression, and carbon gasification. In particular, the shrinkage increased with increasing FC/O ratio as a higher FC/O ratio means more free space would be made by the carbon gasification reaction. In the temperature range higher than 1100°C, the shrinking of VTM-CCB mainly depends on aggregation growth of molten phase and residual solid carbon. Figure 15 shows the reduction shrinking schematic diagram of VTM-CCB at 1300°C. With a FC/O ratio of 0.8, a large amount of molten slag appeared and encapsulated the solid iron, resulting in a decreasing of porosity and an increasing of shrinkage of VTM-CCB. With increasing FC/O ratio from 0.8 to 1.0, the molten slag decreased and solid slag increased, which causes the generation of pores and the decreasing of shrinkage. With further increasing FC/O ratio, although liquid iron appeared due to the sufficient carburization, the solid slag and residual carbon deteriorated the aggregation growth of liquid iron and also promoted the development of pores in VTM-CCB. Therefore, a higher FC/O ratio results in a small shrinkage of VTM-CCB at 1300°C.

Shrinking schematic diagram of VTM-CCB with different FC/O ratio reduced at 1300°C. (Online version in color.)
As a new type of BF ironmaking material, VTM-CCB is charged into the traditional iron-bearing burden consisting of sinter and pellet. Obviously, the volume change of VTM-CCB will influence the reduction swelling, degradation, and smelting behavior of the mixed burden. Therefore, it is necessary to build a mathematical model to predict the shrinking behavior of VTM-CCB during reduction. The VTM-CCB shrinkage with different FC/O ratio have been obtained by reduction experiments.
The fractional linear shrinkage of spherical carbon composite pellet is widely and effectively described by Eq. (3). McAdam et al.28) explored the volumetric shrinking behavior of iron-sand concentrate-coal/char composite pellet and calculated the shrinking activation energy by Eq. (3). Wang et al.29) investigated the volumetric shrinking behavior of ludwigite/coal composite pellet during isothermal reduction and deduced the volumetric shrinking kinetics in the reduction process based on Eq. (3). The shrinking activation energy of ludwigite/coal composite pellet was 276.0±59.52 kJ/mol. Therefore, as a typical type of carbon composite pellet, we applied Eq. (3) to describe the shrinking kinetics of VTM-CCB during isothermal reduction.
| (3) |
Based on Eq. (3) and Arrhenius equation, Eqs. (4) and (5) can be obtained. The value of lnk can be calculated by using the linear fitting between lnSh and lnt. Finally, the value of E can be obtained by Eq. (5).
| (4) |
| (5) |
The radius value of VTM-CCB during reduction can be obtained due to the fact that the shrinkage is uniform and can be converted into spherical pellet of the same volume. Besides, the physical properties such as the size of the briquette, the size distribution and reactivity of the constituent particles of VTM-CCB made out of different FC/O ratios were assumed constant, and the main factors investigated include reduction temperature, reduction time duration, and FC/O ratio of VTM-CCB. The relationships between lnSh and lnt in the reduction duration range from 5 min to 30 min are listed in Fig. 16. It can be seen that the value of lnk at the four selected FC/O ratios are all increased gradually with increasing reduction temperature. Besides, the curves linearity degrees in the temperature range from 900°C to 1300°C are all higher than 0.95, which indicates the linear fitting has a high reliability.
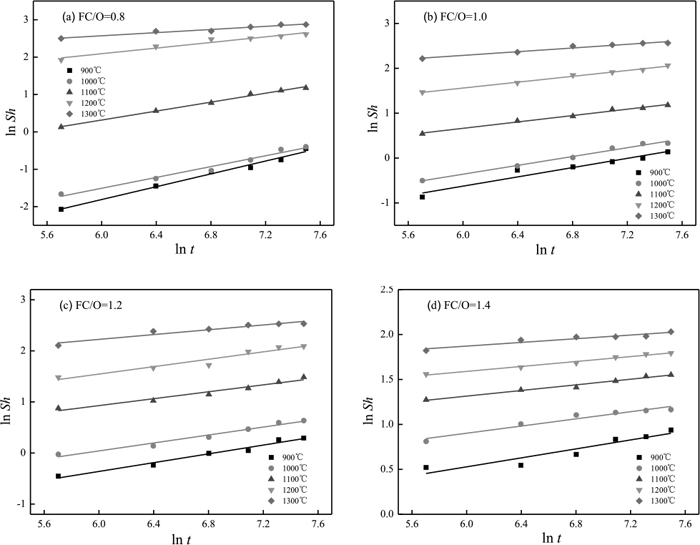
ln Sh vs ln t curves at different FC/O ratios.
The relationships between lnk and 1/T at different FC/O ratio of VTM-CCB are presented in Fig. 17. The shrinking activation energy E of VTM-CCB, calculated based on the Eq. (5), is decreased from 337.86 kJ/mol to 84.85 kJ/mol with increasing FC/O ratios from 0.8 to 1.4. This decreasing tendency of E can be attributed to the shrinking mechanism of VTM-CCB at different FC/O ratio. With a lower FC/O ratio, the shrinking of VTM-CCB is caused by multiple factors, including carbon gasification, iron ore particles sintering, iron whisker suppression and molten phases generation. Especially, the molten slag generation can be viewed as the most important factor in the temperature higher than 1100°C. However, the volumetric shrinking affecting factors of VTM-CCB would simplify with increasing FC/O ratio, and the carbon gasification reaction becomes the decisive shrinking factor of VTM-CCB with a FC/O ratio of 1.4. Therefore, the simplify of shrinking affecting factors results in a noticeable decrease of shrinking activation energy of VTM-CCB during reduction.

Arrhenius plot of the rate constant of shrinkage at different FC/O ratios.
Based on the calculated value of frequency factor k0, shrinking activation energy E, and Eq. (4), the formula for the shrinkage of VTM-CCB during reduction can be obtained as follows:
| (6) |
| FC/O ratio/– | 0.8 | 1.0 | 1.2 | 1.4 |
|---|---|---|---|---|
| a | 26.931 | 15.171 | 11.089 | 7.741 |
| b | 40637.48 | 22738.75 | 16734.42 | 10205.67 |
The volumetric shrinking characteristics and kinetics of vanadium titanomagnetite carbon composite hot briquette (VTM-CCB) during isothermal reduction were investigated in this paper. The following conclusions can be obtained from this study.
(1) The volumetric shrinkage of VTM-CCB is closely related with the FC/O ratio, reduction temperature, and reduction time. With a FC/O ratio of 0.8, the volumetric shrinkage of VTM-CCB reached the maximum value 17.67% when reduced at 1300°C for 30 min.
(2) In the temperature range from 900°C to 1100°C, the volumetric shrinking occurs as a combination of a loss of carbon and oxygen from VTM-CCB as well as sintering of vanadium titanomagnetite particles, suppression of growth of iron whiskers, and the carbon gasification reaction is the primary factor for the shrinking behavior.
(3) In the temperature higher than 1100°C, the shrinking of VTM-CCB is mainly caused by the formation of molten slag, liquid iron, and solid residual carbon content. The volumetric shrinkage of VTM-CCB decreased with increasing FC/O ratio, which is mainly due to the fact that a higher FC/O ratio leads to a decreasing of molten slag generation proportion and a suppression of aggregation growth of liquid iron by the increasing of residual solid carbon.
(4) According to the kinetics analysis, the value of shrinking activation energy of VTM-CCB decreased obviously from 337.86 kJ/mol to 84.85 kJ/mol with increasing FC/O ratio from 0.8 to 1.4. The formula for the shrinkage of VTM-CCB during reduction can be described as Sh=t2/5exp
The authors are especially thankful to National Natural Science Foundation of China (51574067), China Postdoctoral Science Foundation (2016M601321) and National High Technology Research and Development Program of China (No. 2012AA062302 and No. 2012AA062304).